Avocado (Persea americana) is an extremely important fruit crop produced in several areas of the world. Nowadays, it is widely documented as being an important source of energy and vitamins (Araújo et al., 2018). Mexico is the main avocado producer and exporter, with a production of over 2.3 million t, followed by the Dominican Republic, Peru and Indonesia (FAOSTAT, 2020). Nationwide, Michoacán provides over 70% of the production, particularly in the municipalities of Ario de Rosales, Salvador Escalante, Tacámbaro and Tancítaro, due to the volume produced (SIAP, 2019). According to the Food and Fishing Information Service in the municipality of Zitácuaro, the avocado production has increased, since in 2010 it reached 10,027 t and 12,509 t in 2019, which represents an increase of 19.8%.
One of the most devastating diseases on avocado in the world due to its frequency, severity and economic losses is root rot or wilt (Coffey, 1992; Ramírez-Gil et al., 2014). In Mexico, this disease affects all the varieties and is responsible for the deaths of trees in the main avocado-producing areas, covering 5% of the total surface of the crop in Michoacán (Téliz, 2000). Root rot causes necrosis in feeder roots, chlorosis, defoliation and dieback, which reduces yield and leads to the death of trees (Erwin and Ribeiro 1996; Hardham, 2005). The disease is more severe and develops faster in heavy soils, with poor drainage and at an average temperature of 24 ºC (Zentmyer, 1980; Erwin and Ribeiro 1996). Phytopathogenic fungi such as Calonectria ilicicola, Fusarium oxysporum, F. solani, Ilyonectria (=Neonectria) macrodidyma, Rosellinia necatrix, and others have been associated to this disease (Ruano-Rosa and López-Herrera, 2009; Dann et al., 2012; Vitale et al., 2012; Olalde-Lira et al., 2020). Recently, in the Canary Islands, the species P. cinnamomi, P. citricola sensu lato, P. nicotianae, P. niederhauserii, P. palmivora and Phytopythium vexans have been identified as the cause of rot in avocado (Rodríguez-Padrón et al., 2018). In Michoacán, Pythium sp. amazonianum and P. vexans were reported as related to wilt and root rot (Ochoa-Fuentes et al., 2018; Hernández-Pérez et al., 2019). However, P. cinnamomi is the pathogen most frequently associated to root rot in avocado, and it generally infects fine and feeder roots using mobile zoospores (O’Gara et al., 2015; Rodríguez-Padrón et al., 2018). In the genus Phytophthora, it is easier to identify the species P. cinnamomi due to its particular morphological characteristics. In a V8 medium, it develops cotton-like cultures with a prominent cenocytic mycelium, with resistant hyphae with swellings (coraloid), chlamydospores, ovoid and non-papillated sporangia, absent in culture medium. These characteristics are usually enough for its identification (Erwin and Ribeiro, 1996; Robin et al., 2012).
The most commonly used fungicides to control P. cinnamomi are metalaxyl-M (Mtx) and potassium phosphite (Fp) (Ramírez-Gil et al., 2017). Since Mtx was introduced in the market in 1977, it is known to affect the polymerase I complex, inhibiting rRNA synthesis, affecting the growth of hyphae and the formation of haustoria and spores (Müller and Ulrich, 2012). In 1984, Darvas and Becker reported that after constant applications of Mtx on soils, it becomes difficult to control root rot in young avocado plants, suggesting a loss of sensitivity. Studies show that species such as P. cryptogea, P. nicotianae are insensitive or have an intermediate sensitivity to mefenoxam at concentrations of 1 or 100 μg mL-1 i. a. (Hwang and Benson, 2005). However, when testing for sensitivity to Mtx in P. cinnamomi populations isolated from avocado in California, EC50 value were obtained between 0.023 and 0.138 μg mL-1 of i.a., confirming the sensitivity of the isolates to the fungicide (Belisle et al., 2019a).
Potassium phosphite has a complex action mechanism. It is known to have a direct fungistatic effect that reduces the growth and sporulation of the pathogen, allowing the defense system of the host to have more time to kill the invasive organism (Guest and Grant, 1991; Dann and MacLeod, 2021). Studies with Phytophthora spp. confirm the inter- and intraspecific variation in the tolerance to Fp, as well as the promotion of the mycelial growth on isolates exposed to low concentrations (Hunter et al., 2018). In Australia, P. cinnamomi populations displayed variations in sensitivity to Fp, with CE50 values ranging between 4 and 148 μg mL-1 of i.a., (Wilkinson et al., 2001). Recently, in California, in P. cinnamomi populations of lineage A2, genetically different isolates were found, which are less sensitive to potassium phosphite and have high virulence (Belisle et al., 2019b). Linde et al. (1999) suggest that P. cinnamomi isolates with a higher growth rate may be more virulent, and other investigations have concluded that some isolates, less sensitive to potassium phosphite, are also more virulent (Belisle et al., 2019b).
The surface planted with avocado in the municipality of Zitácuaro, Michoacan, has increased in recent years, given the agroclimatic conditions that are adequate for its development. However, root rot is also present in commercial orchards and there are currently no studies on the etiology of the disease in this production region. Therefore, the aim of this study was to phenotypically characterize isolates obtained from avocado tree roots in Zitácuaro, Michoacán and evaluate their sensitivity to fungicides.
Materials and methods
Characteristics of the orchards. In November of 2019, five avocado orchards, located in the municipality of Zitácuaro, Michoacán, were sampled. All orchards are located in a A(C)(w1)(w) semi-warm subhumid climate with abundant rainsfall in the summer, winter rains below 5%, intermediate humidity, according to the Köppen climate system, modified by García (1981), and the established variety is ‘Hass’; in addition, they have heavy, clay-rich soils. Other characteristics of the orchards are indicated in Table 1.
Collection of samples and isolation. Root and soil samples were collected from the rhizosphere, one sample per tree and five trees per orchard, with symptoms of yellowing, defoliation and dieback. The samples were placed in labelled plastic bags in a cooler and transported to the Plant Pathology Laboratory of the Institute of Farming and Forestry Research (Instituto de Investigaciones Agropecuarias y Forestales - IIAF), of the Universidad Michoacana de San Nicolás de Hidalgo (UMSNH). In order to obtain a greater percentage of isolates from roots, the protocols by Erwin and Ribeiro (1996) and Rodríguez-Padrón et al. (2018) were modified. Lateral, partially necrotized roots were selected, washed with tap water and dried using sterilized paper towels. Approximately 2 cm cuts were made, with one healthy part and one necrotized part, placed in inclusion cassettes, which were submerged in commercial chlorine bleach at 2% (v/v) for 45 s and rinsed with sterile distilled water three times. The excess water was removed from the tissue cuts using sterilized paper towels and placed in Petri dishes with a NARPH-V8 (Natamycin 0.02 g L-1, PCNB (Pentachloronitrobenzene) 0.10 g L-1, Ampicillin 0.27 g L-1, Rifampicin 0.01 g L-1, Hymexazol 0.075 g L-1) selective medium. The dishes were incubated at 25 °C in the dark for 24 to 48 h, until the growth of cenocytic mycelium and with the characteristic P. cinnamomi swellings were observed. The cultures were purified by the successive cultivation of hyphal tips on corn meal agar (HMA, Fluka®) culture medium.
Table 1 Characteristics of the avocado root-collecting sites in Zitácuaro Michoacán.
| Huerta | Localidad | Altura msnm | Latitud | Longitud | Humedad | Total de muestras |
|---|---|---|---|---|---|---|
| Benedicto | San Felipe | 1890 | 19° 49' 00" N | 100° 37' 41" W | Temporal | 5 |
| Los llanos | San Felipe | 1890 | 19° 49' 08" N | 100° 37' 52" W | Temporal | 5 |
| Los llanos 2 | Macutzio | 1900 | 19° 49' 17" N | 100° 35' 50" W | Riego | 5 |
| El Martín | Carpinteros | 2176 | 19° 49' 05" N | 100° 31' 58" W | Temporal | 5 |
| El dorado | La soledad | 2266 | 19° 46' 80" N | 100° 29' 50" W | Riego | 5 |
Compatibility type. The compatibility of the isolates obtained was determined using the reference P. cinnamomi isolate A1 (camellia isolate) and A2 (avocado isolate) which were donated by the University of California, Riverside to the Plant Pathology Laboratory (IIAF). In a Petri dish containing scV8 agar medium (900 mL distilled water, 100 mL Campbell´s V8 juice, 1 g CaCO3), a mycelium disc of the isolate with compatibility type A1 was placed, and in another dish, the type A2. Likewise, in each dish, a mycelium disc of the isolate of interest was placed, approximately one centimeter away. The dishes were incubated at 25 ºC in the dark for 10 days. The isolates that formed oospores were assigned the complementary compatibility type in the cross.
Morphological characterization. The pure cultures were cultivated on HMA medium and cV8 agar medium (10 g of CaCO3, 1 L of V8 juice, centrifuged at 4,000 rpm for 20 min) (Fernández-Pavía et al., 2020). The shape of the culture was characterized, along with the appearance of the mycelium. For the production of sporangia, the protocol by Hwang et al. (1975) was modified. Three 5 mm mycelium discs from each isolate were plated in triplicate in Petri dishes measuring 60 x15 mm with 10 mL of V8 agar medium over a sheet of transparent cellophane and exposed to light for 24 h. The cellophane paper with the mycelium was transferred to new Petri dishes, 10 mL of liquid scV8 medium (900 mL of distilled water, 100 mL of V8 juice, 1 g CaCO3), were added and incubated for 24 h at 18 ℃ in the dark. The liquid medium was then poured out, they were rinsed three times and were flooded with a salt solution, following the protocol by Chen and Zentmyer, (1970) (1.64 g of Ca (NO3)2, 0.05 g of KNO3 and 0.48 g of MgSO4 were dissolved in one liter of distilled water and the solution was sterilized for 20 min at a pressure of 15 lb). They were then exposed to white light at 25 ℃ every 24 h, rinsed three times with the salt solution for two consecutive days and the reproductive structures observed were registered (Erwin and Ribeiro, 1996; Abad et al., 2019).
Pathogenicity tests. To perform the pathogenicity tests, four isolates were chosen to be inoculated on avocado fruits with physiological maturity; they were washed with soap and tap water and sprayed with alcohol at 75% to disinfest them. Two mycelium discs, 6 mm in diameter, from isolates ZITR-1-3, ZITR-2-5, ZITR-3-4 and ZITR-5-3, four days old and grown on HMA, were placed on the sides of the fruits; the discs were then covered in Janel™ brand masking tape. Three fruits were used for every isolate, and after inoculation, they were placed in a moist chamber and incubated at 25 °C. As controls, three fruits were inoculated with HMA discs.
Sensitivity to fungicides. The sensitivity of 15 isolates to potassium phosphite (Fp) (Nutriphite plus magnum 40% Gowan®) were evaluated, at final concentrations of 0, 5, 10, 25, 50, 100, 300 and 600 µg mL-1 of i.a. and metalaxyl-M (Mtx) (Ridomil Gold® 480SL) 0, 0.5, 1, 3 and 5 µg mL-1 of i.a. A completely randomized experimental design was used with a factorial arrangement with three replications. The effective concentration at which 50% of the mycelial growth was inhibited (value of EC50) was calculated using the traditional method of dilution in agar (Gray et al., 2018). The fungicide was added to the cV8-A medium at 10% to obtain the final concentrations. Discs measuring 6 mm with the mycelium from the four-day-old isolates cultivated on HMA were placed in the center of the Petri dishes containing the different concentrations. After three days of incubation in the dark at 22 °C, the growth of the culture was measured in two perpendicular directions, the measurement of the inoculum disc was subtracted and the average was obtained. The growth was calculated by dividing the diameter of the culture in the Petri dishes with fungicide by the average growth of the control dishes and it was expressed as a percentage (Hu et al., 2010).
Effect of the temperature and culture medium on the growth of the mycelium. Six isolates were selected, based on the percentage of inhibition to Fp, along with three isolates with low percentage of inhibition and three with high percentage of inhibition (an A [high] and a B [low] were added at the end of the identification code of each isolate used in this test), to determine whether there was or was not a relationship between the velocity of growth and sensitivity. Growth rate (mm per day) was determined at 22 and 25 °C in the cV8 agar and HMA media, following the methodology described by Belisle et al. (2019b). Agar discs, 7 mm in diameter, were cut from the edge of the 4-day-old cultures plated on HMA medium, transferred to the dishes with the corresponding media and incubated in the dark for four days at both temperatures. The diameter of the culture was measured every day.
To calculate the values of the EC50, the average percentage of inhibition of both repetitions was estimated for each concentration of the fungicides based on the percentage observed in the control group, using the formula ICR (%) = (CRT-CRF)/CRT)x100, where ICR is the percentage of inhibition of the radial growth, CRT is the radial growth of the control, and CRF is the radial growth in each of the concentrations. To obtain the EC50, the natural logarithm of each of the studied concentrations was included and a probit analysis was carried out for each isolate related to 50% of its inhibition (Adaskaveg et al., 2015). The experiments were carried out in duplicate.
To determine the effect of the temperature and of the medium on the isolate, the final growth was taken into consideration; the average was obtained and with these values, an analysis of variance and a multiple comparison of averages were carried out (Tukey, 0.05) for a 2x2 factorial experiment. Using the information on the final growth, interaction graphs were created to observe the effect of the factor over the variable. The analyses were carried out and graphs created using R version 4.0.1 (2020-06-06).
Molecular characterization. Isolates ZITR-1-3 and ZITR-5-3 were grown in a green bean medium (green bean and zucchini baby food: Gerber Nestlé®, 339 g, agar 15 g and water 646 mL) for five days. The mycelium was collected, dehydrated at 39 °C for 24 h, stored at -20 °C for 24 h, ground, and the genomic DNA extracted following an extraction protocol based on CTAB (Fernández-Pavía et al., 2020). The DNA was quantified in a spectrophotometer (Varioskan Flash Thermo Scientific NanoDrop 2000®) and diluted to obtain a concentration of 12 ng µL-1. The DNA was amplified by PCR using oligonucleotides for the region of the internal transcribed spacer (ITS). The reactions contained 6.25 μL of GoTaq® Hot Start Master Mix (Promega, Madison, WI, U.S.A.), 0.0675 μL of each of the oligonucleotides ITS4 (TCCTCCgCTTATTgATATgC) and ITS6 (gAAgAAggTgAAgTCgTAACAAgg) (100 pmol μL-1), 3 μL of genomic DNA (12 ng) and ultrapure distilled water, until a final volume of 13 μL was obtained. The amplification reactions were carried out in the following conditions: an initial denaturalization for 2 min at 94 °C, 35 denaturalization cycles for 1 min at 94 °C, alignment for 1 min at 53 °C, extension for 1 min at 72 °C, and a final extension for 10 min at 72 °C. The products of the PCR were sequenced (Macrogen, Seoul, South Korea) and the sequences were edited using the programs PreGap and Gap (http://staden.sourceforge.net). The consensus sequences obtained were analyzed with the BLAST program, from the NCBI [National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov)] to compare with other sequences of oomycetes from this database.
Results
Isolates obtained. Plating was carried out for every root sample out of a total of 25 samples. Fifteen isolates were obtained (Table 2) with morphological characteristics of P. cinnamomi, and in the rest of the dishes, bacterial growth was observed, therefore they were discarded.
Compatibility Type. The crosses of the isolates with a P. cinnamomi strain with compatibility type A1 produced plerotic oospores with amphigynous antheridia. The crosses of the isolates with a strain with compatibility type A2 only produced globose chlamydospores (Figure 1D). The results indicate that the isolates are compatibility type A2.
Morphological and molecular characterization. The isolates developed rosaceous shaped cultures with wavy edges, with abundant, cotton-like, coraloid mycelia, coenocytic hyphae with swellings, with globose chlamydospores on HMA culture medium, whereas in the sV8-A medium, the cultures were star-shaped (Figures 1F-G). The isolates produced sporangia without papillae, ovoid, ellipsoid, persistent and with internal proliferation in the cultures flooded with a salt solution (Figure 1A-E). The characteristics observed coincide with those reported for P. cinnamomi (Erwin and Ribeiro 1996; Hardham, 2005; Abad et al., 2019). The analyzed sequences of the ITS region of the isolates selected for molecular identification ZITR-1-3 and ZITR-5-3 coincide with those reported for P. cinnamomi.
Table 2 Comparison of averages (Tukey, 0.05) of growth diameter and inhibition (%) of isolates of P. cinnamomi collected in Zitácuaro, Michoacán, exposed to potassium phosphite and metalaxyl-M.
| Fosfito de potasio (Fp) | Metalaxil-M (Mtx) | ||||
|---|---|---|---|---|---|
| Concentración (μg mL-1) | Crecimiento (mm) | Inhibición (%) | Concentración (μg mL-1) | Crecimiento (mm) | Inhibición (%) |
| 0 | 16.4 a | 0.0 az | 0 | 17.7 a | 0 a |
| 5 | 16.5 a | -0.7 a | 0.5 | 5.0 b | 72.3 b |
| 10 | 11.2 b | 31.5 b | 1 | 2.3 c | 87.1 c |
| 25 | 8.7 c | 46.8 c | 3 | 0.66 c | 96.3 d |
| 50 | 3.9 d | 75.1 d | 5 | 0.46 c | 97.4 d |
| 100 | 1.0 e | 93.7 e | |||
| 300 | 0.2 e | 98.6 e | |||
| 600 | 0.0 e | 99.6 e | |||
z Means with the same letter are statistically equal
Pathogenicity tests. The tests on the avocado fruits turned out positive. Seventy-two hours after inoculation (hai) dark circular lesions with irregular edges were found on the epidermis. At 96 hai, the diameter of the lesions were between 3 and 6 cm. The control fruits displayed no symptoms and all the isolates were reisolated from the inoculated fruits, thus proving the pathogenicity completing Koch’s postulates (Figure 1H).

Figure 1 Morphological characteristics and pathogenicity of P. cinnamomi. A) Coraloid mycelium; B-C) Sporangia without papillae, ovoid-ellipsoid; D) Globose chlamydospores in groups; E) Internal proliferation of sporangia; F) Cottonlike, star-shaped culture, in a cV8A medium; G) Rosaceous-shaped culture in a HM medium; H) Pathogenicity in fruits of the isolates (downwards) ZITR-2-5, ZITR-3-4, ZITR-5-3, ZITR-1-3 and control.
Sensitivity to fungicides. The effect of fungicides Fp and Mtx on the growth of the culture and the percentage of inhibition of the isolates between treatments was significant (P>0.001). No fungicide-resistant isolates were found. With Fp, no statistical differences were found between isolates for growth (P>0.63) or for inhibition (P>0.982) of the mycelium, unlike with the different concentrations, in which significance was found. Additionally, something in particular that was observed was the promotion of the growth of eight isolates (ZITR-1-3, ZITR-1-5, ZITR-2-1, ZITR-2-4, ZITR-3-2, ZITR-3-4, ZITR-5-1, ZITR-5-3) by Fp at 5 μg mL-1 (1.15-18.61%) (Figure 2). In the comparison of averages, six groups were formed; no significant difference was found in growth between the control (16.76 mm) and the first concentration (16.02 mm). Growth was mainly inhibited at 100, 300 and 600 μg mL-1, with these concentrations it resulted statistically equal, for both growth and for inhibition (Table 2). The values of the effect of Fp on the inhibition of growth ranged between 54.7 and 74.2 % regarding the control, and for the EC50, between 11.8 and 37.7 μg mL-1 with a mean of 24.62 μg mL-1 for potassium phosphite. For Mtx, a lower variation was observed between treatments regarding Fp; at concentrations of 3 and 5 μg mL-1, the mycelium was mostly inhibited; the percentage of inhibition ranged between 80.4 and 100% and the values of EC50 ranged from 0.08 to 0. 34 μg mL-1 with a mean of 0. 215 μg mL-1 of metalaxyl-M. There was no significance in the growth (P> 0.996) or inhibition (P>1.0) of the mycelium among isolates (Table 3).
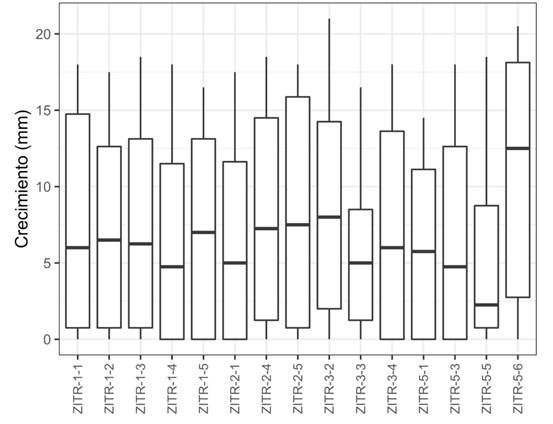
Figure 2 Inhibition of the mycelial growth of P. cinnamomi isolates collected in Zitácuaro, Michoacán against potassium phosphite.
Effect of the temperature and culture medium on the growth of the mycelium. The analyses displayed a significant (P>0.001) effect on the mycelium growth rate at both temperatures, in both nutrient media and their interactions. In the test for the comparison of means, six groups were formed and the isolates with the greatest inhibition (%) to Fp presented a greater growth speed than those with the lowest inhibition. However, isolate ZITRA-3-3, with the highest inhibition (%) displayed the lowest growth rate (11.25 mm) and ZITRB-1-5, with the lowest inhibition (%), presented a higher one (21.75 mm), although the latter also displayed a greater variation in data (Figure 3). The growth of isolates ZITRA-1-4, ZITRA-5-5, ZITRB-3-4 and ZITRB-2-4 fluctuated between 18.66 and 15.91 mm. A higher growth rate was observed in cultures at 25 °C and in the cV8-A culture medium, with the exception of isolate ZITRB-1-5, which displayed a higher growth rate at 22 °C (Figure 4). The morphology of the culture varied according to the culture medium. In the isolates in cV8-A, they displayed star-shaped cultures, and in HMA, rosaceous-shaped cultures.
Table 3 Compatibility type, EC50 and inhibition (%) to potassium phosphite and metalaxyl-M of P. cinnamomi isolates collected in the municipality of Zitácuaro, Michoacán.
| Aislados | Tipo de compatibilidad | % de inhibición (Fp) | CE50 (Fp) (μg mL1) | % de inhibición (Mtx) | CE50 (Mtx) (μg mL1) |
|---|---|---|---|---|---|
| ZITR-1-1 | A2 | 63.3 | 24.5 | 88.7 | 0.18 |
| ZITR-1-2 | A2 | 65.3 | 21.6 | 90.7 | 0.15 |
| ZITR-1-3 | A2 | 62.3 | 37.7 | 87.7 | 0.26 |
| ZITR-1-4 | A2 | 71.1 | 15.7 | 87.8 | 0.2 |
| ZITR-1-5 | A2 | 60.8 | 26.4 | 88.3 | 0.15 |
| ZITR-2-1 | A2 | 63.2 | 21.8 | 83.3 | 0.35 |
| ZITR-2-4 | A2 | 56.5 | 31.6 | 85.8 | 0.17 |
| ZITR-2-5 | A2 | 61.4 | 26.9 | 88.2 | 0.25 |
| ZITR-3-2 | A2 | 54.7 | 34.7 | 83.9 | 0.34 |
| ZITR-3-3 | A2 | 72.7 | 11.8 | 100 | ----- |
| ZITR-3-4 | A2 | 63.2 | 23.8 | 80.4 | 0.22 |
| ZITR-5-1 | A2 | 63.3 | 24 | 88.2 | 0.26 |
| ZITR-5-3 | A2 | 66.8 | 18.7 | 93.3 | 0.24 |
| ZITR-5-5 | A2 | 74.2 | 12.4 | 87.1 | 0.08 |
| ZITR-5-6 | A2 | 55 | 37.7 | 90.2 | 0.17 |
Discussion
This is the first study on P. cinnamomi isolates related to wilt in avocado in eastern Michoacán, in the municipality of Zitácuaro. Other studies on this disease have reported the presence of P. cinnamomi in other areas of the state (Ochoa-Fuentes et al., 2007; Ochoa-Fuentes et al., 2018; Hernández-Pérez et al., 2019). The surface with avocado plantations in Zitácuaro has increased in recent years and could potentially be an important production area in the future. However, the presence of the pathogen P. cinnamomi represents a threat to the crop. This area displays certain agroclimatic characteristics, such as the presence of Acrisol soils, which promote the development of this disease (Zentmyer, 1980; Guillén-Andrade et al., 2007). The sampled trees were adults, established in orchards with high contents of clay and poor drainage, which contributed to the presence of the disease (Zentmyer, 1980; Erwin and Ribeiro 1996; Hardham, 2005). Although in this study only isolates with morphological characteristics of P. cinnamomi in trees with root rot were recovered, the presence of other oomycetes is not discarded.
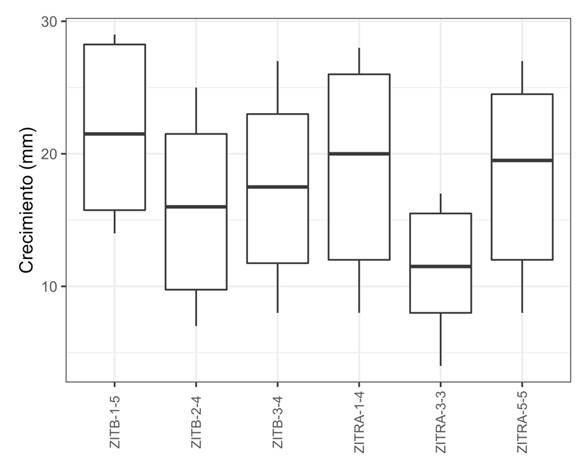
Figure 3 Effect of the temperature and culture medium on the mycelial growth of six P. cinnamomi isolates obtained from avocado roots with rotting
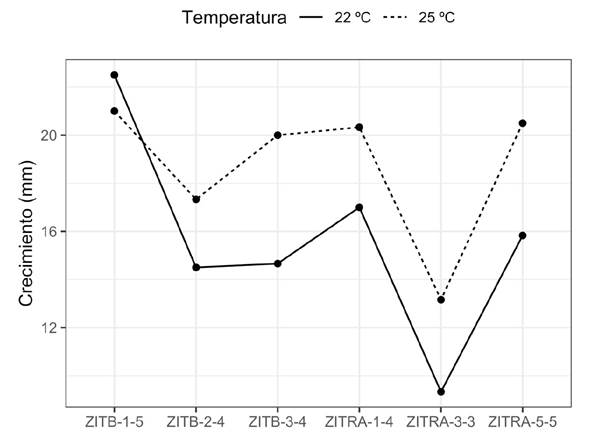
Figure 4 Effect of 22 and 25 ºC temperatures on six P. cinnamomi isolates from avocado roots with rotting.
All isolates corresponded to compatibility type A2, which indicates that the oomycete is reproducing asexually. Despite Ochoa-Fuentes et al. (2005) reporting both compatibility types with molecular markers in Michoacán, they did not prove it with crosses, as performed in this study, which is considered the most accurate way to determine them. The morphological characteristics observed in the isolates coincide with those described for P. cinnamomi according to Abad et al. (2019). This identity was verified with the amplification of the ITS region in selected isolates and the pathogenicity was proven on avocado fruits. P. cinnamomi is one of the 10 main phytopathogenic oomycetes, it infects over 5000 species (Hardham, 2005) and its pathogenicity has been proven (Linde et al., 1999; Rodríguez-Padrón et al., 2018; Belisle et al., 2019b).
For many years, phosphites have been used to control root rot. Avocado producers use potassium phosphite more frequently than metalaxyl-M, due to its higher efficiency and lower cost than others (Adaskaveg et al., 2015; Belisle et al., 2019b). Our results prove that there are no resistant isolates to the fungicides evaluated in Zitácuaro, Michoacán. However, regarding potassium phosphite, a lower sensitivity was observed in the isolates than towards metalaxyl-M. The values obtained for EC50 fluctuated between 11.8 and 37.7 μg mL-1 of potassium phosphite, and for growth inhibition, between 54.7 and 74.2% in comparison with the control, which are similar to those obtained by Wilkinson et al. (2001), who obtained inhibition values ranging from 59 to 100%. Eight of the isolates displayed an enhancement in growth when they were exposed to the concentration of 5 μg mL-1 of i.a of potassium phosphite, and these results coincide with those by Wilkinson et al. (2001) and Hunter et al. (2018). This may be due to the possibility that, at low potassium phosphite concentrations, in the cV8 nutrient-rich medium, it may be easier for P. cinnamomi to alter its growth as a survival strategy, along with the fungistatic effect not affecting the mycelial growth (Guest and Grant, 1991; Hunter et al., 2018). Another reason is the loss in sensitivity of the isolates due to the frequent exposure to the fungicide, since they undergo a pressure of selection, which may eventually lead to the development of resistance, even if there is no sexual recombination (Dobrowolski et al., 2008; Hunter et al., 2018). Not only has P. cinnamomi, been reported as sensitive to potassium phosphite, also P. syringae and P. citrophthora, although the inhibition of growth of the species in vitro is not indicative of the disease control held in the field as being effective (Hao et al., 2021).
On the other hand, although Marin et al. (2021) reported the development of resistance in Phytophthora species to metalaxyl-M (mefenoxam), our study observed an average growth inhibition of 88.24% and a EC50 value of 0.08 and 0. 34 μg mL-1 of metalaxyl-M mL-1, suggesting that the P. cinnamomi populations obtained from avocado are sensitive to the fungicide. In a similar study, the isolates obtained from ornamental plants in nurseries were less sensitive, with a EC50 value of 0.01-0.08 μg mL-1 of metalaxyl (Hu et al., 2010). This may be due to the applications being more controlled and less frequent in the nurseries than in the field. In North Carolina, sensitivity tests performed on P. cinnamomi isolated from Christmas trees gave percentages of inhibition that fluctuate between 89 and 100%, despite metalaxyl-M applications being carried out in the region two or three times a year (Benson and Grant, 2000). This confirms that P. cinnamomi is sensitive to metalaxyl-M, along with the fact that high percentages of inhibition of mycelial growth by metalaxyl-M have also been observed in other species such as P. nicotianae (73. 5%) and P. × pelgrandis (97.5%) (Pánek and Tomšovský et al., 2017).
On the other hand, González et al. (2014) indicated that the V8 culture medium promotes the growth of Phytophthora in regard to other media. These results are consistent with those obtained in this work, since the isolates with the most growth were those plated on V8. A difference in growth was found between both temperatures, with 25 ºC being more favorable, since it is within the optimum growth temperature range of P. cinnamomi that ranges between 24 and 28 ºC (Erwin and Ribeiro 1996).
In Mexico, the sensitivity studies performed on P. cinnamomi isolates to fungicides are scarce. The results of this work provide an estimation of the range of sensitivity to potassium phosphite and metalaxyl-M of P. cinnamomi isolates that cause root rot in avocado produced in the municipality of Zitácuaro, Michoacán. However, it is necessary to carry out further studies which include a greater number of isolates from the main producing municipal areas, in order to help us determine if the populations of the pathogen are becoming tolerant to these fungicides.
Conclusions
The results of this work indicate that in Zitácuaro, Michoacán, P. cinnamomi is the main oomycete present in avocado roots with rot. Out of 25 root samples, 15 isolates were obtained with the morphological characteristics of P. cinnamomi. The isolates were inhibited by more than 98% at concentrations of 300 and 600 μg mL-1 of potassium phosphite and over 96 % at 3 and 5 μg mL-1 with metalaxyl- M.











 texto en
texto en 


