The geminivirus Pepper golden mosaic virus (PepGMV), is a bipartite singled standed virus transmitted by whiteflies and it considered a major pathogen that infects numerous solanaceous crops, including different species of Capsicum in Mexico and Central America (Barboza et al., 2018; Hernández-Espinal et al., 2018; Méndez-Lozano et al., 2001; Nakhla et al., 2005). Based on their genomic and biological properties, three strains of PepGMV are reported as PepGMV-Serrano (PepGMV-Se), PepGMV-Mosaic (PepGMV-Mo) and PepGMV-Distortion (PepGMV-Di) and they cause different symptoms in the infected plants. PepGMV-Se causes a bright golden mosaic, PepGMV-Mo produced a yellow-green mosaic, and PepGMV-Di caused only a mild mosaic and foliar distortion followed by a “recovery” phenotype (Brown et al., 2005), that has been associated with posttranscriptional and transcriptional gene silencing (Rodríguez-Negrete et al., 2009). A geminivirus genetic resistance trait was characterized in the Capsicum chinense BG-3821 accession, and the marker genes for systemic acquired resistance (SAR) were found to be induced after inoculation of resistant leaves with PepGMV (García-Neria and Rivera-Bustamante, 2011). These findings suggest that resistance in plants depends at least in part on the early response to virus infections, such as the induction of genes related to H2O2 production.
Plants respond to pathogen attacks by activating both local and systemic defenses that restrict the growth and spread of the pathogen. In infected leaves, these defenses often involve the hypersensitive response (HR) and the formation of necrotic lesions at the infection site (Lukan et al., 2018; Park et al., 2007). Eventually, the non-inoculated parts of the plant develop a long-lasting SAR (Clark et al., 2007). One of the earliest events in the HR is a burst of oxidative metabolism leading to the generation of superoxide anion radicals (O2-) and hydrogen peroxide (H2O2) (Wohlgemuth et al., 2002). Hydrogen peroxide is an important signal molecule in plant defense against biotic and abiotic stress (Quan et al., 2008; Mejía-Teniente et al., 2019). Plants have developed antioxidant mechanisms for protection against the damage caused by Reactive Oxygen Species (ROS). The major ROS scavenging enzymes in plants include CAT and POX. In potato tubers infected with Erwinia chrysanthemi, for example, a two-fold increase in CAT activity was observed in infected potato tubers compared to mock-inoculated tubers (Miguel et al., 2000). In another study in which susceptible and resistant tomato cultivars were infected with Ralstonia solanacearum, the activities of glutation peroxidase, superoxide dismutase, ascorbate peroxidase, and catalase all increased; however, the antioxidative mechanism was found to be much stronger in the resistant cultivars (Mandal et al., 2011). In tomato plants, the inoculation of saprobic fungi filtrates increased enzyme activity of CAT, POX and other antioxidant enzymes. These increases were efficient to control the severity of the early blight disease caused by Alternaria solani in tomato plants (Rodrigues-Alencar et al., 2020). Plants generally respond to viral infection with ROS generation and an upregulation of antioxidant enzymes. In the compatible interactions of Cucumber mosaic virus and Zucchini yellow mosaic virus with Cucumis sativus and Cucurbita pepo plants, respectively, increased antioxidant activities of CAT, POX, and superoxide dismutase have been reported. Thus, antioxidant enzymes contribute to oxidative stress in systemic plant-virus interactions (Riedle-Bauer, 2000).
Salicylic acid (SA) is a key signal in plant defense against viral infection and it is considered critical for the activation of both local and systemic resistance responses (Klessig et al., 2018). Findings reported in the literature indicate an H2O2-SA interaction in which H2O2 and SA constitute a self-amplifying system: H2O2 induces SA accumulation, and SA enhances H2O2 levels (Van Camp et al., 1998). Salicylic acid participates in the induction of the incompatible interaction between plants and virus. After this induction, plant responses with several mechanisms to limit viral propagation at the infection site. These mechanisms include the increase in the production of ROS, pathogenesis-related proteins, induction of the HR, etc. (Baebler et al., 2014). SA is also responsible for the activation of systemic acquired resistance (SAR) in distal tissues, which lessens the effects of secondary attacks. Salicylic acid can induce resistance to Tobacco mosaic virus (TMV) by affecting its ability to replicate in the inoculated tissue of Nicotiana benthamiana (Chivasa et al., 1997). It is also involved in the inhibition of long-distance movement when Arabidopsis thaliana is infected by Cucumber mosaic virus (CMV). Nevertheless, different host species may use distinct approaches for resisting infection by the same virus (Mayers et al., 2005). The aim of the present study was to investigate the role of hydrogen peroxide, salicylic acid and antioxidant enzymes during the compatible interaction between PepGMV-Mo strain and habanero pepper (Capsicum chinense) grown in vitro.
Materials and methods
Plant materials and treatments
Habanero pepper (Capsicum chinense) seeds were germinated in vitro using basal MS media. The seeds were disinfected with sodium hypochlorite (2%) and 70% ethanol, washed three times with distilled sterile water then incubated at 50 °C for 30 min to eliminate any seed-borne pathogens. The in vitro plants were maintained in a room under controlled environmental conditions (25 to 26 °C, 16:8 h (light/dark) photoperiod, light intensity of 50.26 mmol m-2 s-1).
Plant inoculation with PepGMV-Mo
Infective clones of PepGMV-Mo (DNA A and DNA B), donated by Dr. Rafael Rivera-Bustamante, were used to inoculate habanero pepper seedlings using a previously described biolistic inoculation procedure (Idris et al., 2001). The third or fourth leaves of plants at the four-leaf stage of in vitro plants were directly bombarded at 900 psi He with gold particles (1 μg, BioRad, Hercules, CA) covered with viral DNA as previously described (Hernández-Zepeda et al., 2007a). For mock inoculation, the plants were bombarded with the cloning vector (Bluescript) without the virus genome. Healthy plants without inoculation were used as control. Inoculations using this protocol were done in four separate experiments, each consisting of six inoculated plants for each time point of the time course experiment, for virus-infected, mock and healthy plants.
Plant material and DNA analysis
Plant material was collected from systemic infected tissue at the following time points: 0 and 30 min; 1, 2, 4, 8, 12 and 24 h; and 3, 6, 9, 12, 15, 18, 21 and 24 days). The samples were immediately frozen in liquid nitrogen and stored at -80 °C. Total DNA was extracted for each treatment as previously described (Echevarría-Machado et al., 2005).
A 576 bp fragment was amplified using the primers prAV324 and prAC889 (Hernández-Zepeda et al., 2007a). PCR was performed using RedTaq Polymerase (Bioline) as described in Hernández-Zepeda et al. (2007b). The PCR products were cloned into the pGEMT-Easy Vector (Promega, Madison, WI, USA) according to the manufacturer’s instructions, and the cloned inserts were sequenced by Macrogen Inc. The sequences were edited and aligned using EditSeq and MegAlign (DNAStar version 5.08, Madison, USA) and subjected to a BLASTx search using the NCBI GenBank database.
Southern blot
Five micrograms of total DNA from each treatment were digested with HindIII and loaded onto a 1% agarose gel. After electrophoresis, the DNA was transferred to a Hybond N+ membrane (Amersham Biosciences, Piscataway, NJ) by capillary action using a 20X solution of 0.15 M sodium chloride and 0.015 M sodium citrate (SSC). After this, the membrane was UV-crosslinked. The hybridization was performed with 500 ng of the A genome of PepGMV-Mo as probe. The probe was labeled using AlkPhos Direct™ (Alkphos Direct Hybridization kit, Amersham, Arlington Heights, Illinois, USA).
Hydrogen peroxide determination
A modified version of the ferrous ammonium sulfate/xylenol orange (FOX) method was used to determine the H2O2 contents of the leaf extracts, as previously reported (Cheeseman, 2006). The assay mixture after addition of the sample contained 250 mM ferrous ammonium sulfate, 100 mM sorbitol, 90% ethanol and 100 mM xylenol orange in 25 mM sulfuric acid in a total volume of 3 mL. The assay measured the difference in absorbance between 550 and 800 nm for at least 15 min, and the color was stable for at least 1 h. The standard was prepared by dilution in 30% H2O2 (reagent grade, SIGMA). The concentration of H2O2 in the reagent was calibrated using the absorbance at 240 nm and an extinction coefficient of 43.6 M-1 cm-1.
Salicylic acid determination
Salicylic acid was extracted from leaf tissue (0.3 g) and analyzed as described by Gaffney et al. (1993), with several modifications. The methanol extracts (2.5 mL) were split into two equal parts, with one part analyzed for free SA and the other for SA conjugate. The final samples were resuspended in 500 µL methanol, from which 50 µL was injected into a high-performance liquid chromatographic column (Agilent Technology 1200 series, column Alltima C18 reverse-phase). Step gradient elution was performed using 20 mM sodium acetate (pH 5.0) plus 20% methanol (solvent A) and 20 mM sodium acetate (pH 5.0) plus 70% methanol (solvent B). The separation conditions were the same as those described by Uknes et al. (1993): 10 min gradient from 5 to 30% solvent B, 100% B and re-equilibration at 5% B.
Enzyme activity
Protein extraction was performed using phosphate buffer (pH 7.0), and quantification of total proteins was performed as previously described by Peterson (1977). Catalase activity was determined following the generation of O2 as described by Inamine and Baker (1989). The reaction mixture contained 100 mM potassium phosphate buffer (pH 7.0) and 20 g mL-1 protein extract in 3 mL solution. The reaction was initiated by adding 10 µL of 30% [2.2 mM] (w/v) H2O2 (reagent grade, SIGMA). POX activity was measured with guaiacol (4 mM, e= 26.6 mM-1 cm-1) as a substrate in 0.05 mM C2H3NaO2 (pH 5.0) at 25 °C. The reaction was initiated using 2.2 mM H2O2. The subsequent oxidation of guaiacol was measured as absorbance change at 470 nm in a spectrophotometer (DU 800; Beckman, Munich, Federal Republic of Germany) for 3 min (Berg and Huystee, 1984).
Results
PepGMV-Mo infection and symptom development
To control environmental variables, an in vitro infection protocol was developed for PepGMV-Mo in its compatible host, habanero pepper (Capsicum chinense). Typical symptoms associated with PepGMV-Mo infection, were observed in the inoculated plants (Figure 1G-I), however, the symptoms were slightly attenuated. The symptoms associated with PepGMV-Mo infection were visible nine dpi in the inoculated plants. The symptoms observed were leaf curling and mild yellow-green mosaics in the new leaves (Figure 1G). Symptoms were not observed in healthy and mock-inoculated plants (Figure 1A-C and 1D-F). Yellow mosaic and leaf distortion symptoms continued to develop until 24 dpi (Figure 1I). PepGMV-Mo was detected in the new, systemic leaves of the inoculated plants. The samples were analyzed for the detection of PepGMV-Mo of the infected plants. In the infected plants, a 576 bp fragment of the core Cp gene was detected using PCR (see methods). Amplification did not occur in samples from mock-inoculated and healthy plants. A total of 15 sequences were selected and compared by pairwise nucleotide identity with the original PepGMV-Mo clone. All of the clones sequenced had 98-100% identity with the original PepGMV-Mo DNA-A clone and 95-98% nucleotide identity with other PepGMV-Mo isolates previously reported in GenBank. Southern blot hybridization was used to evaluate the accumulation of viral DNA. The three expected DNA replicative forms, the open circle, double-stranded, and single-stranded forms, were observed in the infected, PepGMV-Mo inoculated plants. While the single-strand form was not observed at 9 dpi, the single- and double-stranded forms were observed at 24 dpi (Figure 1J). These results confirm the validity of the in vitro infection system for evaluating the interaction between habanero pepper and PepGMV-Mo. In particular, all of the replicative forms of PepGMV-Mo were detected in the newly developed leaves of all infected plants.

Figure 1 Pepper plants were biolistically inoculated or not inoculated to create three different treatment groups. A. Healthy control plant at time zero; B. Healthy control plant at 9 dpi; C. Healthy plant at 21 dpi; D. Mock-inoculated plant at 0 dpi; E. Mock-inoculated plant at 9 dpi; F. Mock-inoculated plant at 21 dpi; G. PepGMV-Mo-inoculated plant at time zero; H. PepGMV-Mo-inoculated plant at 9 dpi; I. PepGMV-Mo-inoculated plant at 21 dpi. All of the plants were cultivated in basal MS media. J. Viral DNA accumulation. Lane 1, DNA from PepGMV-Mo-inoculated plants at 9 dpi; lane 2, DNA from PepGMV-Mo-inoculated plants at 21 dpi; lane 3, DNA from mock-inoculated plants at 9 dpi; lane 4, DNA from mock-inoculated plants at 21 dpi. The following replicative forms of viral DNA are shown: ssDNA (single-stranded DNA), scDNA (supercoiled dsDNA) and ocDNA (open circular DNA).
Hydrogen peroxide generation in PepGMV-Mo-infected habanero pepper
After bombardment, H2O2 levels increased significantly in mock- and PepGMV-Mo-inoculated plants 30 mpi. In contrast, no increase in H2O2 was observed in healthy plants, indicating that the mechanical damage due to the inoculation procedure was responsible for the initial oxidative burst. The content of H2O2 in the mock-inoculated and PepGMV-Mo-inoculated plants was 4.6 and 4.8 times greater, respectively, than the content in healthy plants. After this initial increase in H2O2 levels, a gradual decrease was observed in mock- and PepGMV-Mo-inoculated plants up to 8 hpi. At 12 hpi, a second peak was observed in the infected plants, with the H2O2 content 2.4 and 1.8 times greater than the content in the healthy and mock-inoculated plants, respectively (Figure 2A). Thus, the significant difference in H2O2 production could be associated with the presence of the virus in pepper plants. A third peak of H2O2 was observed in virus-inoculated plants at 21 dpi that was two times greater than the levels in mock-inoculated and healthy plants (Figure 2B).
Salicylic acid generation in PepGMV-Mo-infected habanero pepper
The levels of SA increased significantly after biolistic inoculation of PepGMV-Mo in inoculated and mock-inoculated plants. At 30 mpi, the SA content in mock-inoculated and PepGMV-Mo-infected plants was 4.0 and 6.2 times greater, respectively, than the SA content in healthy, non-inoculated control plants. These results clearly indicate that the initial increase in SA and H2O2 levels were attributable to the mechanical damage caused by biolistic inoculation. After this initial increase, an 8-fold decrease was observed in the levels of SA up to 8 hpi, both in mock- and PepGMV-Mo-inoculated plants (Figure 3A). At 12 hpi, 4.5- and 7.8- fold increases were observed in mock- and PepGMV-Mo-inoculated plants, respectively, relative to the levels in healthy plants (Figure 3A). Although increases were observed in both mock- and PepGMV-Mo-inoculated plants, the difference in SA content between them was 58%. This difference suggests that the SA content at this time point reflects the plant response to the viral infection. As observed with H2O2, a third peak of increase of SA content was observed 21 dpi in PepGMV-Mo-inoculated plants. At this time point, the SA content in the infected plants was 3 times higher than the SA content in healthy and mock-inoculated plants (Figure 3B). The increases in SA and H2O2 levels in PepGMV-Mo-infected plants indicate the onset of a late (21 dpi) response corresponding to SAR.

Figure 2 Time-course analysis of the H2O2 levels in habanero pepper plants inoculated with PepGMV-Mo. A. H2O2 content during the early response period after inoculation (0-24 hpi); B. Time-course experiment from 0 to 24 dpi. PepGMV-Mo-inoculated plants (p); mock-inoculated plants (l); healthy plants (n). The data are the mean values ± SE of 24 repetitions from four independent experiments.
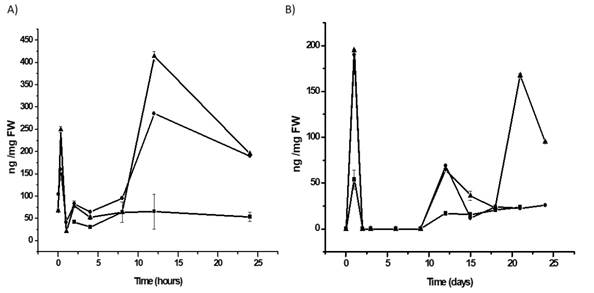
Figure 3. Time course-analysis of the salicylic acid content of habanero pepper plants inoculated with PepGMV-Mo. A. SA content during the early response after inoculation (0-24 hpi); B. Time-course experiment from 0 to 24 dpi. PepGMV-Mo-inoculated plants (p); mock-inoculated plants (l); healthy plants (n). The data are the mean values ± SE of 24 repetitions from four independent experiments.
Antioxidative enzyme activity in PepGMV-Mo-infected habanero pepper. Catalase (CAT) activity.
An increase in catalase activity was observed in PepGMV-Mo-infected habanero pepper plants. The activity of this enzyme increased gradually in PepGMV-Mo-infected plants, with a peak at 4 hpi. At this time point, the CAT activities in the mock- and PepGMV-Mo-inoculated plants were 2.1 and 4.7 times greater, respectively, than the activity in healthy plants (Figure 4A). CAT activity subsequently decreased in PepGMV-Mo-inoculated plants and gradually increased in control plants. At 12 dpi, the CAT activities in PepGMV-Mo-inoculated, mock-inoculated and healthy plants were similar (Figure 4B), suggesting that CAT activity could be regulated by the increase in SA concentration at 8 hpi (Figure 3A).
Guaiacol peroxidase (POX) activity
Two peaks of POX activity were observed during the first 12 hpi. At the first peak at 4 hpi, POX activity was 3.3 times greater in inoculated plants than in mock-inoculated and healthy plants. At the second peak at 12 hpi, POX activity was 3.5 and 5.8 times greater in mock- and PepGMV-Mo-inoculated plants, respectively, compared to healthy plants (Figure 5A). The activity of this antioxidant enzyme gradually increased in PepGMV-Mo-inoculated plants over the course of the experiment. At 9 dpi, POX activity was 1.3 and 4.6 times higher in PepGMV-Mo-inoculated plants than in mock-inoculated and healthy plants, respectively (Figure 5B). At 18 dpi, POX activity was 4.7 times greater in inoculated plants than in both mock-inoculated and control plants (Figure 5B).

Figure 4 Time-course analysis of the specific activity of catalase in habanero pepper plants inoculated with PepGMV-Mo. A. CAT activity during the early response after inoculation (0-24 hpi); B. Time-course experiment from 0 to 24 dpi. PepGMV-Mo-inoculated plants (p); mock-inoculated plants (l); healthy plants (n). The data are the mean values ± SE of 24 repetitions from four independent experiments.
Discussion
In the present study, we identified responses that occur both early and late after inoculation of habanero pepper with PepGMV-Mo. The development of symptoms in the present study was consistent with previous reports for the PepGMV-Mo isolate (Brown et al., 2005) and all the plants were susceptible to virus infection. However, some of the symptoms in the in vitro system were attenuated due to the different variables affecting these plants.

Figure 5 Time-course analysis of the specific activity of peroxidase in habanero pepper plants inoculated with PepGMV-Mo. A. POX activity during the early response after inoculation (0-24 hpi); B. Time-course experiment from 0 to 24 dpi. PepGMV-Mo-inoculated plants (p); mock-inoculated plants (l); healthy plants (n). The data are the mean values ± SE of 24 repetitions from four independent experiments.
Little is known about the involvement of ROS in symptom development and pathogenesis in the context of compatible plant-virus interactions. Although a HR was not observed in PepGMV-Mo-infected plants in the present study, an oxidative burst was observed, with three major peaks of increase of H2O2 from 30 mpi to 24 dpi. The first peak of increase at 30 mpi was due to mechanical damage caused by biolistic inoculation. Intensive ROS production has been reported in wounded cells and cells subjected to mechanical tension (Olson and Varner, 1993), so it is not surprising that oxidative bursts occurred in all of the treatments 30 min after biolistic inoculation. In plant cell-bacterial interactions, the phase I burst is considered a biologically non-specific reaction (Wojtaszek, 1997). The second peak of increase observed 12 hpi in PepGMV-Mo-infected plants is consistent with previous reports that associate the production of ROS during phase II with a specific HR trigger in non-host resistance (Wojtaszek, 1997). Thus, the second peak of increase can be associated with the PepGMV-Mo infection, the plant response to this infection and symptom development. Previous studies, including a report about Plum pox virus (PPV) infection of susceptible peach plants (Hernández et al., 2006), have indicated that increased ROS levels may contribute to symptom development and pathogenesis in compatible plant-virus interactions. In incompatible plant-virus interactions, ROS generation plays an important role in virus resistance. In compatible plant-virus interactions, however, little is known about the involvement of ROS in symptom development and pathogenesis. As previously reported, the generation of ROS is not sufficient to cause a hypersensitive reaction in compatible plant-RNA virus interactions or tissue collapse and restriction of the pathogen at the infection site (Riedle-Bauer, 2000). Similarly, our results demonstrate that in the compatible habanero pepper-PepGMV-Mo interaction, ROS production was not sufficient to confer tolerance, and the disease was able to develop further.
Exogenous SA can induce resistance to viruses even in plants lacking a resistance gene. This resistance is characterized by a decrease in virus yield and a delay in the onset of disease symptoms, and it occurs in the absence of macroscopic cell death or a hypersensitive reaction (Murphy et al., 1999). This suggests that plants possess another mechanism by which SA mediates resistance to viruses. However, increased SA levels at 12 hpi did not inhibit symptom development in the habanero pepper plants infected with PepGMV-Mo in vitro. Our results showed that PepGMV-Mo, a DNA virus, can induce an increase in SA in a compatible interaction along with a bimodal response, as previously reported (García-Neria and Rivera-Bustamante, 2011). Additionally, this response can restrict the spread of certain RNA viruses in compatible infections (Murphy and Carr, 2002; Takahashi et al., 2002). Markers for SA signaling, such as PR-1, are up-regulated during systemic infections by several RNA viruses (Huang et al., 2005). CaMV infection, for example, was found to stimulate SA-dependent expression of PR-1, PR-2, and PR-5 in susceptible Arabidopsis ecotypes (Laird et al., 2004). DNA viruses have also been found to stimulate SA signaling pathways in both compatible and incompatible interactions. Garcia-Neria and Rivera-Bustamante (2011) reported two peaks of increase at day two and day five post inoculation in the levels of SA in both compatible and incompatible PepGMV infections. They showed that the kinetic of SA production was similar in both resistant and susceptible plants although the concentration on resistant plant was two to three fold higher in inoculated infected tissue. In systemic infected tissue, they observed a bimodal response too, however, in systemic infected tissue, levels of SA were lower than in inoculated infected tissue. Susceptible plants showed two peaks of increase in SA content at day five and day 10-post inoculation. In our work we used susceptible plants in the experiments. In our study, two SA peaks of increase in systemic infected tissue were observed, the first at 12 hpi and the second at 21 dpi. These results were similar to the results find by Garcia-Neria and Rivera-Bustamante (2011), with one peak of increase in the first days after the virus inoculation in susceptible plants and other peak of increase at the end of the time course experiment. Our results suggest that SAR is involved in the compatible interaction between PepGMV-Mo and habanero pepper; however, it was not sufficient to confer resistance to the pepper plants.
Our findings also indicate an interaction between H2O2 and SA that results in a self-amplifying system for plant defense: H2O2 induces SA accumulation, and SA enhances the levels of H2O2 (Van Camp et al., 1998). The increase observed in SA levels 12 hpi in PepGMV-Mo-infected plants was accompanied by increased in H2O2 levels. This relationship was confirmed at 21 dpi in infected plants, when an increase in H2O2 levels co-occurred with an increase in SA content.
Plants react to virus infections with an upregulation of radical scavenging enzymes, such as SODs, catalases, and peroxidases (Riedle-Bauer, 2000). In plants, the main enzymatic H2O2 scavenger of photosynthetic cells is CAT, which converts H2O2 into H2O and O2 (Quan et al., 2008). An early increase in CAT activity was observed in the compatible interaction between PepGMV-Mo and habanero pepper plants, resulting in an activity level 2 times greater than that of the control at 4 hpi. After this peak, CAT activity was similar to that of control plants. Our results are in contrast to previously reported decreases in catalase activity in Phaseolus vulgaris infected with WClMV and tobacco infected with TMV (Chen et al., 1993; Clarke et al., 2002; Neuenschwander et al., 1995). In cucumber plants inoculated with Cucumber mosaic virus (CMV), CAT activity was unaffected (Riedle-Bauer, 1998). The decrease in CAT activity after 4 hpi may have led to the observed increase in H2O2 levels and the induction of SAR (Neuenschwander et al., 1995).
Increased POX activity in disease development has been correlated with resistance expression, although increased activity has also been reported in hosts that permit systemic infection (Kozlowska et al., 2001; Riedle-Bauer, 2000). In the present study, peroxidase activity increased throughout the duration of the experiment. A previous study by Milavec et al. (2008) demonstrated that in the early period following inoculation of potato plants with Potato virus Y (PVY), the virus induced an increase of two times in soluble, ionically-bound and covalently-bound POXs activities, in both tolerant and resistant plants at 3 hpi, while in susceptible plants the POX activity decreased in the early period and the increase was not observed before 24 hpi. In contrast with this last observation, in our work, in susceptible plants infected with PepGMV-Mo, the first increase in POX activity was observed 2 hpi compared to mock inoculation and healthy plants, similar to the results obtained in potato plants tolerant and resistant to the PVY (Milavec et al, 2008). It was reported that in mock-inoculated potato plants susceptible to PTYNTN, soluble and ionically-bound peroxidases decreased in relation to intact plants. The authors proposed that these effects could be the result of injury and due to the effect of substances present in the sap of healthy plants (Milavec et al., 2001). In contrast, we observed that total POX activity increases in mock-inoculated, in relation to healthy plants. These differences could be due to the use of different mock inoculation. In the Milavec et al. (2001) report, they used healthy plant´s sap as mock inoculation and we used the Bluescript cloning vector as mock inoculation. This last treatment does not contain the substances present in the sap that could inactivate the POX activity. Riedle-Bauer (2000) reported that in susceptible plants of Cucumis sativa and Cucurbita pepo infected with Cucumber mosaic virus (CMV) and Zucchini yellow mosaic virus (ZYMV), total POX activity increased 14 and six times in infected plants 20 dpi, respectively, in systemic leaves displayed viral symptoms, with an increase in H2O2 accumulation. Similar results were obtained in our work. POX activity in systemic leaves continued to increase through the time course experiment, with a higher activity observed between 18 to 24 dpi. This POX activity increase was related with an increase in H2O2 accumulation at 24 dpi. These results suggest that total peroxidase activity are involved in H2O2 production during plant-virus interactions (Riedle-Bauer, 2000). Peroxidases have long been postulated to produce H2O2 (Milavec et al., 2008; Elstner and Heupel, 1976). More recently, cell wall peroxidases have been found to be responsible for some, if not all, of the H2O2 produced in the oxidative burst (Hernández et al., 1993; Bestwick et al., 1997; Bolwell et al., 1995). Taken together, our data indicate that increased of antioxidant defense is not enough to confer resistance or tolerance to habanero pepper plants in the compatible interaction with PepGMV-Mo and that the symptoms observed may be related to this increase. How the increases in H2O2, SA, and POX activity at 21 dpi are related to symptom remission will require further investigation, preferably in an in vivo system.
Conclusions
Our in vitro results demonstrate that habanero pepper plants respond to the compatible virus PepGMV-Mo by activating the synthesis and accumulation of H2O2 and SA and increasing the activity of CAT and POX. Furthermore, the SAR pathway was activated but insufficient to confer resistance to the habanero pepper plants.











 texto en
texto en 


