Blackberry (Rubus sp.) is a fruit crop grown in various regions throughout the world. However, blackberry production has suffered severe losses caused by diseases. Gray mold, caused by Botrytis cinerea, is considered the most important pre- and post-harvest disease (Li et al., 2012) of blackberries in Mexico and has been commonly reported in different blackberry producing regions (Terrones et al., 2019).
The initial symptoms caused by B. cinerea in blackberries are soft, light-brown areas. The infected tissue later dries and mummifies. Abundant conidiophores and conidia develop at this stage, producing the appearance of gray mold (Carisse et al., 2015). The fungus causes quiescent infections in which the disease manifests after harvest (Calvo-Garrido et al., 2014). Therefore, disease management actions must begin before harvest (Fillinger and Walker, 2016; Kim et al., 2016). Chemical control is the main control method against this disease. The use of fungicides against gray mold represents 8% of the global fungicide market (Fillinger and Walker, 2016). In Mexico, the products authorized for use in the control of blackberry gray mold, in compliance with US and European Union regulations, are fenhexamid, captan, pyrimethanil, fludioxonil, boscalid, azoxystrobin, and iprodione (ANEBERRIES, 2021). These fungicides, which are applied at intervals of 7-14 days, increase production costs and can exert selection pressure on resistant populations (Fernández-Ortuño, 2014).
The need to minimize the use of fungicides has led to the search for control alternatives, including Silicon (Si), which modifies plant cell wall properties (Iwasaki et al., 2002). When reinforced with Si, this mechanical defense significantly reduces the damage caused by insects and pathogens, mitigating the intensity of various diseases (Rodríguez et al., 2015). This method has reduced the incidence and severity of gray mold in cucumber (Cucumis sativus) and strawberry (Fragaria spp.) (Lopes et al., 2014). Furthermore, the foliar application of Si prevents the penetration of phytopathogenic fungi by activating the natural defenses of plants, mainly the expression of resistance genes (Fauteux et al., 2005).
Several studies have focused on the mitigation of plant diseases using Si. However, to date there no formal study has focused on the use of potassium silicate in blackberry plants to control gray mold. The present study aimed to evaluate the control of this disease in the field using nine fungicides from different chemical groups, alone and in combination with potassium silicate to potentiate their effect against B. cinerea.
Nine fungicides from different chemical groups were evaluated in an experimental plot with blackberry plants cv. Tupi in the physiological maturity stage: fluazinam (SHOGUN 500®, Syngenta) at a dose of 1 L ha-1; fenhexamid (ELEVAT®, UPL) at 1.5 kg ha-1; thiophanate-methyl (CERCOBIN M®, Basf) at 1 kg ha-1; captan (CAPTAN 50 WP®, Adama) at 2.5 kg ha-1; pyrimethanil (SCALA 400 SC®, Bayer CropScience) at 2 L ha-1; cyprodinil + fludioxonil (SWITCH® 62.5 WG, Syngenta) at 1 kg ha-1; boscalid (CANTUS®, Basf) at 1 kg ha-1; azoxystrobin (IMPALA® 25 SC, Adama) at 0.75 L ha-1; iprodione (ROVRAL® 50 PH, FMC) at 1 kg ha-1; control solution (sterile distilled water). These treatments were applied alone and in combination with potassium silicate (SUPA SILICA®, Agrisolver) at 0.5 L ha-1. The plot where the study was carried out had a history of presence of gray mold. An isolate of Botrytis cinerea (GenBank access number MG838557) had been previously collected and morphologically and molecularly characterized (Terrones et al., 2019). The fungicides, alone and in combination with potassium silicate, were applied at intervals of 15 days, starting in October 2019 and the second repetition was carried out in June 2020 until the end of the harvest in both cases. A total of six applications were made on each repetition using a motorized sprinkler (MS072H, Maruyama®) with a hollow cone nozzle previously calibrated to deliver a volume of 800 L ha-1.
An evaluation was carried out prior to the applications of the treatments, and six evaluations were carried out afterward, five days after each application. The fungicide applications were made directly in the field at the time of harvest when gray mold symptoms were found with different percentages of incidence and severity. The percentage of incidence was calculated by counting the number of fruits with symptoms of the disease and/or signs of the pathogen among 100 fruits chosen at random for each plant evaluated per experimental unit (treatment). The severity of the disease was determined in the same fruits (n=100) using a diagrammatic scale adjusted by Horsfall and Barratt and generated with 2LOG (Osada-Velázquez and Mora-Aguilera, 1997), with the following classes 0=0; 1= 0.1-3.2-7.8; 2= 7.9-25.4-48; 3= 48.1-63.62-83, 4= 83.1-94.58-100. This scale was used to determine the ranges and midpoint of disease severity (Tovar-Soto et al., 2002). The percentages of incidence and severity were converted to area under the disease progress curve values (AUCPE) with the SAS program version 9.3.
Six harvests were carried out (every 15 days, respecting the safety interval for fungicides). The fruits were weighed on an analytical balance (OHAUS®, USA). The total yield was calculated by adding all the harvested fruits from 14 plants in each repetition and extrapolating the yield per hectare later. Total soluble solids (degrees Brix) were determined at each harvest in randomly selected fruits using a digital palette refractometer PR-101α (ATAGO, Japan). Twenty measurements were made for each repetition, including asymptomatic and symptomatic fruits. The silicon content in the foliage was also evaluated at the end of the experiment by collecting 20 g of foliage from blackberry plants and subjecting the material to wet digestion with perchloric and nitric acid (Alcantar and Sandoval, 1999). The samples obtained from the digestion were analyzed using plasma induction atomic emission spectroscopy (ICP-VARIAN 725-ES).
The experiment was a 10×2 factorial design with 20 treatments, four replications and 80 experimental units, each one consisting of a plot 7 meters long and 2 m wide with 14 blackberry plants distributed along one row. Two repetitions of the experiment were carried out. The first from October to January (2019-2020) and the second one from June to September (2020). The data were subjected to analysis of normality and homogeneity of variances, data independence, analysis of variance and comparison of means (LSD, p ≤ 0.05). All analyses were carried out using the SAS program version 9.3.
In the evaluation of incidence, an interaction between the factors was identified (G.L.= 9, F= 238.09, p<0.0001) in the first repetition of the experiment. In addition, there were significant differences (F= 540.5, p<0.0001) in the AUCPE. The treatment based on azoxystrobin + potassium silicate was significantly different from the rest of the treatments, presenting the lowest AUCPE value (1320), as well as the lowest incidence percentages (Table 1). Interactions between the factors were also identified (G.L = 9, F= 289.04, p<0.0001) in the second repetition, as well as significant differences (F= 376.27, p<0.0001) in the AUCPE. The results showed again that the combination of azoxystrobin + potassium silicate was significantly different from the rest of the treatments, presenting the lowest AUCPE value (1099) and the lowest percentages of incidence of the disease (Table 1; Figure 1).
Table 1 Effect of potassium silicate on the control of blackberry gray mold disease caused by Botrytis cinerea under field conditions during two evaluation cycles.
| Tratamiento | Primera repetición (octubre de 2019 a enero de 2020) | Segunda repetición (mayo a agosto de 2020) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Incidencia | % severidad | ° Brix | Conc. Silicio | Rendimientox | % Incidencia | % severidad | ° Brix | Conc. Silicio | Rendimientox | |||||||
| E7z | ABCPEy | E7z | ABCPEy | Sanas | Enfer. | E7z | ABCPEy | E7z | ABCPEy | Sanas | Enfer. | |||||
| Fluazinam | 39 | 5070bw | 2.2 | 942.9b | 8.99jz | 6.51jkl | 21.11kl | 6.74l | 38 | 4770bz | 1.9 | 834.5b | 9.01i | 6.53ghi | 20.59k | 6.66m |
| Fenhexamid | 33 | 3968cd | 1.6 | 669.5e | 9.14i | 7.08c | 20.49l | 7.97k | 29 | 3593c | 1.3 | 594.7e | 9.14h | 7.06d | 21.67k | 8.12l |
| Tiofanato-metil | 25 | 3188gh | 1 | 636.5e | 9.17hi | 7.07cd | 21.15kl | 9.43fgh | 23 | 2940fg | 1 | 575.2e | 9.16gh | 7.05d | 21.80k | 9.35i |
| Captan | 31 | 4110c | 1.4 | 951.7b | 9.21fg | 6.49kl | 21.86jk | 8.01k | 27 | 3585c | 1.3 | 853.9b | 9.21fg | 6.46i | 23.14ij | 8.02l |
| Pyirimetanil | 28 | 3739e | 1.8 | 925.2bc | 9.19gh | 6.59hi | 21.76jk | 8.55ij | 25 | 3345de | 1.3 | 822.8bc | 9.22fg | 6.60g | 22.78j | 8.65k |
| Fludioxonil | 25 | 3608e | 1.4 | 798.8d | 9.20fgh | 6.59hi | 21.79jk | 8.76i | 22 | 3169e | 1 | 811.3bc | 9.20fgh | 6.62fg | 23.10ij | 8.80j |
| Boscalid | 21 | 3034h | 0.7 | 517.4g | 9.24ef | 7.00d | 22.91i | 9.61ef | 20 | 2629h | 0.6 | 455.6gh | 9.29de | 7.02d | 23.75i | 9.91g |
| Azoxistrobin | 29 | 2029j | 2.4 | 370.3hi | 9.86b | 7.45a | 22.26ij | 11.30c | 28 | 1905k | 2.4 | 329.9ij | 9.99b | 7.50a | 23.59ij | 11.42d |
| Iprodiona | 10 | 1811k | 0.3 | 355.8i | 9.84b | 7.34b | 23.08i | 11.54c | 9 | 1631l | 0.3 | 315.5j | 10.01b | 7.33b | 24.01i | 11.91c |
| Fluazinam + Si | 30 | 3908d | 1.2 | 772.9d | 9.13i | 6.70g | 25.81gh | 8.35j | 22 | 3398d | 0.9 | 688.4d | 9.19fgh | 6.70f | 28.02g | 8.66k |
| Fenhexamid + Si | 23 | 3178gh | 0.8 | 533.4fg | 9.24ef | 6.91e | 26.32g | 9.48efg | 15 | 2779gh | 0.6 | 488.1fg | 9.31cd | 6.97d | 28.39fg | 9.85g |
| Tiofanato-metil + Si | 20 | 2771i | 0.6 | 601.1ef | 9.27de | 6.77f | 26.57fg | 10.26d | 17 | 2419i | 0.6 | 537.6ef | 9.35c | 6.83e | 28.97f | 10.68f |
| Captan + Si | 23 | 3030h | 1.2 | 846.7cd | 9.30cd | 6.64gh | 27.35f | 9.67ef | 18 | 2625h | 1 | 743.2cd | 9.33cd | 6.63fg | 29.22f | 9.84g |
| Pyirimetanil + Si | 23 | 3281fg | 1.4 | 835.3cd | 9.24ef | 6.55ijk | 28.70e | 9.30gh | 19 | 2846fg | 1.3 | 723.6d | 9.33cd | 6.63fg | 30.86e | 9.55h |
| Fludioxonil + Si | 24 | 3364f | 1.2 | 650.2e | 9.23efg | 6.46l | 29.62d | 9.16h | 19 | 2963f | 1.1 | 596.9e | 9.30cde | 6.48hi | 31.94d | 9.60h |
| Boscalid + Si | 22 | 2640i | 0.7 | 461.6gh | 9.29cd | 6.57ij | 37.84c | 10.27d | 16 | 2228j | 0.5 | 402.9hi | 9.35cd | 6.58hg | 39.96c | 10.96e |
| Azoxistrobin + Si | 4 | 1320m | 0.1 | 298.5i | 10.33a | 7.09c | 50.79a | 12.44a | 3 | 1099n | 0.1 | 214.5k | 10.47a | 7.17c | 53.75a | 13.62a |
| Iprodiona + Si | 10 | 1646l | 0.3 | 338.2i | 10.36a | 7.49a | 41.44b | 11.98b | 6 | 1286m | 0.2 | 297.6j | 10.43a | 7.55ª | 43.30b | 12.80b |
| Silicato de potasio | 25 | 3041h | 1.1 | 657.1e | 9.32c | 5.21m | 25.03h | 9.72e | 20 | 2606h | 1 | 587.6e | 9.24ef | 5.31j | 26.22h | 9.83g |
| Testigo | 79 | 7436a | 41.7 | 3841.4a | 8.75k | 5.10n | 19.76m | 3.06m | 69 | 6555a | 31.2 | 2889.3a | 8.78j | 4.60k | 20.27l | 3.27n |
w Mean values followed by the same letters within the same column are statistically equal (*= p≤0.05) according to the least significant difference (LSD) test.Yield in t ha-1.y Area Under the Disease Progress Curve calculated with the seven assessments over time.z Percentage of last evaluation performed.
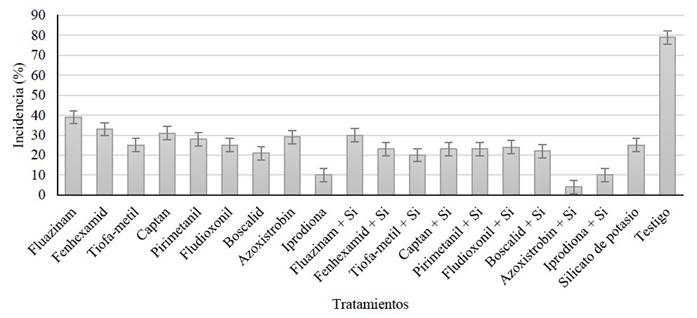
Figure 1 Incidence of gray mold induced by B. cinerea in blackberry fruits sprayed with 20 different treatments in the field. Data from the last evaluation (seventh) of the second repetition of the experiment.
The evaluation of disease severity showed an interaction between the factors (G.L = 9, F= 434.05, p<0.0001), as well as significant differences (F= 502.96, p<0.0001) in the AUCPE during the first repetition of the experiment. The treatments based on iprodione, iprodione + potassium silicate and azoxystrobin + potassium silicate were significantly different from the rest of the treatments, with AUCPE values of 355.8, 338.2 and 298.5, respectively. These treatments also presented the lowest percentages of disease severity (Table 1). In the second repetition, there were interactions between the factors (G.L = 9, F= 317.02, p<0.0001) as well as significant differences (F= 373.06, p<0.0001) in the AUCPE. The treatment of azoxystrobin + potassium silicate showed significantly different results from the rest of the treatments, with the lowest AUCPE value (214.5) and the lowest percentage of the severity of the disease (Table 1; Figure 2).
With the addition of potassium silicate, all fungicides under study promoted a reduction in the incidence and severity of the disease induced by B. cinerea, compared to the application of the fungicides alone. This was reflected in the AUDPC. These results could be because Si modifies the properties of plant cell walls (Iwasaki et al., 2002) by forming a hard outer layer (Bélanger et al., 2003) that significantly reduces the penetration of phytopathogenic fungi (Rodrigues et al., 2015). It has been reported that the foliar application of Si activates the natural defenses of plants, mainly those associated with gene expression (Fauteux et al., 2005). According to the general concepts of Jennings (2007) on fungal nutrition and mycelial growth, when Si adheres to the cell wall, the enzymes released from the hyphae cannot act efficiently to break down cellulose to glucose, affecting the nutrition of the fungus and mitigating the disease caused by it (Datnoff et al., 2011).
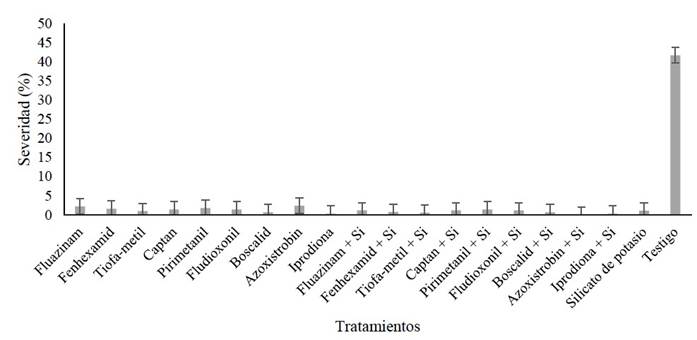
Figure 2 Severity of gray mold disease induced by B. cinerea in blackberry fruits sprayed with 20 different treatments in the field. Data from the last evaluation (seventh) of the second repetition of the experiment.
The application of azoxystrobin reduced the percentages of incidence and severity in the first three evaluations (three applications). However, the fourth evaluation showed poorer results in the control of the disease, which suggests that the pathogen possibly developed resistance since strobilurins, as mentioned above, have a high risk of inducing resistance (Villani and Cox, 2014). This was not observed when azoxystrobin was combined with potassium silicate, possibly due to the modifications in the cell walls induced by Si and its ability to activate the plant’s natural defenses (Bélanger et al., 2003; Rodriguez et al., 2015).
During the first repetition of the experiment, the total soluble solids variable showed interactions between factors in diseased (G.L = 9, F= 42.92, p<0.0001) and healthy (G.L = 9, F= 97.95, p<0.0001) fruits. In addition, significant differences were observed between diseased (F= 611.47, p<0.0001) and healthy fruits (F= 711.52, p<0.0001). The plants treated with azoxystrobin + potassium silicate and iprodione + potassium silicate showed significantly different results from the rest of the treatments, presenting the highest concentration of soluble solids in diseased and healthy fruits (Table 1). Similarly, in the second repetition of the experiment there was an interaction between factors in diseased (G.L = 9, F= 58.42, p<0.0001) and healthy fruits (G.L = 9, F = 104.52, p<0.0001). Significant differences were observed in diseased (F = 347.31, p<0.0001) and healthy fruits (F = 414.06, p<0.0001). The plants treated with azoxystrobin + potassium silicate and iprodione + potassium silicate showed significantly different results from the rest of the treatments with respect to the highest concentration of soluble solids in diseased and healthy fruits (Table 1).
In the present study, the concentration of soluble solids was higher in healthy fruits compared to diseased ones, but the plants treated with azoxystrobin + potassium silicate and iprodione + potassium silicate showed more degrees Brix. Marodin et al. (2014) found that the application of Si improved the physicochemical quality of tomato fruits (Solanum lycopersicum) by increasing the content of soluble solids. Similarly, Jarosz (2012) found a higher amount of soluble solids in cucumbers sprayed with Si. The response of plants to Silicon includes changes in the concentration of different elements, biomass production (including yield), photosynthetic rate, transpiration rate and production of enzymatic and non-enzymatic antioxidants, as well as changes in other components of agronomic or commercial interest such as the content of soluble solids (Guntzer et al., 2012; Haynes et al., 2013).
Regarding the concentration of Silicon, interactions between factors were identified (G.L = 9, F= 324.27, p<0.0001) in the first repetition of the experiment. Significant differences in the values of this variable were also observed (F= 661.90, p<0.0001). The combination of azoxystrobin + potassium silicate was significantly different from the rest of the treatments, presenting the highest concentration of Silicon (50.79 ppm) (Table 1). A similar result was observed in the second repetition, with interactions between factors (G.L = 9, F= 315.13, p<0.0001) and significant differences between treatments (F= 661.28, p<0.0001). The combination of azoxystrobin + potassium silicate was significantly different from the rest of the treatments, presenting the highest silicon concentration (53.75 ppm) (Table 1).
Regarding fruit yield, an interaction between the factors was identified (G.L = 9, F= 176.61, p<0.0001) during the first repetition, as well as significant differences between treatments (F= 429.24, p<0.0001). The plants treated with azoxystrobin + potassium silicate showed significantly different results from the rest of the treatments. This treatment was associated with the highest yield (12.4 t ha-1), followed by the treatments based on iprodione + potassium silicate and iprodione, with yields of 11.9 and 11.5 t ha-1, respectively (Table 1). In the second repetition there was also an interaction between factors (G.L = 9, F= 195.54, p<0.0001) as well as significant differences between treatments (F= 476.31.12, p<0.0001). The treatment of azoxystrobin + potassium silicate was significantly different from the rest of the treatments, presenting the highest yield (13.6 t ha-1), even higher than the result of the first repetition (Table 1).
The plants treated with azoxystrobin + potassium silicate presented the highest yield per hectare. This is pobably because Si alone, as a nutritive element, performs some metabolic and structural functions that have beneficial effects on the physiology of plants. Thus, the accumulation of Si results in an increase in productivity in different plant species. Marodine et al. (2014) evaluated the effect of three silicon sources on tomato yield and found that the yield increased as the dose of silicon increased. Lu et al. (2016) evaluated three different sources of Silicon on the agronomic variables of tomato and determined that the application of nanosilica was associated with increased height and dry and fresh weight of plant organs, and consequently with increased yield. Numerous studies suggest that Silicon increases the yield of crops such as strawberry (Ouellette et al., 2017), tomato (Lu et al., 2016; Marodin et al., 2014; Toresano et al., 2012), cucumber (Abd- Alkarim et al., 2017), courgette (Cucurbita pepo) (Savvas et al., 2009), potato (Solanum tuberosum) (Pilon et al., 2013), wheat (Triticum durum) (Hanafy et al., 2008), and other grasses (Ahmad et al., 2017).
One of the most notable effects of Silicon on plants is the reduction of the incidence and severity of diseases caused by pathogens, which is reflected in different agronomic characteristics, including yield. Silicon applications can work as well as fungicides to suppress plant diseases, which makes this element a valuable addition to an integrated disease management strategy (Fillinger et al., 2016).
In general, the data obtained in the present study showed that blackberry plants treated with a combination of different fungicides + potassium silicate, especially azoxystrobin, had a lower incidence and severity of gray mold disease. This was reflected in the component variables of yield. The conclusion is that potassium silicate enhances the effect of fungicides in field conditions and is a viable alternative for integrated management programs of blackberry gray mold caused by Botrytis cinerea.











 texto en
texto en 


