In Mexico, the cultivation of coffee (Coffea arabica) is an activity with great economic, social and environmental importance. The state of Veracruz is one of the main coffee producers in terms of surface (around 139 thousand hectares), commercialization volume (SAGARPA, 2015), and number of producers (Pérez and Pérez, 2012). The varieties cultivated include Typica, Garnica, Bourbon, Caturra, among others (López-García et al., 2016). The cultivation of Coffea arabica and its varieties is vulnerable to phytosanitary problems that affect grain production and quality. Such is the case of the Typica variety, which is characterized by its tall size, thin trunk and branches widely separated, large leaves with bronze tips, red fruits, acidity, and high quality in the cup due to its complex flavors with notes of flowers and fruits (Hernández-Solabac et al., 2011; López-García et al., 2016). This variety is susceptible to pests and diseases. One of the most predominant and harmful in terms of production is coffee rust, caused by the fungus Hemileia vastatrix (Avelino et al., 2015).
Chemical control, despite being effective when used properly, entails high costs for producers, and sometimes the production organic coffee must meet official standards that prohibit the use of pesticides (Shigueoka et al., 2014; Silva-Acuña et al., 2002). The use of resistant hybrid varieties of arabica coffee is the most economical and environmentally friendly form of control (FAO, 2015). However, the disease resistance of coffee plants can be affected by the genetic variability of the pathogen, its high evolutionary potential, and the probability of mutation events (Várzea and Marques, 2005). Current initiatives and technological trends promote the development of bio-inputs such as bioinsecticides, bioherbicides, and biofungicides, among others (Ordanza-Beneitez, 2017). These include plant defense stimulants, biological control techniques, and derivatives of natural products obtained from plants and microorganisms (Ordanza-Beneitez, 2017; Mesa et al., 2019). The United States Environmental Protection Agency (EPA) has stated that natural products play an important role in protecting crops under the principles of Integrated Pest and Disease Management (IMPE) (Cantrell et al., 2012; Leahy et al., 2014; Sparks et al., 2017).
This is why these types of alternatives have been used in the cultivation of various crops such as corn and beans, to mention a few. For example, essential oil of neem (Azadirachta indica) has been used to control phytopathogenic fungi such as Sclerotium rolfsii, Rhizoctonia solani, Sclerotina sclerotiorum, Lagenidium giganteum, Metarhizium anisopliae, and Fusarium graminearum (Mohamed-Ali et al., 2017; Mohammad et al., 2014; Mohanty et al., 2008; Heidtmann-Bemvenuti et al., 2016). It has also been used on urediniospores of Phakopsora pachyrhizi, leading to a 35% reduction in the severity of Asian soybean rust (Glycine max) under greenhouse conditions. Similarly, the extract of “acuyo” or “hoja santa” (Mexican pepperleaf, Piper auritum) has been classified as a biofungicide agent against species of phytopathogenic fungi such as Helminthosporium and Fusarium spp. (Montes-Belmont, 2009), Colletotrichum acutatum, C. gloeosporioides and Botryodiplodia theobromae (Pineda et al., 2012). The present study evaluated the bio-fungicidal activity of leaf extracts of Piper auritum and A. indica against H. vastatrix using in vitro and in vivo tests.
Fresh leaves (without mechanical damage and stains) of A. indica were collected in Boca del Río, in the state of Veracruz (19° 09’ 00.3” N and 96° 07’ 32.9” W, 16 masl). Fresh leaves of P. auritum were collected in Coatepec, Veracruz (19° 27’ 33.3” N and 96° 57’ 18.8” W, 1200 masl). All leaves were collected during the spring of 2020. The fresh leaves of both species were rinsed with running water to clean them. The leaves were then crushed, and 10 g of each species were weighed using an analytical balance. The crushed leaf was then placed in a clean container, 100 mL of absolute ethanol were added, and the material was left to stand for 72 h. The same operation was carried out in another container but with an apolar solution (a 1:1 mixture of hexane and chloroform). Afterward, the solutions were poured separately into a funnel with filter paper (Wattman # 2) to obtain ethanolic and apolar extracts. Finally, each extract was placed in a water bath at 45 °C to concentrate the extracts (1g to 900 mg of recovered extract) until the solvent evaporated (modified method of Alvarado-Castillo et al., 2017). The extracts were then stored in a refrigerator at 4 °C for later use in in vitro and in vivo tests.
Uredospores of H. vastatrix were collected from naturally infected Typica arabica coffee leaves. The collection of the leaves was carried out between April and July of 2020, in the town of Mahuixtlán, Coatepec, Veracruz, (19° 24’ 31.7” N and 96° 55’ 37.0” W, 980 masl). On the same day they were collected, the leaves were taken to the parasitology laboratory of the Faculty of Agricultural Sciences of the Universidad Veracruzana in Xalapa, Veracruz, to carry out the in vitro test. The effectiveness of the extracts was tested using the method of Alvarado-Castillo et al. (2017). For this test, 100 ± 10 uredospores of H. vastatrix were placed in a humid chamber and were then treated with extracts of P. auritum and A. indica in three concentrations, 1000, 3000 and 5000 ppm (1mL of volume), a control of water:ethanol (1:1 v/v) and a blank of Copper Oxychloride (OXIMET®) and wettable powder (50%) (3.0 g/L of water), which was prepared following the instructions of the manufacturer for application to coffee plants. The humid chambers were placed in an incubator at a regulated temperature of 27 °C for five days. The experimental unit was a Petri dish for each treatment, set up in triplicate. The experiment was observed for 5 days because that is the time necessary for the uredospores to germinate and the germinated tubes to appear, which generally occurs after 6 to 12 hours, without the fungus penetrating and carrying out its biological cycle completely (Avelino and Rivas, 2013; Virginio-Filho and Astroga-Domian, 2015).
The in vitro germination was observed at 24, 72 and 120 hours using an optical microscope (VELAB™ model VE-B6). The number of germinated spores per treatment was quantified. The germination percentage of each treatment was calculated after five days. The percentage of inhibition was calculated using the following formula: %growth inhibition= [(control growth-treatment growth)/control growth] *100 (Taken with modifications from Polanco-Florían et al., 2020).
The in vivo analysis of the effectiveness of the P. auritum and A. indica extracts was conducted on Typica coffee plants with the presence of H. vastatrix. These were obtained from a commercial nursery located in San Marcos de León, Xico, Veracruz. (19° 25’ 17.1” N 96° 57’ 59.2” W, 1100 masl) during the spring of 2020. The plants were taken to the Faculty of Biology, Universidad Veracruzana, Xalapa, Veracruz for analysis. The inclusion criteria used to collect the plants were the following: similar size and number of leaves (approximately 11 to 15 leaves), same age (one year), variety, substrate and no previous treatment with fungicides. The coffee plants were grouped into experimental units of 10 individuals. Different treatment groups were formed: control (water), blank (copper oxychloride), ethanolic extracts of P. auritum and A. indica at three concentrations, 1000, 3000 and 5000ppm. Each treatment had three repetitions. The experimental design was completely randomized. The data were analyzed with an analysis of variance (ANOVA) and the means of each evaluation (24, 72 and 120 h) were compared with a Tukey test (P ≤ 0.05). The normality of the parameters was checked with the Shapiro-Wilks test. All statistical analyses were performed using the STATISTICA® program for Windows.
All coffee plants were kept under the same greenhouse conditions of temperature, humidity, light incidence, and watering. To induce infection with H. vastatrix (previously collected in the town of Mahuixtlán, municipality of Coatepec, Veracruz), around 100 uredospores per square centimeter (previously quantified with the help of a binocular stereoscope VELAB™ VE- S1) were inoculated on the underside of the leaves of Typica coffee plants with the help of a fine-tipped brush. The progress of the infection, including the incubation period (IP) and the latency period (LP), was observed for 20 days using the diagrammatic scale of severity for coffee rust infection (SINAVEF, 2013). It is important to highlight that the first sori were formed during this period, as well as the release of new spores, which confirmed the presence of coffee rust and the beginning of the infection process (Avelino and Rivas, 2013) in 2% of the affected area (Capucho et al., 2011).
The treatments were applied on the underside of the leaves once the first signs of the pathogen were detected (100 mL volume of previously prepared solution) and the progress of the disease was recorded (0, 5, 10, 15 and 20 days after inoculation) (SINAVEF, 2013), that is, during the appearance of new yellow or orange spots on the underside of the leaves, which is one of the indicators of the growth of the disease. The progress of the disease in all the experimental groups was assessed using a diagrammatic scale, according to SINAVEF (2013). Photos of the upper and lower sides of the leaves were taken to classify them according to the diagrammatic scale of disease severity and to record the reactions of H. vastatrix, as reported by SINAVEF (2013) and SENASICA (2019). The SINAVEF scale (2013) was used to evaluate the severity of the disease in the leaves.
The data obtained with the diagrammatic scale were transformed into severity percentages using the Townsend and Heuberger formula (Guillén-Sánchez et al., 2017):
Where,
P - weighted severity average
n - number of leaves for each class in the scale
v - numerical value of each class
N - total number of leaves in the sample
CM - major category
The experimental design was completely randomized, with a factorial arrangement of three repetitions. Factor A corresponded to the commercial fungicide and to the three concentrations of plant extracts, while factor B corresponded to the fungus. An analysis of variance (ANOVA) was used to determine the effect of the treatments. Tukey’s comparison of means test was used to evaluate (P ≤ 0.05) differences, using the STATISTICA® program for Windows.
Figure 1 shows the inhibition effect of all treatments on the germination of H. vastatrix uredospores. The ethanolic and apolar extracts of A. indica and P. auritum inhibited 99% of the germination of H. vastatrix. The yellow coloration usually presented by untreated uredospores was not observed. However, the uredospores treated with copper oxychloride did show a yellow coloration, similar to those in the control group (Figure 1 A and C). A gradual increase in uredospore germination was observed in the latter group, from 18 to 99 % between 24, 72 and 120 hours (Figure 2).
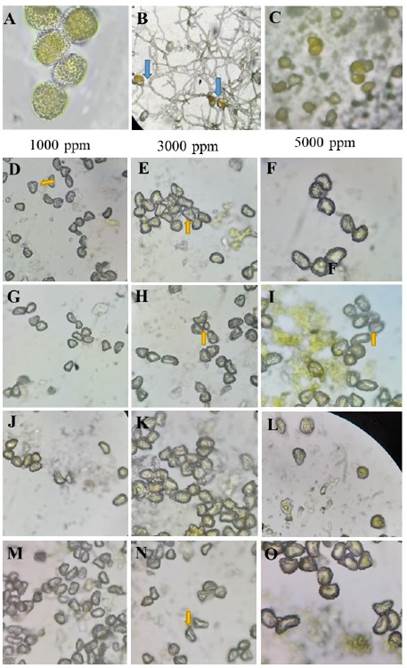
Figure 1 Effect of plant extracts on Hemileia vastatrix uredospores. A) H. vastatrix uredospores without treatment; B) Control; the blue arrow shows the germ tubes of H. vastatrix. C) Blank (Copper oxychloride) inhibits the germination of H. vastatrix. D-F) Ethanolic extract of A. indica; G-I) Apolar extract of A. indica; J-L) Ethanolic extract of P. auritum; M-O) Apolar extract of P. auritum (40X); the yellow arrows indicate decoloration of H. vastatrix and absence of germination.
The treatments with A. indica and P. auritum, in particular at concentrations of 3000 ppm and 5000 ppm, caused damage to the uredospores of H. vastatrix, as evidenced by the discoloration and deformation observed in the outer structure of the uredospores. The uredospores treated with copper oxychloride remained yellowish and did not germinate. Alvarado et al. (2017) mentioned that uredospores that showed discoloration and deformation after being treated with plant extracts could not germinate. Based on the definition of “antifungal” by Gregorí-Valdés (2005), they confirmed that the leaf extracts of A. indica and P. auritum have biofungicidal activity against H. vastatrix uredospores. Both extracts evaluated in the present study might have partially altered the external structures of H. vastatrix uredospores, or simply altered their viability or survival capacity, either directly or indirectly, as described by Gregorí-Valdés (2005).
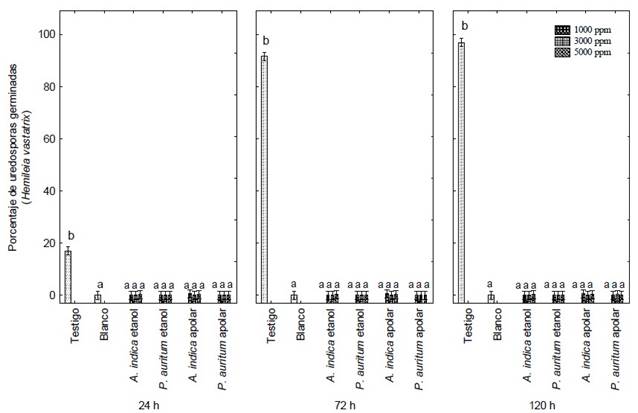
Figure 2 Germination percentage of Hemileia vastatrix uredospores under the action of ethanolic and apolar extracts of Piper auritum and Azadirachta indica, and copper oxychloride (Blank) at 24 h, 72 h and 120 h of exposure. Letters indicate significant differences between means (Tukey, P ≤ 0.05).
The harmful effects of the coffee plant extracts on uredospores could be due to the presence of secondary metabolites with biofungicidal activity in P. auritum, such as monoterpenoids, sesquiterpenoids, and phenylpropanoids; or to the presence of phenolic metabolites such as alkaloids and glycosides in A. indica (Mohammad et al., 2014; Pineda et al., 2012; Nagano and Batalini, 2021). It is also possible that the presence of several secondary metabolites could have a synergic effect that inhibits the germination of H. vastatrix (Babatunde et al., 2019; Mgbeahuruike et al., 2017; Durant-Archibold et al., 2018, Salehi et al., 2019; Mesa et al., 2019). This fungicide activity might work by interacting with the cell wall or membrane of the fungus. However, further studies should be carried out to identify the possible mechanisms of action and the type of secondary metabolites with biofungicide activity.
The apolar extract was omitted in the in vivo tests due to its degree of environmental toxicity. Treatment with the ethanolic extracts of P. auritum and A. indica caused no increase in yellow spots or uredospore sporulation in Typica coffee plants at the three different concentrations evaluated, 1000, 3000 and 5000 ppm (Figure 3). The germination percentage remained constant at around 26.6% during the 20 days of experimentation (SENASICA 2019); that is, no new spore colonies were found on the underside of the leaves and foliar injury was minimal, according to the diagrammatic scale for evaluating the severity of coffee rust in leaves and plants, as reported by SAGARPA (2015) and SENASICA (2019). Therefore, the severity of the disease remained constant from the beginning to the end of the experiment. Only the yellowish spots caused by the uredospores of H. vastatrix were observed (Figure 4 C and D).
In the control treatment, the infection increased progressively until the end of the experiment, up to severity class IV or 69.3% severity (Figure 4 and 5 A). Significant differences were found between treatments when comparing their effect over time (Tukey, P ≤ 0.05). In the coffee plants treated with copper oxychloride, the infection was observed up to 15 days after the application of the treatment. The infection increases from class two to three, that is, from 32 to 52% severity (Figure 4), when some yellowish spots with small fungal pustules appeared on the underside of the leaf of Typica coffee plants. These signs indicate the beginning of the production of uredospores.
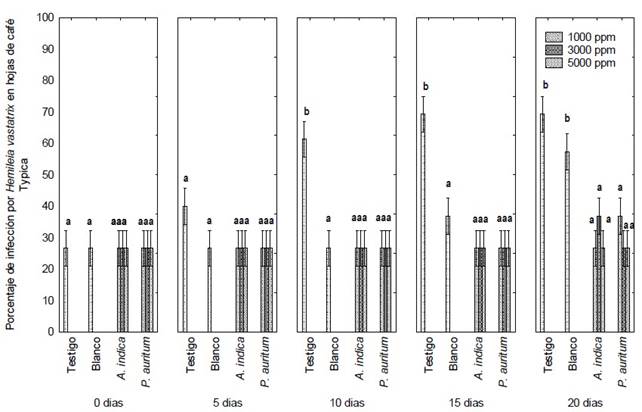
Figure 3 Severity percentage of H. vastatrix in Typica coffee leaves treated with foliar ethanolic extracts of A. indica and P. auritum during 20 days of treatment. On the Y-axis title: Severity of Hemileia vastatrix (%). Letters indicate significant differences between means (Tukey, P ≤ 0.05).
Therefore, the use of ethanolic extracts of A. indica and P. auritum, until now, is one of most the promising extracts as inhibiting agents of coffee rust caused by H. vastatrix in Typica coffee plants under greenhouse conditions. Sánchez-Fernández et al. (2013) indicated the importance of carrying out tests on plants under greenhouse or nursery conditions to assess the pre- and post-emergent activity of different natural compounds.Previous studies have reported that the extracts of P. auritum and A. indica are effective for the management of phytopathogenic fungi in mango, avocado, apple, peach, tomato, potato and rice plantations infected with fungi of the genera Colletotrichum, Botryodiplodia, Alternaria, Curvularia, Sarocladium, Bipolaris, Fusarium (Pineda et al., 2012), Rhizoctonia, Sclerotium (Mohamed-Ali et al., 2017), Sclerotinia (Mohammad et al., 2014), Lagenidium, and Metarhizium (Mohanty et al., 2008). These studies support the use of extracts of P. auritum and A. indica as biofungicides in Typica coffee plants before planting and the effective concentration of the extracts ranges between 1000 and 5000 ppm. The use of these extracts within the framework of sustainable agriculture is a promising alternative due to their effectiveness, low cost and easy degradation due to their natural origin (Rodríguez et al., 2000; Villavicencio-Nieto and Pérez-Escandón, 2010). A classification consensus of these extracts should be established according to their effect: active, moderately active, slightly active and innocuous. This would allow to select the plant extracts with the most promising antifungal activity (Mesa et al., 2019). The results of the present study support the use of extracts from A. indica and P. auritum as biofungicides against H. vastatrix.
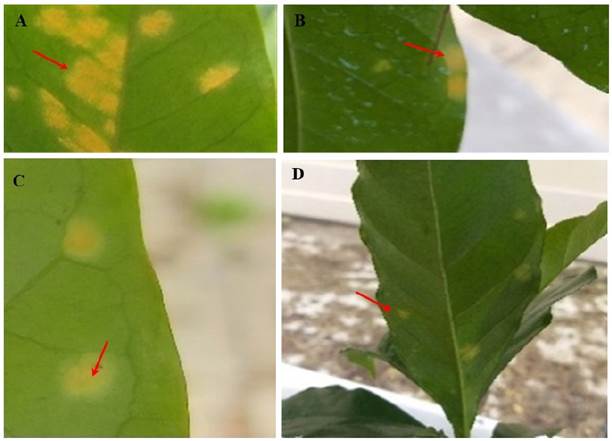
Figure 4 Effect of the application of copper oxychloride and extracts of Azadirachta indica and Piper auritum on Typica coffee leaves. A) Control; red arrows indicate H. vastatrix infection; B) Copper oxychloride; yellowish stains; C and D) Coffee leaves treated for 15 days with extracts of A. indica and P. auritum (red arrows indicate the damage caused by the initial infection, as well as the absence of uredospores due to the application of plant extracts).
The foliar extracts of A. indica and P. auritum, in concentrations of 1000, 3000 and 5000 ppm, inhibited the germination of H. vastatrix during the 120 hours of treatment, causing discoloration and deformation in the external structure of H. vastatrix uredospores. Both extracts showed biofungicidal activity against H. vastatrix uredospores when applied to Typica variety coffee plants, reducing the spread of the disease during 20 days after the application of the extracts. In conclusion, extracts of A. indica and P. auritum in concentrations of 1,000 to 5,000 ppm are an effective alternative for the preventive management of coffee rust, mainly in nursery plants.











 texto en
texto en 


