Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.42 no.1 Texcoco ene. 2024 Epub 27-Ene-2025
https://doi.org/10.18781/r.mex.fit.2305-2
Scientific Articles
Induction of defense response mediated by inulin from dahlia tubers (Dahlia sp.) in Capsicum annuum
1 Biotecnología Vegetal, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, AC (CIATEJ). Unidad Zapopan, Camino Areneno, 1227, Zapopan, Jalisco, CP 45019, México;
2 Biotecnología Médica y Farmacéutica, CIATEJ. Unidad Guadalajara, Avenida Normalistas, 800, Guadalajara, Jalisco, CP 44270, México;
3 Departamento de Bioingeniería, Escuela de Ingeniería y Ciencias, Instituto Tecnológico y de Estudios Superiores de Monterrey. Campus Guadalajara, Ramón Corona, 2514, Zapopan, Jalisco, CP 45201, México.
Objective / Background.
Phytophthora capsici is the causal agent of chili wilt. Among the strategies for its control is the use of resistance inducers. Fructans are molecules with interesting biological properties, including the ability to induce resistance mechanisms in some plants. In this work, the protective effect of four concentrations inulin from dahlia tubers on chili infected with P. capsici was evaluated.
Materials and Methods.
The concentration that showed the highest protection was chosen to evaluate the induction of defense response through the enzymatic activity of β-1,3 glucanases, peroxidases and the production of total phenolic compounds.
Results.
Inulin showed a protective effect against infection at concentrations of 100 to 300 μM, as symptoms decreased and seedlings showed improved vegetative development. It was observed that inulin at 200 μM concentration was able to induce an effective defense response associated with increased activity of β-1,3 glucanases and peroxidases through a local and systemic response in seedlings. This response was differentiated between seedlings treated with inulin and seedlings infected with
P. capsici.
Conclusion.
It was concluded that inulin has the ability to protect chili bell pepper from P. capsici by induction of resistance.
Keywords: elicitors; Phytophthora capsici; fructans; phenolic compounds; PR proteins
Objetivo/Antecedentes.
Phytophthora capsici es el agente causal de la marchitez del chile. Entre las estrategias para su control está el uso de inductores de resistencia. Los fructanos son moléculas que presentan propiedades biológicas interesantes, incluyendo la capacidad de inducir mecanismos de resistencia en algunas plantas. En este trabajo se evaluó el efecto de protección de cuatro concentraciones de inulina de tubérculos de dalia en chile infectado con P. capsici.
Materiales y Métodos.
La concentración que presentó mayor protección se eligió para evaluar la inducción de respuesta de defensa mediante la actividad enzimática de β-1,3 glucanasas, peroxidasas y la producción de compuestos fenólicos totales.
Resultados.
La inulina mostró un efecto protector contra la infección a concentraciones de 100 a 300 μM, ya que disminuyeron los síntomas y las plántulas mostraron mejor desarrollo vegetativo. Se observó que la inulina en concentración 200 μM indujo una respuesta de defensa efectiva asociada al aumento de la actividad de β-1,3 glucanasas y peroxidasas mediante una respuesta local y sistémica en las plántulas. Esta respuesta fue diferenciada entre las plántulas tratadas con inulina y las plántulas infectadas con P. capsici.
Conclusión.
Se concluyó que la inulina tiene la capacidad de proteger al chile de P. capsici por la inducción de resistencia.
Palabras clave: Elicitores; Phytophthora capsici; fructanos; compuestos fenólicos; proteínas PR.
Introduction
Chili (Capsicum annuum) es an economically important crop in Mexico, with a production estimated in over 3.1 million tons a year, making Mexico the fourth chili producer in the world in 2022 (SADER, 2023). However, this crop is affected by different pathogens including viruses, bacteria, oomycetes and fungi, which cause metabolic alterations in the crop, limiting the yield and quality of the fruit (Ramos- Sandoval et al., 2010; Majid et al., 2017). Chili wilting or blight, considered the most destructive disease for the crop (Barchenger et al., 2018), is mainly related to the oomycete Phytophthora capsici, which spreads through irrigation and rain water via oospores and zoospores (Sanogo et al., 2023). Economic losses related to this disease are considered of great importance for farmers, and its control entails a considerable rise in crop production costs (Moreira-Morrillo et al., 2022).
In Mexico, diverse studies have been carried out on the presence, genetic diversity and search for chili varieties resistant to this pathogen (Castro-Rocha et al., 2016; Palma-Martínez et al., 2017; Pons-Hernández et al., 2020). Isolations have been obtained in different chili species and varieties from production areas in Aguascalientes, Chihuahua, Guanajuato, Jalisco and Michoacán, as well as characterizing the distribution of the pathogen and its range of hosts in economically important crops of the Solanaceae and Cucurbitaceae families (Reyes-Tena et al., 2021).
Different alternatives are explored to control chili wilting, such as resistance inducers or plant defense, which may be peptides, polysaccharides, lipids and other types of biomolecules. Its action mechanism is the generation of an induced defense response, or “priming”, which includes physiological, molecular or epigenetic changes as a result of a stimulus related to a process of pathogenesis, where the plant generates a resistance response, which protects it against the later attack of pathogens (Hönig et al., 2023).
Different molecules have been reported as resistance inducers against the genus Phytophthora. From phytohormones such as jasmonic acid and methyl jasmonate, which were proven to delay the progress of the infection by P. infestans in potato (Arévalo-Marín et al., 2021) to low-spectrum fungicides such as potassium phosphite, a functional analog of salicylic acid such as acibenzolar-S-methyl and a non-protein amino acid such as β-aminobutyric acid (BABA) that reduced the lesions and symptoms of the disease related to P. infestans in tree tomato (Solanum betaceum) (Castaño-Monsalve et al., 2015).
The genus Dahlia includes perennial herbaceous plants that are characterized by their inflorescences with ligulate and discoid flowers, tuberous roots, developed from a short rhizome. This genus has 38 species, out of which 35 are endemic to Mexico (Carrasco-Ortiz et al., 2019). The main use of the dahlia is as an ornamental plant, although its medicinal and nutritional benefits are currently being promoted. Particularly the tuberous roots are characterized for their fiber, protein, mineral and vitamin contents, as well as for their percentage of carbohydrates in the form of inulin and other fructans (Hernández-Epigmenio et al., 2022). Depending on the maturity of the root and on the species, the inulin content may fluctuate between 52 and 83 % of de carbohydrates found in the tubers (Santana-Legorreta et al., 2016). In this sense, fructans are polymers composed of fructose units linked by β(2-and/or β(2-6) bonds that generally have a terminal glucose residue, and some derivatives have been reported as inducers of plant defense for some crops (Sun et al., 2013; Zhang et al., 2009; Zhang et al., 2013). For the chili crop (C. annuum), the use of agave (Agave tequilana var. Azul) fructans have been reported, with different degrees of polymerization and concentrations inducing a protective effect against the severe symptoms of infection by P. capsici, reducing the incidence and damage to the root through foliar application (Navarro-López et al., 2019). Applying inulin (200 µM) on the base of the chili plant has also been reported to reduce the incidence of the disease and the damage on the root caused by P. capsici by up to 40 % (López-Velázquez et al., 2019a). This protection by inulin could have been increased by up to 80 % with the use of biodegradable hydrogels that protected the polymer in from lixiviation and degradation in the soil (López- Velázquez et al., 2019b). In all three studies, the protection against the disease was evaluated with the reduction of the incidence, damage to the roots, and the presence of the pathogen in the root, although no tests were conducted to determine if this protection was triggered by the induction of resistance in the plant.
Considering the importance of validating that the protection provided by the application of inulin is related with the induction of plant defense mechanisms, as well as the effects of different doses of the polymer, the aim of this study was to evaluate the protective effect of four concentrations of a reactive grade of inulin from dahlia tubers applied on the base of the plant. The concentration that displayed the greatest protection was further assessed for its induction of an effective biochemical defense response through the enzymatic activity of β-1,3 glucanases, peroxidases and the production of total phenolic compounds.
Materials and Methods
Location and area of study. This study was carried out in the facilities of the Plant Biotechnology Unit of the Center for Research and Assistance in Technology and Design in the state of Jalisco AC. Zapopan Unit, between August and December, 2018.
Plant material. Serrano chili seeds (Capsicum annuum) cv. Camino Real (Harris Moran Seed Company) were used. They were disinfested by submerging for 3 minutes in commercial sodium hypochlorite (NaClO) at 3%. Next, they were rinsed with distilled water and left to germinate on trays with a sterile peat-moss substrate, sand and vermiculite in a proportion of 6:2:1, respectively.
Biological material. The Phytophthora capsici (CH11) strain from the CIATEJ strain collection was used, provided and isolated in 2011 by Dr. Sylvia Fernández Pavía, of the Institute of Agricultural and Forestry Research of the San Nicolás de Hidalgo University of Michoacan (Reyes-Tena et al., 2019). The conditions for planting and generation of the inoculum were the same as those described by López-Velázquez et al. (2019a) .
Clarified v8 culture medium. V8 (Campbell’s) juice was used, clarified by filtration. A mixture was prepared using 50 mL of clarified juice, 0.5 g of calcium carbonate (Sigma-Aldrich, St. Louis, MO, USA) and 15 g of agar (Sigma-Aldrich, St. Louis, MO, USA) in distilled water. This mixture was shaken and diluted to 1 L with distilled water. The medium was sterilized using an autoclave at 121 °C for 20 min (Trinidad-Cruz et al., 2021).
Dahlia tuber inulin solutions. Inulin solutions were prepared from reactive grade inulin from dahlia tubers (Sigma-Aldrich, St. Louis, MO, USA). They were dissolved in distilled water at concentrations of 20, 100, 200 y 300 μM. The solution was sterilized by filtration with 20 μm membranes before their application.
Evaluation of the protection of dahlia inulin in the control of P. capsici. Ten 30-day old chili seedlings were used per treatment, which were transplanted in 275 mL polystyrene cups with a sterile substrate composed of peat-moss, sand and vermiculite in a proportion of 6:2:1. After transplanting, 10 mL of dahlia tuber inulin were placed on the base of the stem. Inulin concentrations were 20, 100, 200 and 300 μM. Ten days after applying the inulin, a second application was performed, and immediately after that, the seedlings were inoculated with 10 mL of the P. capsici zoospore suspension (1×104 zoospores mL-1) (Wang et al., 2013). The experiment ended two weeks after inoculation, photographs were taken to record and the heights and fresh weights of the plants were determined. The level of protection against the disease was also determined, considering the following disease severity scale that was proposed based on the symptoms observed throughout the experiment: 1. Healthy seedling, with no evident signs of the disease; 2. Seedling with chlorosis, darkening in stem and roots, wilting in leaves; 3. Seedling displaying damage on leaves, necrosis in stem and roots, short roots, severe defoliation and smaller size than the control and 4. Dead seedling. A randomized block experimental design was used with 10 repetitions each. The seedlings taken as controls were the ones provided with sterile distilled water instead of inulin and the plants inoculated with only the pathogen. The experiment was carried out in duplicate, separated in space and at the same time.
Determining damage in roots. To determine the damage on the roots, the viability of roots was compared between different treatments. The viability was determined with the reduction of 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma-Aldrich, St. Louis, MO, USA) to 1,3,5-triphenylformazan (TTF) using the method described by Wang et al. (2013) with some modifications (López-Velázquez et al., 2019a). The viability of the roots was obtained by calculating the intensity of the reduction of the TCC (mg g-1 h) = absorbance of the reduced TTC / FW h, where FW is the mass of the fresh root and h is the time of incubation.
Presence of the oomycete in the root. The presence of the oomycete in the root of the chili plant was determined by staining the mycelium with trypan blue (López- Velázquez et al. 2019b). Root samples were taken and bleached with potassium hydroxide at 1.7 M (Sigma-Aldrich, St. Louis, MO, USA) and hydrogen peroxide at 0.5 M (Sigma-Aldrich, St. Louis, MO, USA). They were they dyed using trypan blue at 100 μM (Sigma-Aldrich, St. Louis, MO, USA) for 2 h. The excess dye was discarded by washing with lactoglycerol and the samples were observed under an optic microscope (Olympus, model BH-2, Tokyo, Japan).
Evaluation of the induction of response by defense in chili from the treatment with dahlia inulin against P. capsici. Thirty-day old seedlings were used, which were transplanted into 1 kg polyethylene bags with a sterile substrate. After transplanting, 10 mL of dahlia tuber inulin were applied on the base of the seedling, in the concentration that displayed the greatest protection (200 μM); a treatment with inulin and the oomycete was considered, along with another one, only with the pathogen, and finally, a treatment with distilled water as a control. Ten days after the first application of the inulin, a second application was made, followed immediately by an inoculation of the plant with 10 mL of the P. capsici (1×104 zoospores mL-1) zoospore suspension (Wang et al., 2013).
Enzyme extraction and activity trials. Root and leaf samples were taken from the plant at the moment of induction, on the day of inoculation with the pathogen (considered day 0) and 1, 2, 3, 5, 7 and 9 days after inoculation with the pathogen. The enzyme activity was determined for proteins related to pathogenesis (PR) with the activities of β-1,3 glucanases and peroxidases. The production of phytoalexins was related with the quantification of total phenolic compounds absorbed at 650 nm. The samples were macerated with liquid nitrogen. To determine the β-1,3 glucanases and peroxidases, they were resuspended in a sodium phosphate 0.1 M buffer with a pH 7.0. To quantify the total phenolic compounds, they were resuspended in methanol al 80 % (v/v).
Determining the activity of β-1,3 glucanases. This was carried out with the colorimetric method to detect reducing sugars at 515 nm, as a result of enzymatic hydrolysis and reduction of DNS (3,5-dinitrosalicylic acid, Sigma-Aldrich, St. Louis, MO, USA). Quantification was carried out using a calibration curve with glucose, and the specific activity was reported in nanokatals (nkat) per gram
(g) of total protein (nkat g-1 g-1 TP). One nkat is defined as 1 nmol of D-glucose released from laminarin (Sigma-Aldrich, St. Louis, MO, USA) per second under the conditions of the test (Oliveira et al., 2014).
Determining the activity of peroxidases (POX). This was carried out following the method proposed by Oliveira et al. (2014). We used 10 µL of leaf and root extract for the reactions. The variation of one unit of absorbance per minute was defined as a unit of peroxidase activity (1 AU) and expressed per gram of total protein (AU g-1 g-1 TP).
Determining the induction of total phenolic compounds. This was determined by quantifying total phenolic compounds, following the method proposed by Ainsworth and Gillespie (2007), with some modifications (López-Velázquez et al., 2019a). The quantification was carried out using a standard curve of known concentrations of catechol (0.2 - 1 mM) and the results were expressed as nmol catechol g -1 FW-1.
Statistical analysis. The data were normalized with the Anderson-Darling test. Later, a unifactorial analysis of variance was carried out, using the procedures from the Statgraphics Centurion XVI, version 16.1.11 statistical package (Statistical Graphics, Englewood Cliffs, NJ, USA). For the separation between means, an analysis was performed using the LSD test, with a level of significance of 95 %.
Results
Protection of dahlia inulin in the control of the infection by P. capsici in chili. To determine the degree of protection against the infection from P. capsici by the application of dahlia inulin in serrano chili plants, different parameters were evaluated, such as the reduction of the symptoms of the disease, including the height and fresh weight of the seedling, incidence of the disease, as well as the damage, viability and the presence of the pathogen in the root. The results of the biological effectiveness test showed that plants inoculated with the pathogen (P) had a disease incidence of 80 %, a level 4 disease severity and severe damage to the root, observed as a significantly lower root volume than the control (C) (Figure 1). In addition, a significant reduction was observed in the fresh weight and height of the plant, as well as the viability of the root regarding the control group (Table 1). For the seedlings treated with 20 μM of inulin (I1+P), there was no protection induction response and the symptoms, incidence, disease severity and damage to root were similar to what was observed in plants infected with P. capsici (Figure 1a). The same effect was observed for fresh weight, plant height and root viability (Table 1). The treatment with 100 μM of inulin (I2+P) displayed a positive effect in the induction of protection against the infection by P. capsici. With this treatment, the disease incidence shown was 50 %, severity was rated as level 3 and no significant damage was found in the root (Figure 1b). The fresh weight and seedling height were equivalent to those of the control, although root viability was equal to that of the infected seedlings (Table 1). For the seedlings treated with 200 μM of inulin (I3+P), the induction of the protection against the infection of P. capsici was also observed, although with a severity of level 2 and an incidence of 40 %, being the best protection observed in all treatments (Figure 1).
Finally, the seedlings treated with 300 μM of inulin (I4+P) displayed the induction of protection. Incidence was reduced by 50 %, disease severity was level 3 and no damage was observed in the root (Figure 1). The fresh weight and height of the plant were equivalent to the control, although the viability of the roots was equivalent to that in the infected plants (Table 1). In all the treatments inoculated with the pathogen, the infection was verified with the presence of the P. capsici mycelium, which indicates that the oomycete was able to colonize the roots (Figure 1c). The results highlight that the viability pero mg of the roots from plants treated with inulin did not display significant differences with the plants infected with P. capsici (Table 1). From these results, the treatment with 200 μM of inulin (I3+P) was considered as the one that induced the best protection against the infection by P. capsici in chili plants. This concentration was used for the evaluations of the induction of an effective defense response in chili.
Defense induction in chili seedlings with the application of dahlia inulin against P. capsici
Activity of β-1,3 glucanasas. The response in the root was considered a local defense response and the response in leaves was considered a systemic defense response. In
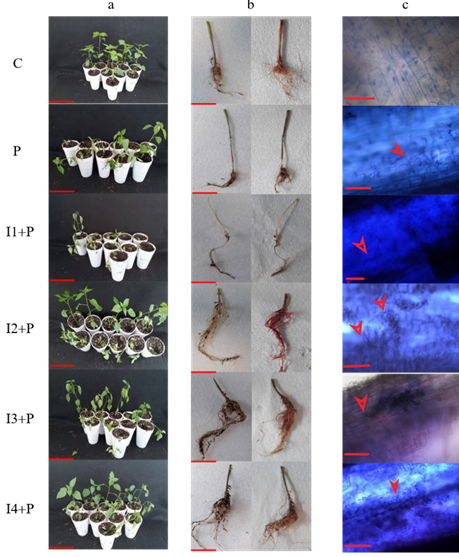
Figure 1 Evaluation of dahlia inulin in the protection of P. capsici infection in chili seedlings. a: Biological effectiveness test of dahlia inulin in chili seedlings, bar equivalent to 10 cm; b: Root damage, bar equivalent to 5 cm; c: Presence of the oomycete in chili roots, bar equivalent to 20 µm, arrows indicate oomycete presence. Treatments as follows: C: Control seedlings, treated with only sterile distilled water; P: Seedlings inoculated with P. capsici; I1+P: Seedlings inoculated with P. capsici and treated with 20 μM of inulin; I2+P: Seedlings inoculated with P. capsici and treated with 100 μM of inulin; I3+P: Seedlings inoculated with P. capsici and treated with 200 μM of inulin; I4+P: Seedlings inoculated with P. capsici and treated with 300 μM of inulin.
Table 1 Growth parameters and protection induced by dahlia inulin in chili seedlings.
| Treatment | Fresh weight (g) | Height of the seedlings (cm) | Root viability (mg g-1 h WF) | Disease severity | Disease incidence (%) |
|---|---|---|---|---|---|
| C | 1.8 ± 0.27a | 27.7 ± 0.66a | 1.7 ± 0.11 c | 1 | 0 |
| P | 0.8 ± 0.18a | 22.5 ± 2.04a | 0.9 ± 0.20a | 4 | 80 |
| I1+P | 0.7 ± 0.18a | 22.1 ± 0.89a | 0.9 ± 0.09ab | 4 | 80 |
| I2+P | 1.3 ± 0.18a | 26.3 ± 1.06a | 1.0 ± 0.16ab | 3 | 50 |
| I3+P | 1.8 ± 0.37bc | 26.9 ± 1.54a | 0.9 ± 0.35ab | 2 | 40 |
| I4+P | 1.5 ± 0.23bc | 28 ± 1.2a | 0.6 ± 0.03a | 3 | 50 |
The treatments are described in figure 1. Means with different letters differ by LSD test for p<0.05.
leaves and roots, the statistical analysis displayed significant differences between treatments and samplings (p < 0.05). Regarding the local response (Figure 2a), the activity of β-1,3 glucanases in seedlings infected with P. capsici (P) increased significantly in regard to the control until day 5 after the inoculation of the pathogen. This activity remained high on day 7 and dropped to the level of the control on day 9. In turn, seedlings treated with inulin and infected with P. capsici (IP) displayed an increase in activity starting on day 2 after the inoculation of the pathogen and remained high until day 7, followed by a significant reduction of activity on day 9. The dahlia inulin was able to locally and prematurely increase the activity of β-1,3 glucanases in the plants inoculated with P. capsici.
Regarding the systemic response, changes were observed in the activity of β-1,3 glucanases on the leaves of treated seedlings (Figure 2b). The activity of β-1,3 glucanases in infected plants (P) increased significantly in regard to the control starting on day 2 after the inoculation of the pathogen. This activity dropped to the level of the control on day 3, remaining on that level on day 5, dropping significantly on day 7 and finally increasing on day 9. On the other hand, in seedlings infected and treated with inulin (IP), the increase in activity was observed starting on the day of inoculation with the pathogen, which remained on days 1, 2, 3 and 5. A reduction was observed that reached the level of the control on day 7, and another significant increase took place on day 9. The dahlia inulin was able to cause a systemic and early increase in the activity of β-1,3 glucanases, in the plants inoculated with P. capsici.
Activity of peroxidases. This study evaluated the activity of enzymes with activity of peroxidases in leaves and roots in chili plants inoculated with P. capsici and treated with dahlia inulin. In regard to the local response, a statistical difference was observed between samplings and treatments (p < 0.05) (Figure 3a). The activity of peroxidases in the seedlings infected with P. capsici (P) fell significantly
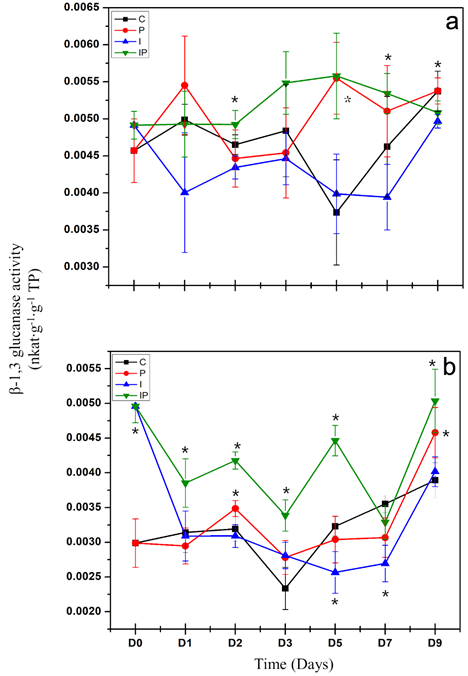
Figure 2 Evaluation of β-1,3 glucanase activity in roots (a) and leaves (b) of chili seedlings treated with dahlia inulin and inoculated with P. capsici. C: Control seedlings, treated with sterile distilled water; P: Seedlings inoculated with P. capsici; I: Seedlings treated with 200 μM of inulin; IP: Seedlings inoculated with P. capsici and treated with 200 μM of inulin. The vertical lines represent the standard deviation. Asterisks represent significant differences with respect to the control.
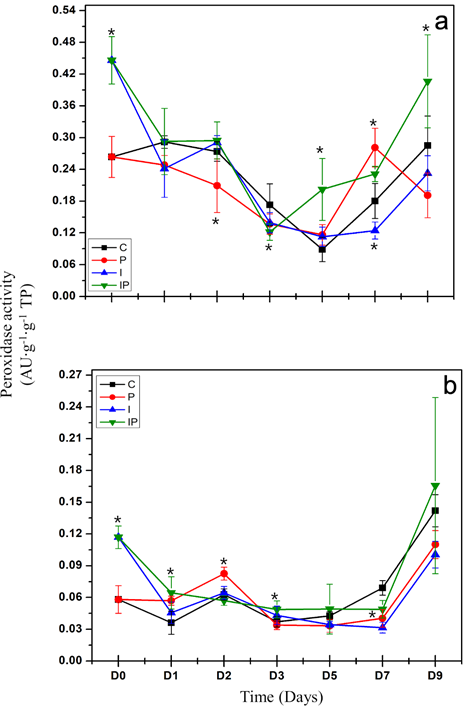
Figure 3 Evaluation of peroxidase activity in roots (a) and leaves (b) of chili seedlings. The treatments are described in figure 2. The vertical lines represent the standard deviation. Asterisks represent significant differences with respect to the control.
on day 2 after the inoculation of the pathogen. This activity remained low, although with levels near to those for the control on days 3 and 5. A significant increase was observed on day 7, along with a new reduction of the control on day 9. In turn, in seedlings treated with inulin and infected with P. capsici (IP), an increase in activity was observed starting on the day of inoculation of the pathogen. This activity decreased to the same level as the control starting on day 1 and remained at this level until day 2. A significant reduction in activity was observed until day 3, as well as a significant increase starting on day 5, which remained on days 7 and 9. The inulin was able to cause a local and early increase in the activity of peroxidases in plants infected with P. capsici.
A systemic response was observed when changes took place in the activity of peroxidases in the leaves (Figure 3b). The activity of peroxidases in seedlings infected with P. capsici (P) increased significantly on day 1 after the inoculation of the pathogen and remained elevated on day 2. A reduction in activity was observed to the level of the control starting on day 3, and remained unchanged on day 5. A significant reduction was observed on day 7, followed by an increase to the same level as the control on day 9. In turn, an increase was observed in the activity of the seedlings treated with inulin and infected with P. capsici (IP), starting on the day of inoculation of the pathogen, which remained stable on day 1. This activity dropped to the same level as the control on day 2, and increased significantly on day 3. A reduction to the level of the control was observed on day 5, followed by a significant reduction on day 7, to then rise to the level of the control on day 9. The inulin was able to increase the peroxidase activity systemically in seedlings infected with P. capsici, and in a differentiated manner compared to untreated plants. Seedlings treated with dahlia inulin displayed an increase in peroxidase activity at both local and systemic levels, which was related to the induction of an effective defense response against infection by P. capsici.
Induction of the production of phenolic compounds. This study related the content of the total phenolic compounds with the production of phytoalexins. Regarding the local response, the seedlings infected with P. capsici (P) displayed no significant differences with the control (C) in the days evaluated (Figure 4a). On the other hand, the seedlings treated with inulin and infected with P. capsici (IP) only displayed a significant increase in the content of phenolic compounds 5 days after the inoculation of the pathogen, and an important reduction on day 7.
On the other hand, a systemic response was observed in the leaves of the chili seedlings (Figure 4b). The content of phenolic compounds in the seedlings infected with P. capsici (P) fell significantly on day 2 after the inoculation of the pathogen, followed by an increase was observed to reach the level of the control on day 3, where it remained for the rest of the days evaluated. On the other hand, the seedlings
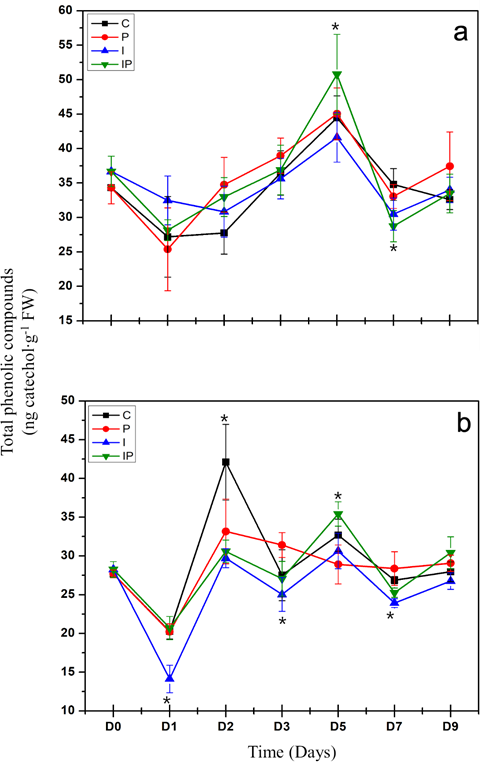
Figure 4 Quantification of total phenolic compounds in roots (a) and leaves (b) of chili seedlings. The treatments are described in Figure 2. The vertical lines represent the standard deviation. Asterisks represent significant differences with respect to the control.
treated with inulin and infected with P. capsici (IP) displayed the same behavior as the plants infected in regard to the control. However, significant differences were observed between the infected seedlings (P) and those treated with inulin (IP). A significant increase was found in the content of phenolic compounds on day 5 and a reduction on day 7. Inulin induced a systemic response, differentiated from the content of total phenolic compounds in the seedlings infected with P. capsici.
According to the results of this study, the increase in the levels of enzymatic activity and the significant differences in the content of phenolic compounds observed locally and systemically was related as a type of effective defense response induced by inulin against infection by P. capsici in serrano chili.
Discussion
The production of green chili in Mexico is an activity of great economic importance. However, this crop is susceptible to fungi and oomycetes that can cause considerable economic losses (Pérez-Acevedo et al., 2017). In order to control them, costly chemical products are used that also have high probabilities of creating long- term resistance, therefore the search for alternatives for the management of these phytopathogens is of great importance. The potential of inulin as an inducer of plant defense is an alternative to be included in the comprehensive management of such diseases, considering its availability, low cost and null toxicity, as well as having an indirect effect that reduces the probability of creating resistance. The effect of protection against infection by P. capsici in chili with the use of inulin, reducing the incidence and symptoms of the disease with one single concentration of 200 μM (López-Velázquez et al. 2019a, 2019b) has been described above. The protection mechanism has been associated with the induction of the effective defense in the plant. However, this plant defense induction effect had not yet been proven in chili plants.
This study observed that a concentration between 100 and 300 μM of inulin had a protective effect against the infection by P. capsici, displaying a reduction in the severity and incidence, as well as a fluctuation on the fresh weight and height of the plant. Additionally, the viability of the root of the plants treated with inulin and the plants infected with the pathogen were reduced. This reduction in viability did not affect plant growth and it was observed that the volume of the root may be larger than in the control. Meanwhile, in the infected plant the root volume is significantly lower and may be related to the significant damage to the root growth.
Different types of responses are triggered, which are related to the protection of the plant, including the activation of the PR proteins and the production of phytoalexins. Its fluctuations and changes in the levels of activity and the accumulation of metabolites in comparison with diseased plants are considered an effective defense response against the pathogen. According to the results of this study, the use of inulin induced an increase in the activity of enzymes β-1,3 glucanases at a local and systemic level. This coincides with the results described for other oligosaccharides with elicitor activity. Sajeesh (2015) reported the induction of defensive enzymes, including β-1,3 glucanases, with the application of chitosan, an oligosaccharide derived from chitin and widely reported in the defense of plants. This response was observed in the control of Phytophthora infestans in potato (Solanum tuberosum).
On the other hand, Burdock fructooligosaccharides (BFO) have been reported as inducers of an effective defense response. Zhang et al. (2009) applied BFO on the leaves of cucumber plants (Cucumis sativus) and later inoculated the pathogen Colletrotrichum orbiculare under greenhouse conditions. Six days after the inoculation of the pathogen, they noticed a significant increase in the activity of β-1,3 glucanases, both in the leaves treated with BFO considered locals, and in those not treated with BFO considered systemic, as opposed to the untreated leaves and inoculated with C. orbiculare. This was equivalent to the observations made in this study, where the activity of β-1,3 glucanases increased in plants treated with inulin and inoculated with P. capsici. In this same study, an increase was observed in the local response, and in less intensity, in the systemic response. In this study, with inulin, the local and systemic responses of the activity of β-1,3-glucanases were found to be similar. In this sense, Sun et al. (2013) reported the increase in the activity of β-1,3 glucanases and protection against the infection of Botrytis cinerea with the postharves application of BFO in grapes in Kyoho (Vitis vinifera ‘Kyoho’). The activity increased in the first days after inoculating the pathogen (days 1, 2 and 3), to later decrease in days 4 and 5. In this study, the local activity of β-1,3 glucanases presented a similar behavior, although the increase began on day 2 and reduction, until day 9. An important difference with reports found in the literature is that polymers are applied on leaves, whereas in this study, they were applied on the base of the plant.
Another group of PR proteins evaluated in this study were peroxidases. Some carbohydrates with eliciting activity have been described to induce the activity of peroxidases (Chaliha et al., 2018), and their activation has also been associated to an effective defense response in chili plants resistant to P. capsici (Wang et al., 2013). The induction of the activity of peroxidases associated to an effective defense response by carbohydrates with elicitor activity has been described for different pathosystems (Chaliha et al., 2018). Zhang et al. (2009) reported the foliar application of the Burdock fructooligosaccharides (BFO) on cucumber, followed by the inoculation of the pathogen C. orbiculare under greenhouse conditions. They noticed that 6 days after the inoculation of the pathogen there was a significant increase in the activity of enzymes with activity of peroxidases, including superoxide dismutase (SOD) and polyphenol oxidase (PPO). Likewise, they observed a greater increase in the local response and a lower intensity in the systemic response. This study observed a similar behavior for the plants treated with inulin. Another carbohydrate that has been reported as a plant defense inducer is trehalose, which was able to induce the activation of peroxidases in wheat (Triticum aestivum) to counteract Blumeria graminis (Reignault et al., 2001). An increase in the activity of peroxidases on a local level was described starting on day 2 after the inoculation of the pathogen in plants treated with trehalose, a response that corresponds with observations made in this study.
Finally, the induction of phytoalexins in chili plants was evaluated from the treatments with inulin. This study associates the content of the total phenolic compounds with the production of phytoalexins (Yu et al., 2022). The production of phytoalexins induced by trehalose in wheat has been reported for the control of B. graminis, associated with the activation of phenylalanine ammonia-lyase (PAL) one day after the treatment (Reignault et al., 2001). By contrast, Sun et al. (2013) reported the reduction of the content of total phenols with the postharvest application of BFO in kyoho grape in the infection by B. cinerea. In this study, the increase in the content of phenolic compounds due to the treatment with inulin on a local level was minimal and it could not be associated with an effective defense response. On a systemic level, a differentiated response was observed between the infected plants and those treated with inulin, which helped monitor the changes in the contents of total phenolic compounds. However, these changes still need to be related to an effective response against the infection with de P. capsici.
In this study, protection could be related with the application of dahlia inulin as an effect of the induction of plant defense, also known as an elicitor (Bektas and Eulgem 2015). The plants treated with inulin displayed an increase in the levels of activity of β-1,3 glucanases and peroxidases at both local and systemic levels, contrasting with plants infected with P. capsici and the control. The use of fructooligosaccharides in agriculture as inducers of plant defense represents an area of opportunity and with great potential. Some studies have been described as related to plant protection of other fructooligosaccharides such as agave fructans in chili, which depend on the degree of polymerization to increase or reduce protection (Navarro-Lopez et al, 2019), although without determining whether the protection was due to the induction of plant defense. In the case of inulin, it will be important to evaluate if this protection can be modified positively depending on its physicochemical properties, origin or purity, which would facilitate its use and technological transfer by reducing production costs, taking advantage of its safety, solubility and stability in storage (Leyva-Porras et al., 2014).
Conclusions
The dahlia tuber inulin displayed a protective effect against infections by Phytophthora capsici, at concentrations ranging between 100 and 300 μM, reducing the incidence and the symptoms of the disease. This protection was related to the induction of an effective defense response with an increase in the activity of the enzymes β-1,3 glucanase and peroxidase at local and systemic levels. This response was differentiated between seedlings treated with inulin and seedlings infected with P. capsici. From these results, the use of dahlia inulin is suggested as a protection agent against the infection of P. capsici in serrano chili.
Acknowledgments
The corresponding author would like to thank the Consejo Nacional de Humanidades, Ciencias y Tecnologías for the scholarship granted, number 61044, to study a Master’s Degree in Biological Innovation in the Center for Research and Assistance in Technology and Design in the state of Jalisco.
REFERENCES
Ainsworth, E.A. , Gillespie, K.M. (2007). Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nature Protocols, 2, 875-877. https://doi.org/10.1038/nprot.2007.102 [ Links ]
Arévalo-Marín, D.F. , Briceño-Robles, D.M. , Mosquera, T. , Melgarejo, L.M. , Sarmiento, F. (2021). Jasmonic acid priming of potato uses hypersensitive response-dependent defense and delays necrotrophic phase change against Phytophthora infestans. Physiological and Molecular Plant Pathology, 115, 101680. https://doi.org/10.1016/j.pmpp.2021.101680 [ Links ]
Barchenger, D.W. , Lamour, K.H. , Bosland, P.W. (2018). Challenges and strategies for breeding resistance in Capsicum annuum to the multifarious pathogen, Phytophthora capsici. Frontiers in Plant Science, 9, 628. https://doi.org/10.3389/fpls.2018.00628 [ Links ]
Bektas, Y. , Eulgem, T. (2015). Synthetic plant defense elicitors. Frontiers in Plant Science, 5, 804. https://doi.org/10.3389/fpls.2014.00804 [ Links ]
Carrasco-Ortiz, M. , Munguía-Lino, G. , Castro-Castro, A. , Vargas-Amado, G. , Harker, M. , Rodríguez, A. (2019). Riqueza, distribución geográfica y estado de conservación del género Dahlia (Asteraceae) en México. Acta Botánica Mexicana, 126, e1354. https://doi.org/10.21829/abm126.2019.1354 [ Links ]
Castaño-Monsalve, J. , Ramírez-Gil, J.G. , Patiño-Hoyos, L.F. , Morales-Osorio, J.G. (2015). Alternativa para el manejo de Phytophthora infestans (Mont.) de Bary en Solanum betaceum Cav. mediante inductores de resistencia. Revista de Protección Vegetal, 30, 204-212. [ Links ]
Castro-Rocha, A. , Shrestha, S. , Lyon, B. , Grimaldo-Pantoja, G.L. , Flores-Marges, J.P. , Valero-Galván, J. , Aguirre-Ramírez, M. , Osuna-Ávila, P. , Gómez-Dorantes, N. , Ávila-Quezada, G. , Luna-Ruiz, J.J. , Rodríguez-Alvarado, G. , Fernández-Pavía, S.P. , Lamour, K. (2016). An initial assessment of genetic diversity for Phytophthora capsici in northern and central Mexico. Mycological Progress, 15, 15. https://doi.org/10.1007/s11557-016-1157-0 [ Links ]
Chaliha, C. , Rugen, M.D. , Field, R.A. , Kalita, E. (2018). Glycans as modulators of plant defense against filamentous pathogens. Frontiers in Plant Science, 9, 928. https://doi.org/10.3389/fpls.2018.00928 [ Links ]
Hernández-Epigmenio, F. , García-Mateos, M.R. , Sosa-Montes, E. , Mejía-Muñoz, J.M. , Fernández-Pavia, Y.L. , Cruz-Álvarez, O. , Martínez-Damián, M.T. (2022). Phenolic profile and nutritional value of Dahlia x hortorum flowers. Revista Chapingo Serie Horticultura, 28, 161-174. https://doi.org/10.5154/r.rchsh.2022.03.004 [ Links ]
Hönig, M. , Roeber, V.M. , Schmülling, T. , Cortleven, A. (2023). Chemical priming of plant defense responses to pathogen attacks. Frontiers in Plant Science, 14, 1146577. https://doi.org/10.3389/fpls.2023.1146577 [ Links ]
Leyva-Porras, C. , López-Pablos, A.L. , Alvarez-Salas, C. , Pérez-Urizar, J. , Saavedra-Leos, Z. (2014). Physical Properties of Inulin and Technological Applications. Polysaccharides. En: Ramawat, K. , Mérillon, J.M. (eds.), Springer, Cham, pp.1-22. https://doi.org/10.1007/978-3-319-03751-6_80-1 [ Links ]
López-Velázquez, J.C. , Navarro-López, D.E. , Qui-Zapata, J.A. , León-Morales, J.M. , Saavedra-Loera, D.I. , García-Morales, S. (2019). Efecto del selenito e inulina en la interacción Capsicum annuum L.-Phytophthora capsici en invernadero. Biotecnología Vegetal, 19, 25-34. [ Links ]
López-Velázquez, J.C. , Rodríguez-Rodríguez, R. , Espinosa-Andrews, H. , Qui-Zapata, J.A. , García-Morales, S. , Navarro-López, D.E. , Luna-Bárcenas, G. , Vassallo-Brigneti, E.C. , García-Carvajal, Z.Y. (2019). Gelatin-chitosan-PVA hydrogels and their application in agriculture. Journal of Chemical Technology & Biotechnology, 94, 3495-3504. https://doi.org/10.1002/jctb.5961 [ Links ]
Majid, M.U. , Awan, M.F. , Fatima, K. , Tahir, M.S. , Ali, Q. , Rashid, B. , Rao, A.Q. , Nasir, I.A. , Husnain, T. (2017). Genetic resources of chili pepper (Capsicum annuum L.) against Phytophthora capsici and their induction through various biotic and abiotic factors. Cytology and Genetics, 5, 296-304. https://doi.org/10.3103/S009545271704003X [ Links ]
Moreira-Morrillo, A.A. , Monteros-Altamirano, Á. , Reis, A. , Garcés-Fiallos, F.R. (2022). Phytophthora capsici on Capsicum Plants: A Destructive Pathogen in Chili and Pepper Crops. En: Baylen, Y.O. (ed.), IntechOpen, pp.1-16. https://www.intechopen.com/chapters/81665. [ Links ]
Navarro-López, D. , López-Velázquez, J.C. , Saavedra-Loera, D. , García-Gamboa, R. , González-Ávila, M. , Ortiz-Basurto, R. , Qui-Zapata, J.A. , García-Morales, S. (2019). Effect of the polymerization degree of agave fructans for the control of Phytophthora capsici. En: Gutiérrez, M.A. (ed.), CIATEJ, pp.107-112. [ Links ]
Oliveira, J.T.A. , Barreto, A.L.H. , Vasconcelos, I.M. , Eloy, Y.R.G. , Gondim, D.M.F. , Fernades, C.D.F. , Freire-Filho, F.R. (2014). Role of antioxidant enzymes, hydrogen peroxide and PR proteins in the compatible and incompatible interactions of cowpea (Vigna unguiculata) genotypes with the fungus Colletotrichum gloeosporioides. Journal of Physiology & Pathology, 2, 1-8. https://doi.org/10.4172/2329-955X.1000131 [ Links ]
Palma-Martínez, E. , Aguilar-Rincón, V.H. , Corona-Torres, T. , Gómez-Rodríguez, O. (2017). Resistencia a Phytophthora capsici Leo en líneas de chile huacle (Capsicum annuum L.). Revista Fitotecnia Mexicana, 40, 359-363. [ Links ]
Pérez-Acevedo, C.E. , Carrillo-Rodríguez, J.C. , Chávez-Servia, J.L. , Perales-Segovia, C. , Enríquez del Valle, R. , Villegas-Aparicio, Y. (2019). Diagnóstico de síntomas y patógenos asociados con marchitez del chile en Valles Centrales de Oaxaca. Revista Mexicana de Ciencias Agrícolas, 8(2), 281-293. [ Links ]
Pons-Hernández, J.L. , Guerrero-Aguilar, B.Z. , González-Chavira, M.M. , González-Pérez, E. , Villalobos-Reyes, S. , Muñoz-Sánchez, C.I. (2020). Variabilidad fenotípica de aislados de Phytophthora capsici en Guanajuato. Revista Mexicana de Ciencias Agrícolas, 11, 1891-1901. https://doi.org/10.29312/remexca.v11i8.2618 [ Links ]
Ramos-Sandoval, R.U. , Gutiérrez-Soto, J.G. , Rodríguez-Guerra, R. , Salcedo-Martínez, S.M. , Hernández-Luna, C.E. , Luna-Olvera, H.A. , Almeyda-León, I.H. (2010). Antagonismo de dos ascomicetos contra Phytophthora capsici Leonian, causante de la marchitez del chile (Capsicum annuum L.). Revista Mexicana de Fitopatología, 28, 75-86. [ Links ]
Reignault, P.H. , Cogan, A. , Muchembled, J. , Lounes‐Hadj, S.A. , Durand, R. , Sancholle, M. (2001). Trehalose induces resistance to powdery mildew in wheat. New Phytologist, 149, 519-529. https://doi.org/10.1046/j.1469-8137.2001.00035.x [ Links ]
Reyes-Tena, A. , Castro-Rocha, A. , Rodríguez-Alvarado, G. , Vázquez-Marrufo, G. , Pedraza-Santos, M.E. , Lamour, K. , Larsen, J. , Fernández-Pavía, S.P. (2019). Virulence phenotypes on chili pepper for Phytophthora capsici isolates from Michoacán, Mexico. HortScience, 54, 1526-1531. https://doi.org/10.21273/HORTSCI13964-19 [ Links ]
Reyes-Tena, A. , Rodríguez-Alvarado, G. , Fernández-Pavía, S.P. , Pedraza-Santos, M.E. , Larsen, J. , Vázquez-Marrufo, G. (2021). Caracterización morfológica de aislados de Phytophthora capsici provenientes de Jalisco y Michoacán, México. Revista Mexicana de Fitopatología, 39, 75-93. https://doi.org/10.18781/r.mex.fit.2007-5 [ Links ]
Sajeesh, P. (2015). Cu-Chi-Tri: A triple combination for the management of late blight disease of potato (Solanum tuberosum L.). Doctoral dissertation, GB Pant University of Agriculture and Technology. Pantnagar, India. [ Links ]
Sanogo, S. , Lamour, K. , Kousik, C.S. , Lozada, D.N. , Parada-Rojas, C.H. , Quesada-Ocampo, L.M. , Wyenandt, C.A. , Babadoost, M. , Hausbeck, M.K. , Hansen, Z. , Ali, E. , McGrath, M.T. , Hu, J. , Crosby, K. , Miller, S.A. (2023). Phytophthora capsici, 100 Years Later: Research Mile Markers from 1922 to 2022. Phytopathology, 113, 921-930. https://doi.org/10.1094/phyto-08-22-0297-rvw [ Links ]
Santana-Legorreta, S. , Villanueva-Carvajal, A. , Morales-Rosales, E.J. , Laguna-Cerda, A. , Dominguez-Lopez, A. (2016). Extracción y evaluación de inulina a partir de dalias silvestres mexicanas (Dahlia coccinea Cav.). ɸYTON, 85, 63-70. [ Links ]
SADER, Secretaría de Agricultura y Desarrollo Rural (2023). Servicio de Información Agroalimentaria y Pesquera. Avance de siembras y cosechas, resumen por estado. http://infosiap.siap.gob.mx:8080/agricola_siap_gobmx/ResumenProducto.do (consulta, Diciembre, 2023). [ Links ]
Sun, F. , Zhang, P. , Guo, M. , Yu, W. , Chen, K. (2013). Burdock fructooligosaccharide induces fungal resistance in postharvest Kyoho grapes by activating the salicylic acid-dependent pathway and inhibiting browning. Food Chemistry, 138, 539-546. https://doi.org/10.1016/j.foodchem.2012.10.058 [ Links ]
Trinidad-Cruz, J.R. , Rincón-Enríquez, G. , Evangelista-Martínez, Z. , Quiñones-Aguilar, E.E. (2021). Biorational control of Phytophthora capsici in pepper plants using Streptomyces spp. Revista Chapingo Serie Horticultura, 27, 85-99. https://doi.org/10.5154/r.rchsh.2020.06.014 [ Links ]
Wang, J.E. , Li, D.W. , Zhang, Y.L. , Zhao, Q. , He, Y.M. , Gong, Z.H. (2013). Defence responses of pepper (Capsicum annuum L.) infected with incompatible and compatible strains of Phytophthora capsici. European Journal of Plant Pathology, 136, 625-638. https://doi.org/10.1007/s10658-013-0193-8 [ Links ]
Yu, H.L. , Kim, J.Y. , Lim, S.H. , Kang, H.W. (2022). The defense response of pepper (Capsicum annuum L.) induced by exopolysaccharide from Schizophyllum commune. Physiological and Molecular Plant Pathology, 118, 101810. https://doi.org/10.1016/j.pmpp.2022.101810 [ Links ]
Zhang, H. , Liu, Z. , Xu, B. , Chen, K. , Yang, Q. , Zhang, Q. (2013). Burdock fructooligosaccharide enhances biocontrol of Rhodotorula mucilaginosa to postharvest decay of peaches. Carbohydrate Polymers, 98, 366-371. https://doi.org/10.1016/j.carbpol.2013.06.008 [ Links ]
Received: May 10, 2023; Accepted: December 11, 2023











 texto en
texto en 


