Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.42 no.1 Texcoco ene. 2024 Epub 27-Ene-2025
https://doi.org/10.18781/r.mex.fit.2302-2
Review articles
Aspergillus oryzae: An opportunity for agriculture
1 Universidad Autónoma Agraria Antonio Narro. Calzada Antonio Narro Núm. 1923, Col. Buenavista, Saltillo, Coahuila, México. CP. 25315;
2 Departamento de I+D, CULTA SA de CV. Boulevard Luis Echeverría Álvarez 1700, Col. Altavista, El Mante, Tamaulipas, México..
Aspergillus oryzae is a filamentous fungus capable of degrading various substances employing enzymes, which is why it is widely used in the biotechnological industry, pharmaceutical products, enzymes for industrial use, bleaching agents, anti- pollution textile treatments. However, few works focus on these microorganism’s field applications. This manuscript reviews the potentially beneficial applications of A. oryzae and some by-products in agriculture as biological control, growth inducer, and bioremediation for soils contaminated with heavy metals.
Keywords: Bioremediation; nematicide; insecticide; microorganisms; metabolites; non-toxigenic
Aspergillus oryzae es un hongo filamentoso, capaz de degradar diversas sustancias empleando enzimas, por lo que es ampliamente utilizado en la industria biotecnológica, productos farmacéuticos, enzimas para uso industrial, agentes blanqueadores y tratamientos textiles anticontaminación. Sin embargo, son pocos los trabajos centrados en las aplicaciones de campo de este microorganismo. El presente manuscrito revisa las posibles aplicaciones con potencial benéfico de A. oryzae y algunos subproductos en la agricultura como control biológico, inductor de crecimiento y biorremediador de suelos contaminados con metales pesados.
Palabras clave: Biorremediación; nematicida; insecticida; microorganismos; metabolitos; no toxígeno
Introduction
The fungi of the Aspergillus spp. genus are considered a complex group of ascomycetes that compose 350 accepted species (Kocsubé et al., 2016). They are described as filamentous fungi, able to secrete a wide range of secondary metabolites and enzymes, whose function is to degrade and recycle biopolymers from plant tissues (El-Enshasy, 2007). The Aspergillus spp. genus is generally found in stored seeds, plants in decomposition and soil, where they develop as saprophytes (Mousavi et al., 2016).
Although fungi from the Aspergillus spp. genus are not considered important sources of phytosanitary diseases, they are responsible for alterations in plants and stored products; since they are opportunist molds, they prosper under storage conditions (Awuchi et al., 2021). This genus is also recognized for its production of mycotoxins, with around 300 and 400 identified, such as aflatoxins, secalonic acids, cyclopiazonic acid, aflatrem, citrinin, stregmatocystin, glycytoxin, ochratoxin A (OTA) and terrein (Navale et al., 2021), with a potential health risk for humans and animals, as well as affecting the environment and having a negative effect on the world’s economy (Bueno et al., 2015). An important example of this type of toxic secondary metabolites are aflatoxins, which represent a hazard for farmers in postharvest and are considered indicators of biological soil degradation (Marshall et al., 2020). They bring about qualitative nutritional and sensory changes in plant- based products, since the infection can produce unpleasant flavors or odors, rotting and discoloration (Kozakiewic, 1989).
Some fungi of the Aspergillus spp. genus, such as A. flavus, A. nidulans, A. nomius and A. parasiticus, are agronomically important, since they produce aflatoxins (AF) (Hesseltine et al., 1970; Gomi, 2014). Mainly B1, B2, G1 and G2 have proven to be strong carcinogenic, cytotoxic and potentially mortal biotoxins for humans and cattle (Ráduly et al., 2019). A. flavus has been reported as the cause of contamination with aflatoxins AFB1 in any stage of the peanut supply chain (imports, manufacturing and retail) in countries such as Malaysia, where the tropical climate conditions are favorable for the growth of this fungus (Norlia et al., 2018, 2019). In Mexico, maize has been affected by contamination with aflatoxins, in the same way as grains such as rice, barley, bean, sorghum, wheat, some oleaginous plants and dried fruits are susceptible to these biotoxins, produced by A. flavus (AFB1 and AFB2), A. parasiticus and A. nomius (AFG1 and AFG2) (Anguiano-Ruvalcaba et al., 2005; Escobar et al., 2023).
It is worth highlighting the existence of non-toxicogenic strains within Aspergillus spp., which do not produce aflatoxins and which can be applied in the planting area, to then be installed, compete and displace the toxigenic strains, resulting in the reduction of aflatoxins (Marshall et al., 2020; Senghor et al., 2020). Non-toxicogenic A. niger, A. sojae and A. oryzae strains do not produce compounds that contain, essentially, a furan ring attached to the coumarin nucleus, important in the biosynthesis path of aflatoxins, and do not produce cyclopiazonic acid jointly with aflatoxins such as A. flavus (Dorner et al., 2000; Padrón et al., 2013).
Aspergillus spp. strains that do not produce aflatoxins can be used as fungal biocontrol agents in the prevention of contamination with biotoxins (Barberis et al., 2019). Strains A. westerdijkiae 107, A. fumigatos C143, A. tamarii C122 and A. niger C187 have proven, in terms of inhibition and production of OTA, to have favorable results, with the strain A. niger C187 displaying an inhibition of 100% in the production of OTA and in the growth of A. ochraceus, A. westerdijkiae, A. carbonarius and A. niger in coffee grains (de Almeida et al., 2019). Likewise, they are used in the pharmaceutical industry and in industrial processes such as the fermentation of foods, since they are abundant sources of enzymes such as proteases, amylases and amylglucosidases, and others (Schuster et al., 2002; Olempska- Beer et al., 2006; Samson et al., 2014; Gómez et al., 2016). The production of polygalacturonase (Exo-PGs), a consortium of enzymes required for the hydrolysis of pectin, is one of the applications of the strain A. sojae ATCC 20235, useful in the depectinization and clarification of fruit juices, the extraction of oils from the skins of vegetables and citrus fruits, and treating wastewater (Tari et al., 2008).
Therefore, because A. oryzae has non-toxigenic strains, it figures as one of the most important species, due to its potential use as a biotechnological tool in degrading metabolic processes of diverse starches and proteins; in the metabolism of amino acids and amino acid and sugar absorption transporters (Machida et al., 2005, 2008; Watarai et al., 2019; Daba et al., 2021). A. oryzae is considered by the FDA as “generally recognized as safe” (GRAS), which refers to any substance intentionally added to foods, which must be subjected to revision and approval before its commercialization, unless the substance is generally recognized among qualified experts (Gad, 2005; FDA, 2019). Therefore, the WHO endorses the security in the use of A. oryzae (He et al., 2019), considering this microorganism adequate for its application in the food industry, such as the fermentation of foods, the production of alcohol and vinegar, in the pharmaceutical and cosmetics industries via the formulation of drugs and depigmenting agents. These applications are due to the production of enzymes and secondary metabolites such as lipases, cellulases, pectinases, β-galactosidase, amylases, kojic acid, malic acid, fumaric acid, pheluric acid and others (Daba et al., 2021).
Therefore, this study focuses on reviewing investigation and literature studies on the diverse products derived from A. oryzae, their contributions and applications in the agricultural areas, such as bioremediators, growth enhancers and biological control agents. It is worth highlighting that, although the study of A. oryzae has focused mostly in the industrial area, this study only considered those studies and investigations in which their application is directed to the agronomic part. It focuses primarily on kojic acid and A. oryzae strains involved in its production, since the fermentation process presents sustainable characteristics, and its applications are novel for the agricultural area.
The main objective of this revision is to publish the potential of A. oryzae in scarcely studied areas of agricultural importance. Although A. oryzae has been studied on a large scale in industrial, food and medical areas, studies on its agronomic potential are few, and in Mexico its study is practically inexistent, hence part of this revision seeks the development in the future of scientific studies on A. oryzae aimed at the agricultural sector, contributing to the development and care of the Mexican countryside.
Morphology and description of Aspergillus oryzae; origin, isolation and development
Aspergillus spp. presents hyaline septate hyphae, with a 45° dichotomic ramification (Cuervo-Maldonado et al., 2010). Growth forms extended mycelia that cover the entire surface of the culture media (Gomi, 2014) (Figure 1). The balloon-shaped vesicle has a diameter between 100 and 200 µm with a structure formed by oval-shaped conidia, 5 to 8 µm in length that contains four soft and slightly coarse nuclei. The phialides are found in the vesicle and may be uniseriate or biseriate sterigmata. The shoots are colorless and 1 to 5 mm in length, with a rugged texture (Moubasher, 1993; Powell et al., 1994).
Ahlburg (1876) first isolated A. oryzae from köji, the material fermented by the mold of A. oryzae planted in a steamed rice solid medium (Machida et al.,
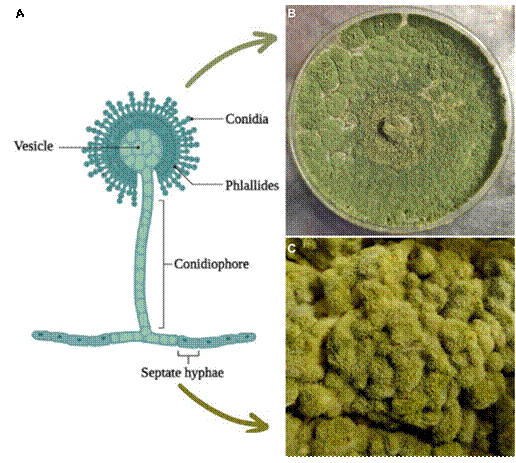
Figure 1 Morphology of A. oryzae; A) Parts and structures of the fungus (Adapted from “Structure of Aspergillus spp.”, 2023), B) A. oryzae planted in a PDA medium and C) Growth of A. oryzae in steamed rice (köji).
2008). This fungus belongs to the Erotyomicetes class; Order: Eurotiales; Family: Trichocomaceae (Daba et al., 2021). The use of A. oryzae in the production of sake (fermented rice alcoholic beverage), vinegar, miso (soybean paste) and soy sauce, has been reported for at least two millennia (Furukawa, 2012; Chang et al., 2014). In general terms, it is considered safe and no strains that produce aflatoxins are known (Machida et al., 2005).
The genes that codify the enzymatic pathway for the biosynthesis of aflatoxins are grouped in a 74 Kb region of the DNA in A. flavus. This group is found in A. oryzae, but it does not seem to be functional (Yu et al., 2004). A. oryzae and A. flavus are morphologically similar. Several studies suggest they are ecotypes, which refers to a same species which have a different expression in different environments, due to the interaction of their genes with the environment in which they are found (Kurtzman et al., 2018). This indicates that A. oryzae was the result of the domestication of A. flavus after centuries of planting it (Payne et al., 2006).
Because Aspergillus oryzae was reported as a domesticated microorganism, it cannot be found in nature. However, there are some reports that mention the isolation of A. oryzae from foods, plants and soils, appearing less frequently (Klich, 2002). A historical file described that A. oryzae should be isolated from a spike of rice, indicating that it could have existed in nature before its domestication (Murakami, 1980).
This fungus grows in several media, including potato dextrose agar, where it grows particularly fast in 7 days at 25 °C (Moubasher, 1993). Its stage of sporulation begins on day 7; when growth reaches 7 to 8 cm, yellow ring begins to form, and which will gradually turn green (Daba et al., 2021). The ideal conditions for the development of A. oryzae include a slightly acidic pH between 5 and 6, its temperature must range between 32 and 36 °C (±1 °C), and variations in temperature above 44 °C inhibit its growth. These fungi show an efficient development in media with water activity above 0.8 and they rarely grow below this range (Gomi, 2014).
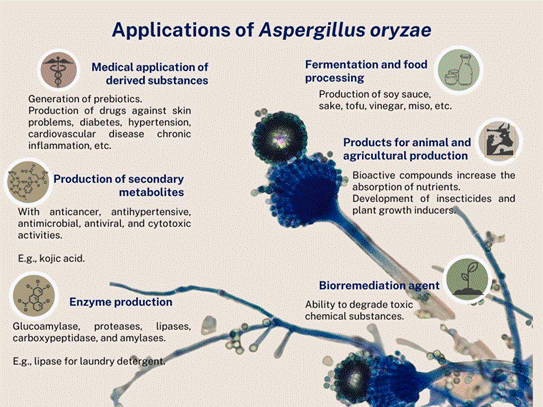
Applications of Aspergillus oryzae and its possible implementation in agriculture
The versatility of A. oryzae is reflected in the wide variety of areas in which it can be applied (Figure 2), since it is highly effective in the manufacturing of biotechnological products, due mainly to its metabolic and enzymatic diversity (El- Enshasy, 2007).
Lee and collaborators (2016) provided a metabolic profile obtained during the fermentation of köji with A. oryzae, which comprises the secondary metabolites secreted in the fermentation process, classifying them into; a) sugars (xylose, fructose and glucose); b) polyols (glycerol, erythritol, xylitol, sorbitol y myo- inositol); c) organic acids (succinic acid, glyceric acid, fumaric acid, malic acid,
kojic acid, citric acid and gluconic acid); d) phenolic acids (4-hydroxybenzoic acid and ferulic acid); e) amino acids (alanine, proline, glycine, serine, threonine, aspartate and GABA); f) fatty acids (palmitic acid, linoleic acid, oleic acid and pinellic acid); and g) vitamins (vitamin B3). Each one of these compounds has different antimicrobial, antioxidant, anticarcinogenic and antiviral properties, as well as hormonal compounds and metal chelators (Frisvad et al., 2018; Daba et al., 2021).
The application of A. oryzae in the production of malic acid and fumaric acid (Xu et al., 2012; Brown et al., 2013) proposes the possibility of the creation of a biorefining process for the production of organic acids and enzymes, replacing the currently used polymers derived from crude oil (Brink et al., 2023). The biorefining process can be enhanced by incorporating agricultural by-products, inexpensive non-food substrates, reducing production costs and providing an option free of any chemical products (Jiménez-Quero et al., 2020).
The microbial enzymes used in the industry have proven to be better in their application, as well as inexpensive and respectful to the environment in comparison with chemical products (Whiteley and Lee, 2006). They have technical-economic advantages, meaning shorter production times, better space per enzyme unit produced and unlimited potential in terms of availability of new enzymes (Scriban, 1985).
There are reports on the application of A. oryzae in the process of fermentation of grape pomace with the production of enzymes (cellulase, pectinase and tannase), which facilitate the aqueous extraction of polyphenols (gallic acid, sinapic acid and ferulic acid) with antioxidant and prebiotic properties, such as food additives, where A. oryzae has a greater production and selectivity of tannase under humid conditions, having a positive effect on the antioxidant activity, which can be influenced by the production of galic acid (Meini et al., 2021). On the other hand, A. oryzae can stimulate ruminal fermentation by improving the consumption and digestion of the food and dry matter in cattle, by applying it as a microbial additive in the cattle feed (Sosa et al., 2022). At the same time, the efficiency in the increase of volatile fatty acids has been proven, making A. oryzae an element of improvement to potentialize the diets of ruminants in a different way. It also exerts an influence on the supply of enzymes in maize, oat hay and alfalfa hay silage (Kong et al., 2021).
Technological progress has taken advantage of the potential of A. oryzae (Matsunaga et al., 2002) for industrial use in the development of detergents, pigments and antioxidants, (Christensen et al., 1988; Machida et al., 2008; Panchanawaporn et al., 2022). Likewise, its application in the fermenting of foods (Machida et al., 2008; Yasui et al., 2020) and the implementation in the production of metabolites such as organic acids and plant growth regulators are important areas of study (El- Enshasy, 2007; Siddiqui, 2016). It can also be useful in biological activities such as in veterinary science as probiotics for poultry and livestock feed digestive (Lee et al., 2006; Murphy, 2021; Podversich et al., 2023).
A.oryzae as a soil bioremediator, growth enhancer and biological control
A. oryzae can be an alternative for the development of a sustainable and eco- friendly agriculture, specifically in Mexico, where their applications on the field are not a topic of study. The following information describes some areas of opportunity where this microorganism can be applied on the field, in order to pave the way for possible scientific studies aimed at the Mexican countryside.
Endophytic plant fungi are those which live on plant tissues and cause no visible harm. Due to this, a mutualistic relationship (endophyte-host) is occasionally identified, which unleashes the production of bioactive substances (secondary metabolites, enzymes etc.) which exert an influence on growth enhancement, the survival of the host under diverse environmental conditions, the reduction of susceptibility to diseases, and helping control pest insects and plant pathogen agents (El-hawary et al., 2020; Murali et al., 2012; Sharma and Singh, 2021).
Although A. oryzae is not commonly reported as a natural endophyte, information has been provided on its isolation in Ginkgo biloba roots in China (Machida et al., 2005).Sun and collaborators (2018), in the study of the inoculation of Raphanus sativus seeds with the strain A. oryzae BNCC341706, established it as a fungus with endophytic properties, since it did not affect the germination of the inoculated seed and instead promoted the growth of the R. sativus culture, which reached a height of 116 mm in comparison with the control, which had a height of 99.6 mm. Another effect of the use of A. oryzae was reflected on the health of its main pest insect Plutella xylostella, affecting its consumption parameters, weight of larvae and pupae, which opens the possibility of treating cruciferous seeds and the control of pest insects using A. oryzae.
Likewise, as bioremediating agents, endophytic fungi have proven to be efficient in the degradation of contaminants, leaving no traces of toxic by-products (Skinder et al., 2022), which is advantageous, due to its biomass, long life cycle and network of hyphae (Sun et al., 2012), along with its ability to degrade chemically toxic substances by modification or acting upon its chemical bioavailability (Bornyasz et al., 2005). In the case of A. oryzae as a bioremediating agent, the in vitro study of the A. oryzae strains AM1 and AM2 displayed the ability to degrade atrazine (90%), endosulfan (56 and 76%) and chlorpyrifos (50 and 73%), while also obtaining an adequate development under high concentration of pesticides, which generates the possibility of degrading this type of chemical products (Barberis et al., 2019).
The OTA is a microtoxin that affects human health and agricultural products, which has led to a search for control measures, therefore, biodegradation has been proposed as a promising method. The strain A. oryzae M30011 is able to degrade OTA by up to 94% in 72 h, at a pH of 8, a temperature of 30 °C and a concentration of the inoculant of 104 UFC mL-1. On the other hand, a reduction in the levels of aflatoxins is an important matter, since they are a threat to worldwide food security (Xiong et al., 2021). The strain A. oryzae M2040 has been proven capable of inhibiting the production of AFB1 by 87%, and the proliferation of A. flavus, under in vitro conditions, and in peanuts by successfully displacing the aflatoxin- producing fungus by secreting antimycotic compounds, which have not been reported (Alshannaq et al., 2018). These studies back the potential of A. oryzae in the agricultural and food industry.
The potential of the use of A. oryzae has been emphasized in the development of research work as a growth enhancer and a biological control agent, shown in Table 1.
The ability of A. oryzae to secrete enzymes is an alternative for the development of microbiological compounds, since biological activities are carried out which can be adapted in the area of agronomy. An example of this is the antifungal activity of xylanase produced by the strain A. oryzae MN894021, which displayed a reduction
Table 1 Use of A. oryzae as a growth enhancer, pest control agent and bioremediator od contaminated soils.
| Application | Strain | Crop / Pest | Results | Reference |
|---|---|---|---|---|
| Arsenic biorremediator and growth enhancer | FNBR_L35 | Oat (Avena sativa), Calendula (Calendula officinalis), Ashwagandha (Withania somifera) | Effects of bioaccumulation and biovolatilization of arsenic in concentrations of 100 to 10,000 ppm in a period of 21 days andenhancement of plant growth | Singh et al.,2015 |
| Entomopathogen | XJ-1 | Locusta migratoria | Mortality in third instar of the insect | Zhang et al.,2015 |
| Growth enhancer and control agent | BNCC341706 | Radish seeds (Raphanus stativus), Puntella xylostella | Greatest plant height. Inhibition of feeding and low weight of larvae and pupae | Sun et al., 2018 |
| Removal of glyphosate | AM1 and AM2 | In vitro | Degradation of 50% in glyphosate concentrations, long periods of incubation and permanence of the fungus | Carranza et al.,2019 |
| Entomopathogen | USMN05 USMM03 NRRL2097 | Spodoptera litura | Mortality of 20% and inability to produce aflatoxins | Fitriana et al.,2021 |
of 75, 90 and 100% in the incidence of Botrytis cinerea, Fusarium solani, F. chlamydosporum, F. incranatum, Macrophomina phaseolina, Rhizotocnia solani and Sclerotinia sclerotiorum in broad bean seeds covered with xylanase, providing protection against the invasion of these phytopathogenic fungi (Atalla et al., 2020). The results obtained from the xylanase produced by the strain A. oryzae MN894021 coincide with the activity of the xylanase from Trichoderma harzianum kj831197 against Corynespora cassiicola, Alternaria spp., F. oxysporum and Botrytis fabae (Ellatif et al., 2022).
The control of phytopathogens is an important challenge for agriculture. The development of sustainable, environmental, easy and eco-friendly control processes is constant in current research; an option is the biogenic synthesis of bioparticles (Zhang et al., 2020). The strain A. oryzae MTCC3107 has been implemented in the formulation of silver nanoparticles (AgNP), whose antimicrobial potential against Sclerotinia sclerotium reflected an inhibition of 100% at a concentration of 100 µL mL-1. The role of A. oryzae in the formulation is due to the secretion of amylase, which catalyzed the AgNP production process, making it a green synthesis process (Gupta and Saxena, 2023).
The study and publication of information related to the potential of A. oryzae serve as a support for future investigations in the area of phytopathology, since it has been relatively scarcely studied. Despite A. oryzae having a wide margin of produced bioactive substances, those applied in this area are few. Some activities and metabolites are presented in Table 2.
Table 2 Activity of bioactive substances produced by A. oryzae against plant pathogens.
| Bioactive substances | Strains | Application | Result | Reference |
|---|---|---|---|---|
| Kojic acid | NRRL 447, 552, 552, 1730 Y30038 (S-03) | Prevention of contamination by toxins in agricultural products | Reduction of aflatoxins in peanut | Dorner et al., 1998 |
| Kojic acid | * | Insecticide: Glyphodes pyloalis | Inhibition of phenyloxidase activity | Sharifi et al., 2013 |
| Oryzaeins A-D | KM999948 | Antiviral: TMV | Rates of inhibition of 22.4 - 30.6% | Zhou et al., 2016 |
| Xylanase | MN894021 | antifungal: Alternaria alternata, Fusarium oxysporum, Phoma destructor, Rhizotocnia solani and Sclerotium rolfsii | Reduction of the live growth Reduction in percentages of incidence of root rotting | Atalla et al., 2020 |
| Kojic acid | * | Antifungal activity: Sclerotinia slerotiorum | Inhibiyion of chitin and melanin synthesis Reduction of oxalic acid of the virulence factor | Zhu et al., 2022 |
*Strain not provided by the author.
Kojic acid, secondary A. oryzae metabolite; alternative for the control of plant pathogens
Within the main secondary metabolites produced by A. oryzae, kojic acid is one of the most relevant (Figure 3) (Yamada et al., 2014). Its application in the control of phytopathogenic agents and pest insects is a relatively new topic; however, the investigation reports show that this application may be a feasible alternative for the control of pests in crops.
A. oryzae has bactericidal, fungicidal and insecticidal effects (Mohamad et al., 2010). It acts in relation with the inhibition of oxidative enzymes in both plants and arthropods. Studies have shown that kojic acid efficiently inhibits the rate of formation of pigmented products and absorption of oxygen when compounds such as catecholamines (DL-DOPA, dopamine and norepinephrine), are oxidized by the enzyme tyrosinase (Kahn, 1995; Kahn and Ben-Shalom, 1997).
Mahmoud and collaborators (2023) analyzed the insecticidal activity of kojic acid produced by the strain A. oryzae ASU44 (OL314732), against Aphis
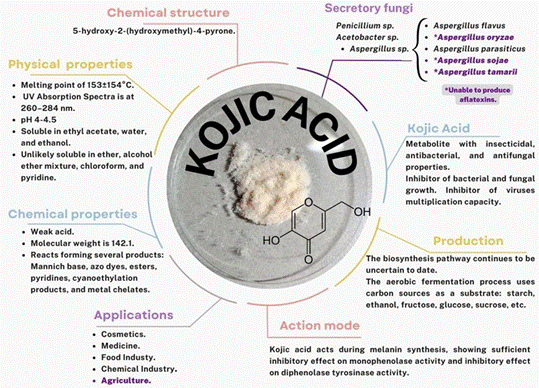
Figure 3 Characteristics and basics of kojic acid based on reports by Phasha et al. (2022) and Siddiquee (2018).
gossypii, vector of the Cotton leafroll dwarf virus (CLRDV) (Mahas et al., 2022).
They evaluated the difference between the kojic acid extracted from the strain A. oryzae ASU44 (OL314732) and synthetic kojic acid, and indicated that the kojic acid produced by A. oryzae ASU44 (OL314732) was more efficient against Aphis gossypii, with a medium lethal concentration (CL50) of 11.2 ppm, a lethal concentration (CL90) of 50.3 ppm, and a lethal time of (LT90) of 7 days, since the results are lower than those for synthetic kojic acid, highlighting their application as an efficient and inexpensive in vitro evaluation model (Mahmoud et al., 2023). Likewise, the antifungal activity of kojic acid has been evaluated with A. terrus, A. flavus, A. parasiticus, A. fumigatus, Penicilium and Sclerotinia sclerotiorum (Kim et al., 2012; Kim and Chan, 2014; Zhu et al., 2022). In the case of S. sclerotiorum, kojic acid inhibits the biosynthesis of melanin, which affects the development of sclerotia and the biosynthesis of chitin and β-1,3-glucanos, which alters the cell walls and the growth of the mycelium, reducing in its entirety the symptoms of S. sclerotiorum in soybean pods with 50 mM de ácido kójico. It has the ability to prevent and inhibit symptoms of S. sclerotiorum. In turn, it is more effective than commercial fungicides (carbendazim and prochloraz) (Zhu et al., 2022).
More frequently, phytoparasitic nematodes have been pointed out as the cause of important economic losses in several crops, fluctuating around $77 billion dollars worldwide, which raises concerns in agriculture, horticulture and forestry (Yadav, 2017; Seo et al., 2019). For example, Meloidogyne spp., the gall-forming nematodes, is responsible for annual losses of up to $100 billion dollars. Kim and collaborators (2016) established a method to control these nematodes using kojic acid as the active ingredient, which was produced by the strain A. oryzae EML- DML3PNa1 obtained from white dogwood (Cornus alba). During their experiments, an inhibiting effect was displayed on the hatching of eggs and the development of larvae, and the use of kojic acid was suggested along with a dispersing, penetrating or surfactant agent, in order to improve absorption and the effect of the product on the crop (Kim et al., 2016). The nematicidal action of the kojic acid reported a mortality of 87.6% in juvenile Meloidogyne incognita under conditions of 20% of a filtrate of a fermentation broth. It displayed inhibition in the incubation of the nematode and a mortality dependent on the dose, with mean effective concentration values (CE ) of 195.2 µg mL-1 and 238.3 µg mL-1, respectively, 72 h after exposure, which suggests that is has potential as a biological control agent (Kim et al., 2016).
Interest in safe agricultural products for human health and free of contaminants is on the rise, due to awareness on residual toxicity caused by the use of pesticides. The implementation of microorganisms with nematocidal activity is recommended, since they are respectful with the environment because they are obtained from natural products, as in the case of kojic acid, therefore its implementation in agronomy opens a door for the development of sustainably inexpensive, environmentally friendly products that, above all, do not harm the health of people who apply them.
Conclusions
This study highlights the potential of the A. oryzae strains and its derivatives (enzymes and secondary metabolites), considering the ability to compete with commercial chemical products as pesticides, since it presents insecticidal, fungicidal and nematocidal characteristics, which represent an inexpensive and sustainable alternative, since the way in which it is produced excludes the use of costly products. Developing new investigation work and opting for the application of products based on A. oryzae at a greenhouse level is required to confirm its adaptability in conditions outside the laboratory and verify if the benefits of A. oryzae are maintained or reduced in such a way that they can be used in the field. Finally, the study of A. oryzae and its by-products is scarcely studied in Mexico, making it a debatable topic to be exploited for the benefit of the Mexican countryside, since it opens a new aspect of study for the development of products that benefit crops and for the control of phytopathogenic agents.
Acknowledgement
Our thanks to the Antonio Narro Autonomous Agrarian University (Universidad Autónoma Agraria Antonio Narro - UAAAN), to the PhD Agricultural Parasitology Program, as well as to the National Council of Humanities, Sciences and Technolo- gies (Consejo Nacional de Humanidades, Ciencias y Tecnologías- CONAHCYT)
REFERENCES
AlshannaqAF, GibbonsJG, LeeMK, HanKH, HongSB y YuJH. 2018. Controlling aflatoxin contamination and propagation of Aspergillus flavus by a soy-fermenting Aspergillus oryzae strain. Scientific Reports 8(1): 16871. https://doi.org/10.1038/s41598-018-35246-1 [ Links ]
Anguiano-RuvalcabaGL, Vargas-CortinaAV y Guzmán-De PeñaD. 2005. Inactivación de aflatoxina B1 y aflatoxicol por nixtamalización tradicional del maíz y su regeneración por acidificación de la masa. Salud Pública de México 47(5): 369-375. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0036-36342005000500007&lng=es&nrm=iso&tlng=es [ Links ]
AtallaSMM, AhmedNE, AwadHM, El GamalNG y El-ShamyAR. 2020. Statistical optimization of xylanase production, using different agricultural wastes by Aspergillus oryzae MN894021, as a biological control of faba bean root diseases. Egyptian Journal of Biological Pest Control 30(1): 125. https://doi.org/10.1186/s41938-020-00323-z [ Links ]
AwuchiCG, OndariEN, OgbonnaCU, UpadhyayAK, BaranK, OkpalaCOR, KorzeniowskaM y GuinéRPF. 2021. Mycotoxins affecting animals, foods, humans, and plants: Types, occurrence, toxicities, action mechanisms, prevention, and detoxification strategies—a revisit. Foods 10(6). https://doi.org/10.3390/foods10061279 [ Links ]
BarberisCL, CarranzaCS, MagnoliK, BenitoN y MagnoliCE. 2019. Development and removal ability of non-toxnic Aspergillus section Flavi in presence of atrazine, chlorpyrifos and endosulfan. Revista Argentina de Microbiología 51(1): 3-11. https://doi.org/10.1016/J.RAM.2018.03.002 [ Links ]
BornyaszMA, GrahamRC y AllenMF. 2005. Ectomycorrhizae in a soil-weathered granitic bedrock regolith: Linking matrix resources to plants. Geoderma 126(1-2): 141-160. https://doi.org/10.1016/J.GEODERMA.2004.11.023 [ Links ]
BrinkHG, Geyer-JohnsonM, SwartRM y NicolW. 2023. Malic acid production by Aspergillus oryzae: the immobilized fungal fermentation route. Biofuels, Bioproducts and Biorefining 17(2): 363-379. https://doi.org/10.1002/BBB.2440 [ Links ]
BrownSH, BashkirovaL, BerkaR, ChandlerT, DotyT, McCallK, McCullochM, McFarlandS, ThompsonS, YaverD y BerryA. 2013. Metabolic engineering of Aspergillus oryzae NRRL 3488 for increased production of L-malic acid. Applied Microbiology and Biotechnology 97(20): 8903-8912. https://doi.org/10.1007/s00253-013-5132-2 [ Links ]
BuenoD, IstamboulieG, MuñozR y MartyJL. 2015. Determination of mycotoxins in food: A review of bioanalytical to analytical methods. Applied Spectroscopy Reviews 50(9): 728-774. https://doi.org/10.1080/05704928.2015.1072092 [ Links ]
CarranzaCS, RegñicoliJP, AluffiME, BenitoN, ChiacchieraSM, BarberisCL y MagnoliCE. 2019. Glyphosate in vitro removal and tolerance by Aspergillus oryzae in soil microcosms. International Journal of Environmental Science and Technology 16(12): 7673-7682. https://doi.org/10.1007/s13762-019-02347-x [ Links ]
ChangPK, BhatnagarD y ClevelandTE. 2014. Aspergillus Introduction. Encyclopedia of Food Microbiology 1: 77-82. https://doi.org/10.1016/B978-0-12-384730-0.00010-0 [ Links ]
ChristensenT, WoeldikeH, BoelE, MortensenSB, HjortshoejK, ThimL y HansenMT. 1988. High level expression of recombinant genes in Aspergillus oryzae. Applied Microbiology and Biotechnology 6(12): 1419-1422. https://doi.org/10.1038/NBT1288-1419 [ Links ]
Cuervo-MaldonadoSI, Gómez-RincónJC, RivasP y Orlando GuevaraF. 2010. Actualización en Aspergilosis con énfasis en Aspergilosis invasora. Infectio 14: 131-144. https://doi.org/10.1016/S0123-9392(10)70131-4 [ Links ]
DabaGM, MostafaFA y ElkhateebWA. 2021. The ancient koji mold (Aspergillus oryzae) as a modern biotechnological tool. Bioresources and Bioprocessing 8(1): 1-17. https://doi.org/10.1186/S40643-021-00408-Z [ Links ]
de AlmeidaÁB, CorrêaIP, FuruieJL, de Farias PiresT, do Rocio DalzotoP y PimentelIC. 2019. Inhibition of growth and Ochratoxin A production in Aspergillus species by fungi isolated from coffee beans. Brazilian Journal of Microbiology 50(4): 1091-1098. https://doi.org/10.1007/s42770-019-00152-9 [ Links ]
DornerJW, HornBW y ColeRJ. 2000. Non-toxicogenic strain of Aspergillus oryzae and Aspergillus sojae for biocontrol of toxicogenic fungi. United States Patent. (Patent No. 6027724). https://patentimages.storage.googleapis.com/84/e6/5f/65765dbc491a4f/US5347263.pdf [ Links ]
El-EnshasyHA. 2007. Filamentous fungal cultures - process characteristics, products, and applications. En: YangS-T (ed.). Bioprocessing for Value-Added Products from Renewable Resources, n.1 (p.225-261). 670p. [ Links ]
El-hawarySS, Moawa-dAS, BahrHS, AbdelmohsenUR y MohammedA. 2020. Natural product diversity from the endophytic fungi of the genus Aspergillus. RSC Advances 10(37): 22058-22079. https://doi.org/10.1039/D0RA04290K [ Links ]
El-ShafieAK. 1996. Soil fungi in Qatar and other Arab countries. Economic Botany 50: 242. [ Links ]
EllatifSA, Abdel RazikES, Al-SurhaneeAA, Al-SarrajF, DaighamGE y MahfouzAY. 2022. Enhanced production, cloning, and expression of a xylanase gene from endophytic fungal strain Trichoderma harzianum KJ831197.1: Unveiling the in vitro antifungal activity against phytopathogenic fungi. Journal of Fungi (Basel, Switzerland) 8(5). https://doi.org/10.3390/jof8050447 [ Links ]
EscobarKV, RamónP, CárdenasFR y MonroyBLD. 2023. Detección de micotoxinas (aflatoxinas) en alimentos primarios y procesados para humanos y animales de granja, en Riobamba-Ecuador. Siembra 10(1): 1-7. https://doi.org/10.29166/SIEMBRA.V10I1.4126 [ Links ]
FitrianaY, SuharjoR, SwibawaIG, SemengukB, PasaribuLT, HartamanM, RwandiniRA, IndriyatiI, PurnomoP y SolikhinS. 2021. Aspergillus oryzae and Beauveria bassiana as entomopathogenic fungi of Spodoptera litura Fabricius (Lepidoptera: Noctuidae) infesting corn in Lampung, Indonesia. Egyptian Journal of Biological Pest Control 31(1): 1-12. https://doi.org/10.1186/S41938-021-00473-8/FIGURES/7 [ Links ]
FrisvadJC, MøllerLLH, LarsenTO, KumarR y ArnauJ. 2018. Safety of the fungal workhorses of industrial biotechnology: update on the mycotoxin and secondary metabolite potential of Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei. Applied Microbiology and Biotechnology 102(22): 9481-9515. https://doi.org/10.1007/s00253-018-9354-1 [ Links ]
FurukawaS. 2012. Sake: quality characteristics, flavour chemistry and sensory analysis. En: PiggottJ (ed.). Alcoholic Beverages, n.1 (p.180-195). Woodhead Publishing. 461p.. https://doi.org/10.1533/9780857095176.2.180 [ Links ]
GadSE. 2005. Generally recognized as safe (GRAS). En: WexlerP (ed.). Encyclopedia of Toxicology, Vol 2. (p.417-420). Gad Consulting Services, Inc.. [ Links ]
GómezS, FernándezFJ y VegaMC. 2016. Heterologous expression of proteins in Aspergillus. En: GuptaVK (ed.). New and Future Developments in Microbial Biotechnology and Bioengineering, n.1 (p.55-68). Spanish National Science Council (CIB-CSIC). https://doi.org/10.1016/B978-0-444-63505-1.00004-X [ Links ]
GomiK. 2014. Aspergillus | Aspergillus oryzae. En: ElsieverInc (ed.). Encyclopedia of Food Microbiology, Vol 2. (p.92-96). https://doi.org/10.1016/B978-0-12-384730-0.00011-2 [ Links ]
GuptaT y SaxenaJ. 2023. Biogenic synthesis of silver nanoparticles from Aspergillus oryzae MTCC 3107 against plant pathogenic fungi Sclerotinia sclerotiorum MTCC 8785. Journal of Microbiology, Biotechnology and Food Sciences 12(4): 1-4. https://doi.org/10.55251/JMBFS.9387 [ Links ]
HeB, TuY, JiangC, ZhangZ, LiY y ZengB. 2019. Functional genomics of Aspergillus oryzae: Strategies and progress. Microorganisms 7(4): 103. https://doi.org/10.3390/MICROORGANISMS7040103 [ Links ]
HesseltineCW, SorensonWG y SmithM. 1970. Taxonomic studies of the aflatoxin-producing strains in the Aspergillus flavus group. Mycologia 62(1): 123-132. [ Links ]
Jiménez-QueroA, PolletE, AvérousL y PhalipV. 2020. Optimized bioproduction of itaconic and fumaric acids based on solid-state fermentation of lignocellulosic biomass. Molecules (Basel, Switzerland) 25(5). https://doi.org/10.3390/molecules25051070 [ Links ]
KahnV. 1995. Effect of kojic acid on the oxidation of DL-DOPA, norepinephrine, and dopamine by mushroom tyrosinase. Pigment Cell Research 8(5): 234-240. https://doi.org/10.1111/J.1600-0749.1995.TB00669.X [ Links ]
KahnV y Ben-ShalomN. 1997. Effect of maltol on the oxidation of DL-DOPA, Dopamine, N-Acetyldopamine (NADA), and Norepinephrine by mushroom tyrosinase. Pigment Cell Research 10(3): 139-149. https://doi.org/10.1111/j.1600-0749.1997.tb00475.x [ Links ]
KimJH, ChanKL. 2014. Augmenting the antifungal activity of an oxidizing agent with kojic acid: control of Penicillium strains infecting crops. Molecules (Basel, Switzerland) 19(11): 18448-18464. https://doi.org/10.3390/molecules191118448 [ Links ]
KimJH, ChangPK, ChanKL, FariaNCG, MahoneyN, KimYK, MartinsM de L y CampbellBC. 2012. Enhancement of commercial antifungal agents by kojic acid. International Journal of Molecular Sciences 13(11): 13867-13880. https://doi.org/10.3390/ijms131113867 [ Links ]
KimT, YeongJJ, JeongJS, LeeH, BaeCH, YeoJ, LeeH, KimI, ParkH y KimJC. 2016. Nematicidal activity of kojic acid produced by Aspergillus oryzae against Meloidogyne incognita. Journal of Microbiology and Biotechnology 26(8): 1383-1391. https://doi.org/10.4014/jmb.1603.03040 [ Links ]
KlichMA. 2002. Biogeography of Aspergillus species in soil and litter. Mycologia 94(1): 21-27. [ Links ]
KocsubéS, PerroneG, MagistàD, HoubrakenJ, VargaJ, SzigetiG, HubkaV, HongSB, FrisvadJC y SamsonRA. 2016. Aspergillus is monophyletic: Evidence from multiple gene phylogenies and extrolites profiles. Studies in Mycology 85: 199-213. https://doi.org/10.1016/J.SIMYCO.2016.11.006 [ Links ]
KongF, LuN, LiuY, ZhangS, JiangH, WangH, WangW y LiS. 2021. Aspergillus oryzae and Aspergillus niger co-cultivation extract affects in vitro degradation, fermentation characteristics, and bacterial composition in a diet-specific manner. Animals : An Open Access Journal from MDPI 11(5). https://doi.org/10.3390/ani11051248 [ Links ]
KozakiewicZ. 1989. Aspergillus species on stored products. Mycological Papers 161: 188. [ Links ]
KurtzmanCP, SmileyMJ, RobnettCJ y WicklowDT. 2018. DNA relatedness among wild and domesticated species in the Aspergillus flavus group. Mycologia 78(6): 955-959. https://doi.org/10.1080/00275514.1986.12025355 [ Links ]
LeeDE, LeeS, JangES, ShinHW, MoonBS y LeeCH. 2016. Metabolomic profiles of Aspergillus oryzae and Bacillus amyloliquefaciens during rice koji fermentation. Molecules 21(6). https://doi.org/10.3390/molecules21060773 [ Links ]
LeeK, LeeSK y LeeBD. 2006. Aspergillus oryzae as probiotic in poultry—A review. International Journal of Poultry Science 5(1): 1-3. 10.3923/ijps.2006.1.3 [ Links ]
MachidaM, AsaiK, SanoM, TanakaT, KumagaiT, TeraiG, KusumotoKI, ArimaT, AkitaO, KashiwagiY, AbeK, GomiK, HoriuchiH, KitamotoK, KobayashiT, TakeuchiM, DenningDW, GalaganJE, NiermanWC y KikuchiH. 2005. Genome sequencing and analysis of Aspergillus oryzae. Nature 438(7071): 1157-1161. https://doi.org/10.1038/NATURE04300 [ Links ]
MachidaM, YamadaO y GomiK. 2008. Genomics of Aspergillus oryzae: Learning from the history of koji mold and exploration of its future. DNA Research: An International Journal for Rapid Publication of Reports on Genes and Genomes 15(4): 173-183. https://doi.org/10.1093/DNARES/DSN020 [ Links ]
MahasJW, HamiltonFB, RobertsPM, RayCH, MillerGL, SharmanM, ConnerK, BagS, BlytheEK, ToewsMD y JacobsonAL. 2022. Investigating the effects of planting date and Aphis gossypii management on reducing the final incidence of cotton leafroll dwarf virus. Crop Protection 158: 106005. https://doi.org/https://doi.org/10.1016/j.cropro.2022.106005 [ Links ]
MahmoudGA, ZohriANA, Kamal-EldinNA y AbdelhamidNMR. 2023. Application of Aspergillus oryzae ASU44 (OL314732) and their kojic acid as pesticides against cotton aphid, Aphis gossypii. Bulletin of Pharmaceutical Sciences. Assiut 46(1): 63-82. https://doi.org/10.21608/bfsa.2023.300763 [ Links ]
MarshallH, MeneelyJP, QuinnB, ZhaoY, BourkeP, GilmoreBF, ZhangG y ElliottCT. 2020. Novel decontamination approaches and their potential application for post-harvest aflatoxin control. Trends in Food Science & Technology 106: 489-496. https://doi.org/https://doi.org/10.1016/j.tifs.2020.11.001 [ Links ]
MatsunagaK, FurukawaK y HaraS. 2002. Effects of enzyme activity on the mycelial penetration of rice koji. Journal of the Brewing Society of Japan 97(10): 721-726. https://doi.org/10.6013/JBREWSOCJAPAN1988.97.721 [ Links ]
MeiniMR, CabezudoI, GalettoCS y RomaniniD. 2021. Production of grape pomace extracts with enhanced antioxidant and prebiotic activities through solid-state fermentation by Aspergillus niger and Aspergillus oryzae. Food Bioscience 42: 101168. https://doi.org/https://doi.org/10.1016/j.fbio.2021.101168 [ Links ]
MohamadR, MohamedM, SuhailiN, SallehMM y AriffA. 2010. Kojic acid: applications and development of fermentation process for production. Biotechnology and Molecular Biology Reviews 5(2): 24-37. [ Links ]
MousaviB, HedayatiM, HedayatiN, IlkitM y SyedmousaviS. 2016. Aspergillus species in indoor environments and their possible occupational and public health hazards. Current Medical Mycology 2(1): 36-47. https://doi.org/10.18869/ACADPUB.CMM.2.1.36 [ Links ]
MuraliM, AmrutheshKN, SudishaJ, NiranjanaSR y ShettyHS. 2012. Screening for plant growth promoting fungi and their ability for growth promotion and induction of resistance in pearl millet against downy mildew disease. Journal of Phytology 4(5): 30-36. [ Links ]
MurphyMM. 2021. Effect of Aspergillus niger and oryzae on the intake and digestibility of Coastal Bermudagrass and Teff hay in horses. Journal of Equine Veterinary Science 100(103516). https://doi.org/10.1016/j.jevs.2021.103516 [ Links ]
NavaleV, VamkudothKR, AjmeraS y DhuriV. 2021. Aspergillus-derived mycotoxins in food and the environment: Prevalence, detection, and toxicity. Toxicology Reports 8: 1008-1030. https://doi.org/10.1016/j.toxrep.2021.04.013 [ Links ]
NorliaM, JinapS, Nor-KhaizuraMAR, RaduS, SamsudinNIP y AzriFA. 2019. Aspergillus section Flavi and aflatoxins: occurrence, detection, and identification in raw peanuts and peanut-based products along the supply chain. Frontiers in Microbiology 10: 2602. https://doi.org/10.3389/fmicb.2019.02602 [ Links ]
NorliaM, Nor-KhaizuraMAR, SelamatJ, Abu BakarF, RaduS y ChinCK. 2018. Evaluation of aflatoxin and Aspergillus sp. contamination in raw peanuts and peanut-based products along this supply chain in Malaysia. Food Additives and Contaminants. Part A, Chemistry, Analysis, Control, Exposure and Risk Assessment 35(9): 1787-1802. https://doi.org/10.1080/19440049.2018.1488276 [ Links ]
Olempska-BeerZS, MerkerRI, DittoMD y DiNoviMJ. 2006. Food-processing enzymes from recombinant microorganisms—a review. Regulatory Toxicology and Pharmacology 45(2): 144-158. https://doi.org/10.1016/J.YRTPH.2006.05.001 [ Links ]
PadrónMHY, DelgadoHS, ReyesMCA y VázquezCG. 2013. The genus Aspergillus and their mycotoxins in maize in Mexico: Problems and perspectives. Revista Mexicana de Fitopatología 31(19): 126-146. [ Links ]
PanchanawapornS, ChutrakulC, JeennorS, AnantayanonJ, RattanaphanN y LaotengK. 2022. Potential of Aspergillus oryzae as a biosynthetic platform for indigoidine, a non-ribosomal peptide pigment with antioxidant activity. PLoS ONE 17(6). https://doi.org/10.1371/JOURNAL.PONE.0270359 [ Links ]
PayneGA, NiermanWC, WortmanJR, PritchardBL, BrownD, DeanRA, BhatnagarD, ClevelandTE, MachidaM y YuJ. 2006. Whole genome comparison of Aspergillus flavus and A. oryzae. Medical Mycology 44: 9-11. https://doi.org/10.1080/13693780600835716 [ Links ]
PhashaV, SenabeJ, NdzotoyiP, OkoleB, FoucheG y ChuturgoonA. 2022. Review on the use of kojic Acid—a skin-lightening ingredient. Cosmetics 9(3): 64. https://doi.org/10.3390/COSMETICS9030064 [ Links ]
PodversichF, TarnonskyF, BollattiJM, SilvaGM, SchulmeisterTM, MartinezJJV, HerediaD, IpharraguerreIR, BargoF, GonellaDiazaA, DubeuxJCB, FerrarettoLF y DiLorenzoN. 2023. Effects of Aspergillus oryzae prebiotic on animal performance, nutrients digestibility, and feeding behavior of backgrounding beef heifers fed with either a sorghum silageor a byproductsbased diet. Journal of Animal Science 101. https://doi.org/10.1093/jas/skac312 [ Links ]
PowellKA, RenwickA, PeberdyJF y Federation of European Microbiological Societies . 1994. The Genus Aspergillus: from taxonomy and genetics to industrial application. Plenum Press, New York USA. 380p. [ Links ]
RádulyZ, SzabóL, MadarA, PócsiI y CsernochL. 2019. Toxicological and medical aspects of Aspergillus-derived mycotoxins entering the feed and food chain. Frontiers in Microbiology 10(2908): 1-23. https://doi.org/10.3389/fmicb.2019.02908 [ Links ]
Samson, R.A. , Visagie, C.M. , Houbraken, J. , Hong, S.B. , Hubka, V. , Klaassen, C.H.W. , Perrone, G. , Seifert, K.A. , Susca, A. , Tanney, J.B. , Varga, J. , Kocsub, S. , Szigeti, G. , Yaguchi, T. , Frisvad, J.C. (2014). Phylogeny, identification and nomenclature of the genus Aspergillus. Studies in Mycology, 78, 141-173. https://doi.org/10.1016/j.simyco.2014.07.004 [ Links ]
Schuster, E. , Dunn-Coleman, N. , Frisvad, J.C. , Van Dijck, P.W.M. (2002). On the safety of Aspergillus niger—a review. Applied Microbiology and Biotechnology, 59 (4-5), 426-435. https://doi.org/10.1007/s00253-002-1032-6 [ Links ]
Senghor, L.A. , Ortega-Beltran, A. , Atehnkeng, J. , Callicott, K.A. , Cotty, P.J. , Bandyopadhyay, R. (2020). The atoxigenic biocontrol product Aflasafe SN01 is a valuable tool to mitigate aflatoxin contamination of both maize and groundnut cultivated in Senegal. Plant Disease, 104 (2), 510-520. [ Links ]
Seo, H.J. , Park, A.R. , Kim, S. , Yeon, J. , Yu, N.H. , Ha, S. , Chang, J.Y. , Park, H.W. , Kim, J.C. (2019). Biological control of root-knot nematodes by organic acid-producing Lactobacillus brevis WIKIM0069 isolated from kimchi. Plant Pathology Journal, 35 (6), 662-673. https://doi.org/10.5423/PPJ.OA.08.2019.0225 [ Links ]
Sharifi, M. , Ghadamyari, M. , Sajedi, R.H. , Zavareh, M. , Sheikhnejad, H. (2013). Insecticidal effects of 4-hexylresorcinol on the lesser mulberry snout moth, Glyphodes pyloalis Walker. Archives of Phytopathology and Plant Protection, 46 (4), 423-435. https://doi.org/10.1080/03235408.2012.743387 [ Links ]
Sharma, P. , Singh, S.P. (2021). Role of the endogenous fungal metabolites in the plant growth improvement and stress tolerance. In: Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology , Volume 3. 381-401. https://doi.org/10.1016/B978-0-12-821734-4.00002-2 [ Links ]
Siddiquee, S. (2018). Recent advancements on the role of biologically active secondary metabolites from Aspergillus. In: Gupta, V.K. , Rodriguez-Couto, S. (eds.). New and Future Developments in Microbial Biotechnology and Bioengineering: Penicillium System Properties and Applications, 69-94. [ Links ]
Siddiqui, S. (2016). Protein production: Quality control and secretion stress response. In: Gupta, V.K. (eds.). New and Future Developments in Microbial Biotechnology and Bioengineering: Aspergillus System Properties and Applications, 257-266. [ Links ]
Singh, M. , Srivastava, P.K. , Verma, P.C. , Kharwar, R.N. , Singh, N. , Tripathi, R.D. (2015). Soil fungi for mycoremediation of arsenic pollution in agriculture soils. Journal of Applied Microbiology, 119 (5), 1278-1290. https://doi.org/10.1111/JAM.12948 [ Links ]
Skinder, B.M. , Nabi, M. , Gojree, B.A.S. , Dar, G.H. , Ganai, B.A. (2022). Endophytic Microbes: Bioremediation of soil contaminants. In: Dar, G.H. , Bhat, R.A. , Qadri, H. , Hakeem, K.R. (eds.). Microbial Consortium and Biotransformation for Pollution Decontamination, 243-258. [ Links ]
Sosa, A. , Marrero, Y. , González, N. , Albelo, N. , Moreira, O.B. , Cairo, J. , Galindo, J. (2022). Effect of Aspergillus oryzae on ruminal fermentation, feed intake and dry matter digestibility in cows fed forage-based diets. Animal Biotechnology, 33 (7), 1519-1524. https://doi.org/10.1080/10495398.2021.1914069 [ Links ]
Sun, B.T. , Akutse, K.S. , Xia, X.F. , Chen, J.H. , Ai, X. , Tang, Y. , Wang, Q. , Feng, B.W. , Goettel, M.S. , You, M.S. (2018). Endophytic effects of Aspergillus oryzae on radish (Raphanus sativus) and its herbivore, Plutella xylostella. Planta, 248 (3), 705-714. https://doi.org/10.1007/s00425-018-2928-4 [ Links ]
Sun, J. , Zou, X. , Ning, Z. , Sun, M. , Peng, J. , Xiao, T. (2012). Culturable microbial groups and thallium-tolerant fungi in soils with high thallium contamination. The Science of the Total Environment, 441, 258-264. https://doi.org/10.1016/J.SCITOTENV.2012.09.053 [ Links ]
Tari, C. , Dogan, N. , Gogus, N. (2008). Biochemical and thermal characterization of crude exo-polygalacturonase produced by Aspergillus sojae. Food Chemistry, 111 (4), 824-829. https://doi.org/10.1016/j.foodchem.2008.04.056 [ Links ]
U.S. Food and Drug Administration (2019). Generally Recognized Safe (GRAS) | FDA. https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (consulta, noviembre 2022). [ Links ]
Watarai, N. , Yamamoto, N. , Sawada, K. , Yamada, T. (2019). Evolution of Aspergillus oryzae before and after domestication inferred by large-scale comparative genomic analysis. DNA Research, 26 (6), 465-472. https://doi.org/10.1093/DNARES/DSZ024 [ Links ]
Xiong, K. , Zhi, H.W. , Liu, J.Y. , Wang, X.Y. , Zhao, Z.Y. , Pei, P.G. , Deng, L. , Xiong, S.Y. (2021). Detoxification of Ochratoxin A by a novel Aspergillus oryzae strain and optimization of its biodegradation. Revista Argentina de Microbiología, 53 (1), 48-58. https://doi.org/10.1016/j.ram.2020.06.001 [ Links ]
Xu, Q. , Li, S. , Huang, H. , Wen, J. (2012). Key technologies for the industrial production of fumaric acid by fermentation. Biotechnology Advances, 30 (6), 1685-1696. https://doi.org/10.1016/j.biotechadv.2012.08.007 [ Links ]
Yadav, U. (2017). Recent trends in nematode management practices: the Indian context. International Research Journal of Engineering and Technology, 10 (12), 483-489. [ Links ]
Yamada, R. , Yoshie, T. , Wakai, S. , Asai-Nakashima, N. , Okazaki, F. , Ogino, C. , Hisada, H. , Tsutsumi, H. , Hata, Y. , Kondo, A. (2014). Aspergillus oryzae-based cell factory for direct kojic acid production from cellulose. Microbial Cell Factories, 13 (1), 1-8. https://doi.org/10.1186/1475-2859-13-71 [ Links ]
Yasui, M. , Oda, K. , Masuo, S. , Hosoda, S. , Katayama, T. , Maruyama, J.I. , Takaya, N. , Takeshita, N. (2020). Invasive growth of Aspergillus oryzae in rice koji and increase of nuclear number. Fungal Biology and Biotechnology, 7 (1), 1-15. https://doi.org/10.1186/S40694-020-00099-9 [ Links ]
Yu, J. , Chang, P.K. , Ehrlich, K.C. , Cary, J.W. , Bhatnagar, D. , Cleveland, T.E. , Payne, G.A. , Linz, J.E. , Woloshuk, C.P. , Bennett, J.W. (2004). Clustered pathway genes in aflatoxin biosynthesis. Applied and Environmental Microbiology, 70 (3), 1253-1262. [ Links ]
Zhang, D. , Ma, X. , Gu, Y. , Huang, H. , Zhang, G. (2020). Green synthesis of metallic nanoparticles and their potential applications to treat cancer. Frontiers in Chemistry, 8 (799), 1-18. https://doi.org/10.3389/fchem.2020.00799 [ Links ]
Zhang, P. , You, Y. , Song, Y. , Wang, Y. , Zhang, L. (2015). First record of Aspergillus oryzae (Eurotiales: Trichocomaceae) as an entomopathogenic fungus of the locust, Locusta migratoria (Orthoptera: Acrididae). Biocontrol Science and Technology, 25 (11), 1285-1298. https://doi.org/10.1080/09583157.2015.1049977 [ Links ]
Zhou, M. , Zhou, K. , He, P. , Wang, K.M. , Zhu, R.Z. , Wang, Y.D. , Dong, W. , Li, G.P. , Yang, H.Y. , Ye, Y.Q. , Du, G. , Li, X.M. , Hu, Q.F. (2016). Antiviral and cytotoxic isocoumarin derivatives from an endophytic fungus Aspergillus oryzae. Planta Medica, 82 (5), 414-417. https://doi.org/10.1055/s-0035-1558331 [ Links ]
Zhu, G.Y. , Shi, X.C. , Wang, S.Y. , Wang, B. , Laborda, P. (2022). Antifungal mechanism and efficacy of kojic acid for the control of Sclerotinia sclerotiorum in soybean. Frontiers in Plant Science, 13 (845698), 1-11. https://doi.org/10.3389/fpls.2022.845698 [ Links ]
Received: September 02, 2023; Accepted: October 10, 2023











 texto en
texto en