Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Atmósfera
versión impresa ISSN 0187-6236
Atmósfera vol.17 no.4 Ciudad de México oct. 2004
Characterization of atmospheric particles:
analysis of particles in the Campo de Gibraltar
A. UMBRÍA
Consejería de Medio Ambiente, Junta de Andalucía. Delegación de Cádiz, España
M. GALÁN, M. J. MUÑOZ and R. MARTÍN
Departamento de Ingeniería Química, Tecnología de Alimentos y Tecnologías del Medio Ambiente,
Facultad de Ciencias. 11510 Puerto Real (Cadiz). España
Received July 28, 2003; accepted January 12, 2004
RESUMEN
El campo de Gibraltar es una área situada en el sur de España, alrededor de la bahía de Algeciras ((36° 7'N, 5°27'W). La zona, en la confluencia de los continentes europeo y africano, tiene un alto interés desde el punto de vista geográfico y botánico. La presencia de un polo industrial, unido a los fuertes vientos que caracterizan la zona contribuyen a una rápida dispersión de partículas, tanto las naturales como las originadas por actividades humanas. En el presente trabajo se caracterizan distintas partículas atmosféricas, captadas tanto en las propias fuentes de emisión como en estaciones de inmisión. Se emplea conjuntamente la microscopía electrónica de barrido (SEM) y la espectrometría de dispersión de rayos X (EDX), con lo que se consigue simultáneamente la caracterización morfológica y química. Ambas son fundamentales a la hora de distinguir el origen, natural o antropogénico, de las partículas atmosféricas. Por último se muestra una clasificación general de partículas atmosféricas, basada en sus características físicas y químicas, que incluyen morfología, características superficiales y composición elemental.
ABSTRACT
Campo de Gibraltar is a highly industrialized area situated in the South of Spain around Algeciras bay (36° 7'N, 5°27'W). This area has an advantageous situation from a geographical, geological and botanical points of view. However, high wind speeds and a substantial degree of turbulence characterize this area, a situation that contributes to the rapid and wide dispersion of atmospheric pollutants and natural particles that are emitted in the atmosphere. The work described here involved the use of scanning electron microscopy (SEM) and X-ray Dispersive Energy Spectrometry (EDX) to characterize the atmospheric particles (both natural and anthropogenic). The electron microscopy method provides, simultaneously, knowledge about the morphology and the chemical composition of the particles. These are the fundamental parameters that need to be investigated to determine the origin of the particles. A systematic method has been developed that is applicable to the study of particles collected in emission sources, as well as to particles collected in inmission stations. This simple method allows the origin of the particles to be determined. Finally, a general classification of particles has been devised in terms of their physical and chemical characteristics, including morphology, surface characteristics and elementary composition.
Key words: Particulate matter, anthropogenic and natural sources, morphological and chemical analysis, south-western Mediterranean.
1. Introduction
Campo de Gibraltar is a highly industrialized area situated in the South of Spain around Algeciras bay (36° 7'N, 5°27'W). This area contains one of the largest industrial parks in Spain at present. The industries located here are mainly of the heavy type and include refineries, thermal power plants and steelworks, all of which are considered as heavy polluters in terms of the atmosphere.
For this reason, Campo de Gibraltar is one of the areas in Spain that is responsible for the largest amounts of emissions of atmospheric pollutants, which are emitted in a continuous way from fixed facilities. The Regional Ministry of the Environment, as the appropriate body in this area, drafted the "Corrective Plan for Atmospheric Discharges" in 1990 with the purpose of not surpassing the legally allowed concentrations of pollutants in any case.
In this context, it is of fundamental importance to understand the origin, i.e. natural or anthropogenic, of the particles in the atmosphere. Another equally important goal is the identification of the specific origin of the anthropogenic pollutants, those that are possible to control, and to identify those that are emitted from stationary or mobile sources. Such an identification process should be carried out even when different sources of polluting particles originate from very similar processes, as is the case in the present study.
Two theoretical models can be considered in this study: the receptor model (Watson, 1984) and the chemical element balance receptor model (Watson, 1979). These models allow identification of the sources of particle emission that contribute to their concentration in the atmosphere. The models are based on the different compositions of the environmental aerosol and the emission sources (Henry, 1984), and their goal is to give information about the sources or air pollution, depending on the data provided to the model. It is not always is possible to establish this identification, due to the conditions that must be hold for the solution of a general mixing problem (Henry, 1997).
The sources of particulate pollutants can also be differentiated by the experimental characterization of the particles that they emit. The characterization of particles, based on the morphology and the chemical composition, is an appropriate method for the study of particles in the atmosphere.
Particle shape analysis must involve the use of microscopy techniques. Optical techniques are useful in the preliminary analyses and in the perception of particle color. Using light microscopy, some particle types can be easily recognized. For example, certain vegetable particles or fly-ash particles form the high temperature combustion of fossil fuels (Battarbee et al., 1997). However, there are also many types which could not be allocated unequivocally to a particular source type by optical microscopy alone. In these cases, electron microscopy techniques are the appropriate tools for the complete characterization of the atmospheric particles (Mamane, 1986 and 1988).
The work described here addresses the objectives discussed above, and describes a specific method that is able to characterize the solid particles, taking advantage of systems already in place and supplementing them. Particles arising from different emission sources and inmission samples have been characterized with the purpose of establishing a general classification and allow identification of different populations that are part of the atmospheric environment. The techniques employed were scanning electron microscopy (SEM) combined with X-ray Dispersive Energy Spectrometry (EDX) (Casuccio, 1983).
2. Experimental
2.1. Sampling
Samples of sixteen artificial emission sources in the area were collected. These samples include emissions from fuel/oil and fuel/gas combustion in petrochemical industries; emissions from mineral coal combustion in energy production plants, and emissions from steelwork activities. An isokinetic sampler was used with a filter in the head and a seven-stage cascade impactor, in accordance with the US EPA method #5 (Environmental Protection Agency) for sampling of stationary sources. The samples were captured in glass microfiber filters as well as in cellulose fibers for study by EDX.
The inmission samples were taken with ambient air samplers with five separation stages with glass fiber filters (Kimoto mod. CPS 105), and a sedimentable particles sampler. In the latter case, monolayer extraction over a polyethylene membrane was performed.
2.2. Analysis procedure
The morphologic analysis of the samples was performed using a scanning electron microscope (Jeol JSM series 800 with X-ray probe) and an optical microscope (Leyca Quantimet 500) in conjunction with a JVC image acquisition system and Q500MC software.
The samples were prepared as 5 x 5 mm pieces, mounted on graphite supports for SEM, and covered with a fine layer of gold or graphite (100 Å thickness). Three to five photomicrographs were taken in different fields for each sample, with magnifications varying from 15 0x to 5000x, depending on the individual size and the size distribution of the particles under analysis.
The microphotographs were digitized for morphologic analysis (including size). The samples for optical microscopy were examined visually and the appropriate parameters in terms of natural color were noted. These data were subsequently merged with the digitized images. The use of computer analysis allowed the physical diameters to be measured, classification into sizes, general enhancement, elimination of defects, pseudocolor application and optimization of the image.
In order to establish the chemical composition of the particles, thirty particles from each sample were analyzed by EDX, including those taken in cellulose filters. These latter samples were studied with the aim of establishing the real quantities of silicon present, since samples taken on glass fibers can experience diffusion of this element toward the particles. In many cases, different points within a particle were analyzed with the purpose of establishing the anisotropic character of the sample.
3. Results and discussion
3.1. Morphologic analysis
The shape, size, texture and aspect are differentiating factors in the macroscopic world, and the human being intuitively appreciates them. In the microscopic world these features are also characteristic of particles. However, depending on the magnification used in the analysis, it is easy to move away from the intrinsic relationships of each unit. It would be expected that working at the maximum possible magnification would be better in terms of analysis. However, this is not necessarily the case and as one looks more closely at the smaller details, the wider differentiating characteristics can easily be overlooked. Indeed, the factors that characterize a given particle, in most cases, are not its elementary form but the organization of its structure.
Figure 1 shows the texture of a particle taken with a high magnification. Although the observed texture is an important morphologic feature, it could be very similar to that of other particles or, alternatively, it could simply be mistaken with that of the supporting medium. The next image (Fig. 2), however, would be the ideal one to study because the shape, the elementary structures, the global situation, and even the support that sustains them, can be clearly appreciated.

Usually, different magnification levels are used for this type of particle, beginning with the lowest (150x, which discriminates several dozens of microns) and ranging to the highest magnification (5000x, which allows details smaller than 1 micron to be discriminated). Complete structural information can be obtained in this way.
For the characterization of atmospheric particles it is necessary to establish a number of standard approaches, at diverse resolution levels, in order to provide the correct perspective. The first level of discrimination that must be attained is to distinguish the origin of the particles as natural or anthropogenic. The reason for this requirement has already been discussed in the introduction. Natural and anthropogenic particles both show some morphological characteristics that can be used to discriminate between them.
3.1.1. Natural particles
The land, sea and biomass are the large generators of natural particles, and the characteristics of these particles can vary markedly depending on the geographical area in which they originate. Natural mineral particles (Fig. 3) originate from the land and they usually form part of the local environment. Nevertheless, particles imported from other areas can be present, sometimes to surprisingly high levels (Mattsson, 1996; Ryal, 2002).

The structure and composition of natural particles can be diverse and they usually present a relative two-dimensional aspect, with length and width prevailing over thickness. It is often found that such particles have outlying edges, causing fracture lines of their primitive state (Fig. 4). In the case of particles that are very old and highly eroded, the tendency is to conform to ovoid shapes.

Less abundant, but nonetheless important, types of particle are those arising from the biomass and crystallizations of marine origin. The former type are usually symmetrical and very structured, with pollen being the most significant example due to its abundance and mobility. There is a multitude of morphologic varieties of this type of particle (Figs. 5 and 6).


The natural crystallizations (Fig. 7) also follow some characteristic trends and often have basic and regular structures that depend on the chemical composition of the material. The origin of such particles is usually marine and these are composed fundamentally of chlorides and smaller quantities of sulfates. It is common to find these systems polluted by other elements that are widespread in the environment, such as calcium or silicon.

It is believed that these crystallizations do not exist as solid particles in the atmosphere, rather that they are the result of the condensation and subsequent evaporation of liquid phases on specific surfaces. This mechanism is also applicable to anthropogenic emissions. The crystallizations of artificial origin (combustion, coalition of metals, etc.) are not frequently found and are easily distinguished from the natural ones on the basis of the different chemical composition - they tend to contain trace elements such as vanadium, chromium or nickel. In addition, sulfates prevail over chlorides, the latter being more abundant in particles of natural origin.
In the group classified as natural particles we also find spores, fungus (Fig. 8), virus and bacteria, and even microfossils (Fig. 9), although these represent a very small proportion of atmospheric solid particles.


3.1.2. Anthropogenic particles
The particles arising from combustion are characterized, in most cases, by a spherical shape due fundamentally to the melting process that occurs during their formation. This is a basic axiom and establishes a structural difference between artificial particles, often arising from strongly energetic processes (like combustion or foundry), and natural particles.
However, this axiom is not all-encompassing because there are natural spherical particles, like some spores, pollens, volcanic emanations, etc. In addition, there are also artificial particles that do not present a spherical form, even if they have been subjected to a thermal process. Examples of these include some ashes or scum. In these cases, it would be necessary to undertake a more indepth study.
When a given sample is studied, a varied and sometimes complex morphologic range is often observed. For example, particles can be found that have the same origin, but have undergone different modifications as a result of physical-chemical actions, either within or outside the source focus. Such processes include divisions, breaks, recondensations, or partial dissolution (Fig. 10).

The vast majority of anthropogenic particles usually present a surface that contains a multitude of hollows that give them a cavernous appearance (Fig. 11), although these hollows vary in number, size and distribution. The hollows are formed when gaseous or liquid material is expelled from the interior of the particle, due to an increase in internal pressure or to an external depression. Convection currents favor this process, which causes weakening in certain areas of the surface.
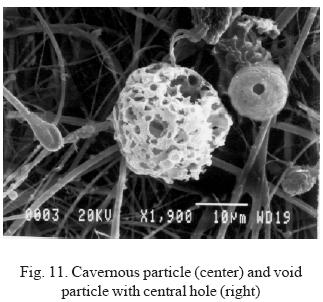
These particles are usually void due to the ejection of the internal material and to the mechanical stability of the surface. However, many of them collapse by fracturing as a result of their fragility. The expulsion of the internal material and the properties of the surface layer are critical factors in determining the characteristics of these particles. If the speed at which expulsion takes place is high, particles are generated with a net-like appearance and they are very spongy, as if a sudden boiling process had occurred in their interior. In other cases, the structure has not survived the forces generated and the particle is totally or partially destroyed, giving rise to other families of particles with different morphology. Generally, if the speed of material ejection is slower, the probability of consolidation of the particle increases.
On occasions, some particles are found with a single central hole (Fig. 11). These are usually smaller diameter particles and perhaps come from smaller drops, which would have stronger cohesion forces in the surface layer. For this reason, these particles can resist the expulsion of internal material and therefore maintain their size.
The generation of surface convection currents is decisive in the morphology of certain types of particle. Figure 12 clearly shows the marks left by these currents, generated during the combustion process. The marks indicate that the combustion process had occurred in the liquid phase - or near the liquid phase - while the particle was suspended in the gas phase.

Another aspect to consider when studying the morphology of the particles is their size distribution. A combustion focus emits particles with certain characteristics that depend on the installation type, on the type of fuel and the operation conditions. When operation conditions vary (within the normal operation range), the distribution of sizes is affected but different particles are not generated, except under special conditions.
The distribution of particle sizes is therefore important information that can pinpoint an emission focus. The physical size can be established by direct measurements, using microscopic techniques (Fig. 13), while the aerodynamic size is determined by using cascade impactors (Fig. 14). These studies also allow the differentiated analysis of the different fractions.


Crystallizations have been found in some samples from fuel-oil combustion (Fig. 15). It is difficult to determine whether these crystallizations were already perfectly formed prior to their arrival on the reception supports or whether they crystallized on the filter. Two different phenomena could explain the formation on the reception filters and in the last stages of the cascade impactors:
• Emission gases usually carry liquid particles that are deposited in the reception filters. These are capable of solubilizing some mineral compounds. When the liquid evaporates, crystallizations occurs.
• In the later stages of the cascade impactor, the speed of gases increases and, consequently, pressure decreases. An adiabatic condensation of the humidity from the gases is caused and subsequent evaporation allows crystallization of the solubilized salts.
3.2. Chemical analysis
Particles of different origin can present morphologic and chemical differences. Complete characterization of the particles should encompass these two aspects as they are complementary areas. Analysis of the elementary composition by means of EDX will contribute additional information of great potential in differentiating between and deducing the origin of the captured particles in the inmission stations.
3.2.1. Natural particles
Natural particles are usually more chemically complex than anthropogenic ones - except in the cases of crystallization - and are almost exclusively formed by chlorides or sulfates of sodium, potassium and magnesium.
The natural mineral particles have a matrix based on silicon, calcium or aluminum. The clays, calcites and silicates are very abundant in this group. Particles originating from biomass are characterized by a carbonaceous matrix, and if they are from a vegetable source they will contain chlorophyll and appreciable quantities of chlorine are detected.
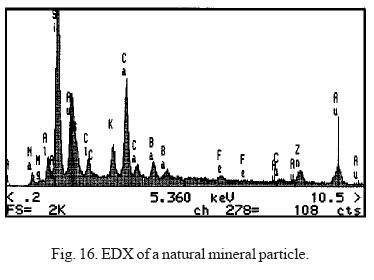
Figure 16 shows an X-ray dispersion spectrum of a typical mineral particle. A matrix based on calcium and silicon can be observed, with other abundant elements in this class, like iron and aluminum, forming chlorides or sulfates.
3.2.2. Anthropogenic particles
Anthropogenic particles show different oxidation levels depending on the process from which they come (e.g. combustion, foundry, calcination). In particles formed by combustion, carbon, aluminum, silicon and sulfur are the major elements and are often accompanied by trace metals. The liquid fuels from the heavy fraction of petroleum usually lead to structures supported by carbon, while, paradoxically, those from mineral coal combustion preferentially tend toward silicon or aluminum-silicon particles. This phenomenon is due to the different composition of the combustibles and to the different methods of burning of them. The EDX spectrum of a particle from fuel-oil combustion is shown in Figure 17 (with a carbonaceous matrix), while the EDX of a particle from the combustion of mineral coal is shown in Figure 18 (with a typical silicon matrix).


Foundry particles, which arise from steelwork factories, contain a great variety of trace metals and, frequently, iron is the most abundant and characteristic element. The spectrum of a particle consisting of an iron-chromium matrix, with sulfur, silicon and a low carbon content is shown in Figure 19. The particles arising from melting ovens (e.g. in cement plants and glass factories) are chemically the most complex. The characteristic elements in these particles are usually calcium or silicon.

In the combustion processes occurring at a given focus, a predominant population of particles shows similar chemical characteristics. However, some appreciable differences can also exist depending on the size of the fraction that is evaluated. It is often found that the largest particles have a higher content in carbon, sulfur or other elements. As the particle diameter diminishes, the carbon is replaced by silicon, the sulfur level drops and evidence of the presence of trace metals is seen. This phenomenon is probably produced by a concentration effect due to the migration of the more volatile elements (in their more oxidized forms) to the gas phase. For example, sulfur can be oxidized to SO2 and SO3, and carbon to CO and CO2. The less volatile elements, such as silicon, remain in the solid fraction and increase in concentration in the smaller particles.
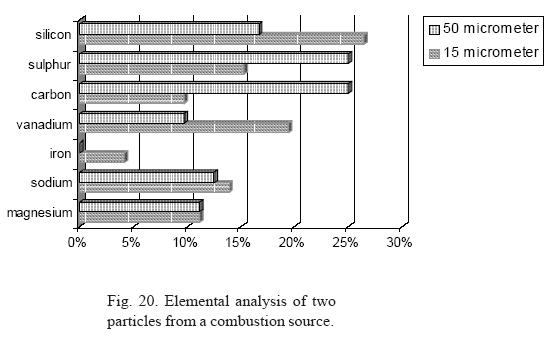
In Figure 20 one can see, as an example, the chemical composition by weight percent of two particles (one of 50 µm and the other of 15 µm) from a combustion focus. A decrease can be observed in the percentage of carbon and sulfur, shifting the balance mainly in favor of silicon. Small increases in vanadium and the appearance of small quantities of iron can also be observed.
4. Classification of the studied particles
On the basis of the information presented above, a general classification of the most habitual solid particles in the atmosphere can be proposed. Such a classification represents one of the first stages in establishing approaches for the evaluation of the air quality in terms of particulate material contents. The proposed classification is as follows:

5. Conclusions
In the study of the atmospheric particles it is of fundamental importance to understand their origin, i.e. natural or anthropogenic. Another goal is the identification of the specific origin of the anthropogenic pollutants. Electron microscopy techniques are appropriate tools for the complete characterization of the atmospheric particles, based on the analysis of the morphology and the chemical composition.
The structure of natural particles can be diverse and they usually present a relative two-dimensional aspect. Those arising from the biomass are usually symmetrical and very structured, and multitude of morphologic varieties. The natural crystallizations often have basic and regular structures that depend on the chemical composition of the material.
The particles arising from combustion are characterized, in most cases, by a spherical shape and a surface that contains a multitude of hollows, giving them a cavernous appearance These particles are usually void. Size distribution of the particles can be used to identify their emission focus.
The natural mineral particles have a matrix based on silicon, calcium or aluminum. Particles originating from biomass are characterized by a carbonaceous matrix. In particles formed by combustion, carbon, aluminum, silicon and sulfur are the major elements. Foundry particles, which arise from steelwork factories, contain a great variety of trace metals.
6. References
Batarbee, J.L., N.L. Rose and X. Long, 1997. A continuous high resolution record of urban airborne particulates suitable for retrospective microscopical analysis. Atmos. Env. 31, 171-181. [ Links ]
Casuccio G.S., P.B. Janocko, R.J. Lee, J.F. Kelly, S.L. Dattner and J.S. Mgebroff, 1983. The use of computer controlled scanning electron microscopy in environmental studies. J. Air Pollut. Control Ass. 33, 937-943. [ Links ]
Dzubay T.G., (1988) Development of composite receptor methods. U.S. EPA Report n° EPA/600/ 3-88/026 Research Triangle Park, NC 27711. [ Links ]
Dzubay T.G., R.K. Stevens, G.E. Gordon, I. Olmez, A.E. Sheffield and W.J. Courtney, 1988. A composite receptor method applied to Philadelphia aerosol. Envir. Sci. Technol. 22, 46-52. [ Links ]
Henry, R.C. 1997. History and fundamentlas of multivariate air quality receptor models. Chemometrics and Intelligent Laboratory Systems 37, 37-42. [ Links ]
Henry W.M. and K.T. Knapp, 1984. Compound forms of fossil fuel fly ash emissions. Envir. Sci. Technol. 14, 450-456. [ Links ]
Mamane Y., 1988. Estimate of municipal refuse incinerator contribution to Philadelphia aerosol. Atmos. Environ. 22, 2411-2418. [ Links ]
Mamane Y. and T.G. Dzubay, 1988. Fly ash concentrations in Philadelphia aerosol determined by electron microscopy. Water, Air and Soil Pollut 37, 389-405. [ Links ]
Mamane Y., J.L. Miller and T.G. Dzubay, 1986. Characterization of individual fly ash particles emitted from coal and oil-fired power plants. Atmos. Environ. 20, 2125-2135. [ Links ]
Martínez, M. 1978. Evaluación de un problema de polución atmosférica: análisis de las emisiones. Técnicas de defensa del medio ambiente. 1, 345-346 [ Links ]
Mattsson, J.O. and T. Nihlev, 1996. The transport of Saharan dust to Southren Europe: a scenario. J. Arid Environ. 32, 111-119. [ Links ]
Ryall, D.B., R.G. Derwent, A.J. Manning, A.L. Redington, J. Corden, W. Millington, P.G. Simmonds, S.O. O'Doherty, N. Carslaw and G.W. Fuller, 2002. The origin of high particulate concentrations over United Kingdom, March 2000. Atmos. Envir. 36, 1363-1378. [ Links ]
Watson, J.G. (1979). Chemical element balance receptor model methodology for assessing the sources of fine and total particulate matter. University Microfilms International, Ann Arbor, MI. [ Links ]
Watson, J.G., J.A. Cooper and J.J. Huntzicker (1984). The effective variance weighting for least squares calculations applied to the mass balance receptor model. Atmos. Environ. 18, 1347-1355. [ Links ]















