Introduction
Growth habit is usually the first characteristic used to describe a plant. Whether a plant is a tree, a herb or a liana seems like a simple question, and yet, many times the answer is not straightforward because there are multiple, sometimes contradictory, or poorly delimited definitions of growth habits. The term habit comes from the Latin word habitus, which means external appearance (Font Quer, 2001); in botany, the habit refers to the external aspect of a plant (Vázquez-Sánchez et al., 2012). Defining growth habits can be quite challenging, even for a single species. For example, 80 randomly selected records of Blakea quadrangularis Triana (Melastomataceae) deposited in the Herbarium of Universidad de Antioquia (HUA, Colombia), were registered as trees (44%), shrubs (39%), vines (12%), lianas (3%), hemiepiphytes (1%), and herbs (1%). For growth habits to be a more useful concept in taxonomy and functional analysis, it is necessary to clarify their definition.
Defining growth habits has turned into a complex matter due to multiple factors. One source of confusion is the proliferation of terms. For example, a climbing plant is commonly described as a liana, vine, twiner, creeper, sprawler, or scrambler (Putz, 1984; Mendoza and Ramírez, 2006). However, these terms are not mutually exclusive and are related to different features: how they grow, climb, or hold to the substrate. Another issue is that growth habits have been confused with habitats where plants grow. For example, the following terms have been considered as growth habits: epiphytic, when plants grow on another plant without subtracting water or nutrient from it (Font-Quer, 2001); parasitic, when plants grow on another plant absorbing its resources; and aquatic, when plants live in the water. However, these terms may not be referring to growth habits because they do not allude directly to plant appearance, but instead to their habitat (Simpson, 2006).
Stem anatomy can be useful to delimit growth habits. Plants with different growth habits vary in their stem stiffness, which is related to the lignification of their stem (Isnard et al., 2012). By definition, some habits can be distinguished by the plant stem anatomy, i.e. herbs are plants that lack woody tissue at any level of their aerial stems (Eiten, 1991). Plants with the lianous growth habit can show anomalous cambial growth, vessels dimorphism, and abundant unlignified parenchyma (Obaton, 1960; Carlquist, 1985; Angyalossy et al., 2011; 2014). Trees and shrubs self-supporting constraints lead to the development of xylem with abundant fibers and longer axial elements (Rowe and Speck, 2004). Despite the evident relationship between stem anatomy and growth habits, there are few studies on how stem anatomy can distinguish growth habits. The morphological observations that define growth habits can greatly benefit from a detailed study of anatomy that helps to better characterize these habits.
The family Melastomataceae is an excellent group to investigate how the stem anatomy varies among plants with contrasting growth habits. It includes nearly 5000 species in 170 genera, standing as one of the largest families of Angiosperms (Penneys and Judd, 2011). Melastomataceae species exhibit a wide variety of growth habits. However, these habits are poorly defined due to various issues, one of them the use of different terminology in growth habit classifications. For example, Vliet (1981) suggested only two anatomically recognizable growth habits (erect stem plants, and climbers). Renner (1993) recognized six habits (trees, treelets (small stature trees), shrubs, herbs, root climbers, and facultative epiphytes). Almeda (2001) reduced to five the growth habits recognized within the family (trees, shrubs, herbs, scandent climbers, and epiphytes). Later, Mendoza and Ramírez (2006) increased to eight the growth habits observed in the family (tree, shrub, suffrutex (=a tall herb), herb, liana, vine (=a liana with a non-woody stem), epiphyte (=a plant with roots that does not actively extract water or nutrients from the ground or from the live tissues of the host), and hemiepiphyte (=epiphyte with roots reaching the ground later in life)). A unified classification of growth habits can clarify the discrepancies among these different groupings.
The stem anatomy variation of Melastomataceae is considerable. Most of the studies on the stem anatomy of members of the family have been realized on trees and shrubs, the most common growth habits in the family. In Melastomataceae, the length of xylem axial cells tends to be longer in trees than in shrubs (Ter Welle and Koek-Noorman, 1981). In some lianas of the family, the proportion of non-lignified xylem parenchyma and the diameter and number of vessels per mm2 tend to be larger than in trees and shrubs (Koek-Noorman et al., 1979; Ter Welle and Koek-Noorman, 1981; Vliet, 1981). In epiphytes of the family, there is a cortical parenchyma in the stem that was interpreted as a hygroscopic tissue that helped to prevent desiccation, a common issue in epiphytes (Reginato et al., 2009). Other studies on the anatomy of the family have focused on the relationship between anatomical variations and environmental factors such as light radiation, precipitation, and average annual temperature (Ely et al., 2005a, b; Gluzezak, 2005; Gardoni et al., 2007; Bosio, 2008). In general, the anatomical descriptions at the growth habit level in Melastomataceae are rare, and the comparisons of anatomical features among species with different growth habits are even more unusual and limited to brief annotations. Moreover, some publications do not even recognize the existence of special adaptations related with growth habits in Melastomataceae (Carlquist, 1985).
In this study, we analyzed whether stem anatomy characteristics vary consistently across growth habits of Melastomataceae. To do so we compared 18 species of this family, which were chosen to sample separate growth habits within the family, accounting for phylogeny by including representatives from different tribes. We used here a growth habit classification that we consolidated from a literature review, trying to reconcile multiple schemes of classification of habits across vascular plants. The stem anatomy of the 18 species was analyzed using 125 characters observed from histological preparations.
Materials and methods
Specimen collection
We collected one specimen from each of 18 species of Melastomataceae (Table 1) for descriptions and comparisons. We chose these species aiming to sample across different ecosystems of Colombian Andes (tropical wet forest, tropical rainforest, tropical moist forest, and paramo), different clades of Melastomataceae (five out of the 11 major clades recognized by Clausing and Renner (2001)), and especially all the different growth habits known to occur in the family. Voucher specimens were identified, described, and deposited at the Herbarium of University of Antioquia (HUA); duplicate specimens were sent to the Herbarium of the Universidad Católica de Oriente (HUCO), both in Colombia.
Table 1: List of species collected for this study and the ecosystem where they were sampled. All collections were performed in Colombia. Voucher specimens were deposited in the Herbarium of the Universidad de Antioquia (HUA).
| Species | Voucher HUA | Locality | Ecosystem |
|---|---|---|---|
| Adelobotrys adscendens Triana | 193782 | Cerro La Judia, Florida blanca, Santander | tropical moist forest |
| Arthrostemma ciliatum Pav. ex D. Don | 193781 | Frontino, Antioquia | tropical rainforest |
| Blakea fasciculata Gleason | 193791 | Frontino, Antioquia | tropical rainforest |
| Blakea granatensis Naudin | 193792 | Cerro La Judia, Florida blanca, Santander | tropical moist forest |
| Blakea quadrangularis Triana | 193788 | Cerro Bonifacio, Carmen de Viboral, Antioquia | tropical moist forest |
| Blakea rosea (Ruiz & Pav.) D. Don | 193793 | San Luis, Antioquia | tropical wet forest |
| Blakea watsonii (Cogn.) Penneys & Almeda | 193787 | San Luis, Antioquia | tropical wet forest |
| Chaetolepis microphylla (Bonpl.) Miq. | 193789 | Belmira, Antioquia | tropical moist forest |
| Clidemia epiphytica (Triana) Cogn. | 193790 | San Luis, Antioquia | tropical wet forest |
| Graffenrieda emarginata (Ruiz & Pav.) Triana | 193785 | Cerro Bonifacio, Carmen de Viboral, Antioquia | tropical moist forest |
| Heterocentron subtriplinervium (Link & Otto) A. Braun & C.D. Bouché | 193794 | Medellín, Antioquia | tropical moist forest |
| Meriania nobilis Triana | 193779 | Cerro Bonifacio, Carmen de Viboral, Antioquia | tropical moist forest |
| Miconia jahnii Pittier | 193786 | Cerro Bonifacio, Carmen de Viboral, Antioquia | tropical moist forest |
| Miconia salicifolia Naudin | 193795 | Urrao, Antioquia | paramo |
| Monochaetum multiflorum (Bonpl.) Naudin | 193783 | Cerro Bonifacio, Carmen de Viboral, Antioquia | tropical moist forest |
| Monolena primuliflora Hook. f. | 192632 | Amalfi, Antioquia | tropical wet forest |
| Tibouchina kingii Wurdack | 193780 | Cerro Bonifacio, Carmen de Viboral, Antioquia | tropical moist forest |
| Tibouchina lepidota (Bonpl.) Baill. | 193784 | Cerro Bonifacio, Carmen de Viboral, Antioquia | tropical moist forest |
Anatomical sample preparation
Stem portions of the fifth most apical internode were collected for analysis. The portions were fixed with FAA (10% of 40% formaldehyde, 50% of 96% ethanol, 5% of 96% acetic acid, and 35% of distilled water). Transverse, radial and tangential sections of each stem were obtained by free-hand slicing. Sections were stained with toluidine blue 0.05% and observed and photographed using a setting formed by an Olympus BH2 microscope (Olympus, Tokio, Japan), an Olympus e620 camera (Olympus, Tokio, Japan), and a NDPL-1 (2X) camera adapter (Amscope, Los Angeles, USA). This setting was located in the Palynology Laboratory of the Universidad de Antioquia, Medellín, Colombia.
Anatomical analysis
To describe the stem anatomy from the anatomical preparations we used 125 characters based on Metcalfe and Chalk (1950), Dickison (1975), Carlquist (1985), Wheeler et al. (1989), and Evert (2006). These characters describe the epidermis (12 characters), peridermis (15), cortex in general (3), cortical parenchyma (8), cortical collenchyma (6), cortical sclerenchyma (6), vascular system in general (8), phloem (9), xylem vessels (14), xylem fibers (6), xylem axial parenchyma (6), xylem rays (9), miscellaneous xylem characters (7), pith in general (4), medullary parenchyma (8), and medullary sclerenchyma (4). The quantitative characters were the tangential diameter of solitary vessels, the number of vessels per mm2, and the vessel axial length. The number of measurements per specimen were 30 for the tangential diameter of solitary vessels, 10 for the number of vessels per mm2, and 20 for the vessel axial length. See the appendix for further details on these characters.
Description of growth habits
Growth habit of analyzed specimens was determined using field observations (Table 2), according to the six growth habits that we condensed from definitions in the literature (Eiten, 1991; Renner, 1993; Moffet, 2000; Almeda, 2001; Font-Quer, 2001; Mendoza and Ramírez, 2006; Simpson, 2006):
Table 2: Established growth habits for analyzed species. Note: “1st” primary, “2nd” secondary, “-” non-registered character.
| Species | Rooting | Position of stem base | Stem orientation | Type of growth | Type of branchs | Height of 1st aerial branch (m) | Principal axis evident | Flower (inflorescence) position | Heigth of plant (m) | Support structures | Growth habit |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adelobotrys adscendens Triana | Soil | Soil | Prostrate | 2nd | Woody aerial | 4 | Yes | Terminal | 8 | Adventitious roots | Vine |
| Arthrostemma ciliatum Pav. ex D. Don | Soil | Soil | Decumbent | 1st | Non-woody basal | 0 | Yes | Terminal | 1 | - | Herb |
| Blakea fasciculata Gleason | Soil | Soil | Fickle stem supported | 2nd | Woody aerial | 0.4 | Yes | Axillary | 15 | Adventitious roots | Liana |
| Blakea granatensis Naudin | Soil | Soil | Prostrate | 2nd | Woody aerial | 3 | Yes | Axillary | 6 | Adventitious roots | Vine |
| Blakea quadrangularis Triana | Soil | Soil | Fickle stem supported | 2nd | Woody aerial | 2 | Yes | Axillary | 5 | Adventitious roots | Liana |
| Blakea rosea (Ruiz & Pav.) D. Don | Soil | On Phorophyte | Decumbent | 2nd | Woody basal | 0 | Yes | Axillary | 4 | Roots | Hemiepiphyte |
| Blakea watsonii (Cogn.) Penneys & Almeda | Soil | On Phorophyte | Decumbent | 2nd | Woody basal | 0 | Yes | Axillary | 7 | Roots | Hemiepiphyte |
| Chaetolepis microphylla (Bonpl.) Miq. | Soil | Different substrates | Erect | 2nd | Woody basal | 0 | No | Terminal | 0.3 | - | Herb |
| Clidemia epiphytica (Triana) Cogn. | Soil | Soil | Prostrate | 2nd | Woody aerial | 0.5 | Yes | Axillary | 10 | Adventitious roots | Vine |
| Graffenrieda emarginata (Ruiz & Pav.) Triana | Soil | Soil | Erect | 2nd | Woody aerial | 2 | Yes | Terminal | 7 | - | Tree |
| Heterocentron subtriplinervium (Link & Otto) A. Braun & C.D. Bouché | Soil | Soil | Decumbent | 2nd | Woody aerial | 0.3 | Yes | Terminal | 1 | - | Herb |
| Meriania nobilis Triana | Soil | Soil | Erect | 2nd | Woody aerial | 3 | Yes | Terminal | 5 | - | Tree |
| Miconia jahnii Pittier | Soil | Soil | Erect | 2nd | Woody aerial | 1.5 | Yes | Terminal | 4 | - | Tree |
| Miconia salicifolia Naudin | Soil | Soil | Erect | 2nd | Woody aerial | 0.2 | Yes | Terminal | 3 | - | Shrub |
| Monochaetum multiflorum (Bonpl.) Naudin | Soil | Soil | Fickle stem supported | 2nd | Woody aerial | 2.5 | Yes | Terminal | 5 | - | Liana |
| Monolena primuliflora Hook. f. | Superficial | Different substrates | Decumbent | 1st | - | - | Yes | Axillary | 0.15 | Roots | Epiphyte |
| Tibouchina kingii Wurdack | Soil | Soil | Erect | 2nd | - | - | Yes | Terminal | 1 | - | Herb |
| Tibouchina lepidota (Bonpl.) Baill. | Soil | Soil | Erect | 2nd | Woody aerial | 1.5 | Yes | Terminal | 6 | - | Tree |
Trees/shrubs: characterized by germination and growth on the ground, the presence of stem and branches with secondary growth (Simpson, 2006), self-support and erect orientation (Font-Quer, 2001; Figs. 1A-B).
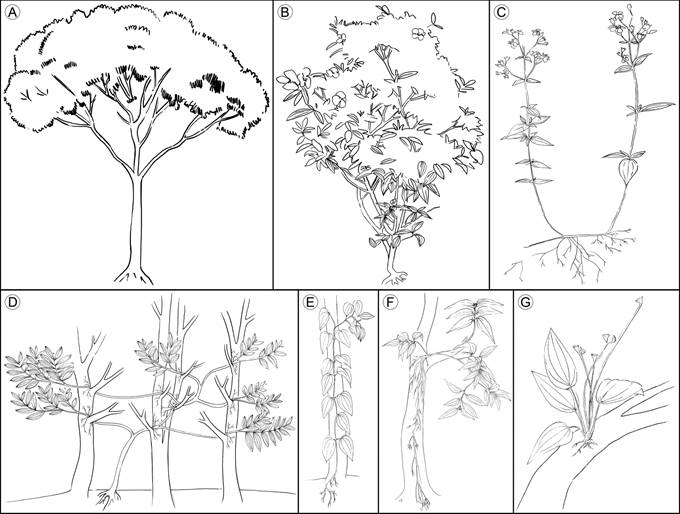
Figure 1 Growth habits of vascular plants compiled from the literature. A. trees; B. shrubs; C. herbs; D. lianas; E. vines; F. hemiepiphytes; G. epiphytes. Illustrations by Elizabeth Builes.
Herbs: characterized by the thin and brittle stem, mainly unbranched, with decumbent or prostrate orientation. If present, branches are basally inserted (Simpson, 2006), and, if they have an erect orientation, their height is less than two meters (Mendoza and Ramírez, 2006) (Fig. 1C). Herbs show the most variable features, making it a group which is hard to define (Eiten, 1991).
Lianas: exhibit a stem with initial erect orientation, when they are surrounded by abundant vegetation, they can lean or climb on a phorophyte or other object to ascend; some can develop adventitious roots on supported branches (Rowe and Speck, 2004). The growth strategy of this group is similar to that of vines, differing in the vertical orientation of the liana’s stem, their self-support in early stages of development, and their thicker stems (Rowe and Speck, 2004). The species of this group are also described as climbers, scandent shrubs, and creepers (Putz, 1984; Moffet, 2000) (Fig. 1D).
Vines: characterized by the prostrated orientation of stem, never self-supported. Early in life, the stem creeps until it finds vertical support to grow vertically, usually developing adventitious roots (Moffet, 2000; Rowe and Speck, 2004). In these plants, the principal stem is thin and flexible (Fig. 1E).
Hemiepiphytes: stem base above the ground, supported on a host plant, with roots reaching the ground (Moffet, 2000; Mendoza and Ramírez, 2006). The stem base is thicker than the rest of the stem and branches, acting as the root insertion point, basally prostrated and usually erect at the branch level. The rest of the stem is short and ramified. Commonly, species identified as hemiepiphytes can grow on the ground, with stem and branches vertically oriented like a shrub (Fig. 1F).
Epiphytes: roots and adventitious roots attach to the substrate without reaching the ground (Moffet, 2000; Mendoza and Ramírez, 2006), although some species can also grow on the ground (facultative epiphytes (Renner, 1993)) (Fig. 1G).
Statistical analyses
Group comparisons of quantitative characters (tangential diameter of solitary vessels, the number of vessels per mm2, and the vessel axial length) were performed with nested ANOVAs, one for each variable, with growth habit as an independent factor, and species within growth habit as the nested factor. When group differences were significant, the Tukey test was used for multiple comparisons of groups. Due to the remarkable differences in diameter and number of vessels of Chaetolepis microphylla (Bonpl.) Miq., we repeated the analyses excluding that species. Our results were almost identical after excluding that species; therefore, we show here the analyses without it. Statistical analyses were conducted using R (R Core Team, 2017).
Results
Anatomical descriptions
Trees/shrubs
Epidermis: Trichomes are present in Graffenrieda emarginata (Ruiz & Pav.) Triana, Miconia jahnii Pittier and Miconia salicifolia Naudin only. Dendritic hairs are showed by Graffenrieda emarginata, Miconia jahnii, and Miconia salicifolia; glands are present only in the two former species. Epidermis is absent in the fifth internode of Tibouchina lepidota (Bonpl.) Baill. and Miconia salicifolia, where it is replaced by periderm. Epidermis in Meriania nobilis Triana, Graffenrieda emarginata, and Miconia jahnii is single-layered and with epicuticular wax like granules (Figs. 2A-D).

Figure 2 Trees/shrubs: Epidermis. A. dendritic hair in Miconia jahnii Pittier; B. glandular hair in Graffenrieda emarginata (Ruiz & Pav.) Triana; C. epidermis and cortex in Meriania nobilis Triana; D. epidermis and cortex in Graffenrieda emarginata (Ruiz & Pav.) Triana; E. periderm and cortex in Miconia jahnii Pittier; F. lenticel in Tibouchina lepidota (Bonpl.) Baill. (Bra, stone cell; Col, angular collenchyma; Cut, cuticle; Epi, epidermis; Fed, phelloderm; Fel, phellogen; Sub, phellem).
Periderm: Periderm is absent in the fifth internode of Graffenrieda emarginata and Meriania nobilis. Scaly bark is only present in Tibouchina lepidota. Periderm in Tibouchina lepidota, Miconia jahnii and Miconia salicifolia is superficial, single-layered and the formation is complete around the stem. The phellem is one- to six-layered with cells in radial disposition without intercellular spaces; the cells have pits only in Tibouchina lepidota. The phellogen is simple and one-layered. The phelloderm is one- to three-layered. Lenticels only occur in Tibouchina lepidota periderm (Figs. 2E, F).
Cortex: The cortex is mainly formed by parenchymatous cells with isodiametric shape, intercellular spaces, diverse materials, and druse accumulation (except in Tibouchina lepidota and Miconia jahnii). Photosynthetic parenchyma only occurs in Meriania nobilis cortex. The collenchyma is of the angular type, forming a continuous multiple-layered tissue around the stem inside to epidermis/periderm in Meriania nobilis, Graffenrieda emarginata, and Miconia salicifolia. Stone cells with simple and ramiform pits are found in Meriania nobilis, Graffenrieda emarginata and Miconia jahnii. In Meriania nobilis and Graffenrieda emarginata, the stone cells form a continuous three-layered tissue around the endodermis. In Miconia jahnii the stone cells form clusters ranging from endodermis to periderm. Vascular bundles compound by just xylem are only seen in Tibouchina lepidota (Figs. 2C-E, 3).

Figure 3 Trees/shrubs: Cortex. A. transverse section of Graffenrieda emarginata (Ruiz & Pav.) Triana; B. cortex of Tibouchina lepidota (Bonpl.) Baill.; C. cortex of Meriania nobilis Triana. (Bra, stone cell; Col, angular collenchyma; Dru, druse; End, endodermis; HVC, cortical vascular bundle; Per, pericycle).
Endodermis and pericycle: The endodermis is only absent in Miconia salicifolia. In the rest of the species, the endodermis forms a continuous one- to three-layered tissue around the stem. Casparian strip is only present in Tibouchina lepidota and Miconia jahnii. The pericycle forms a continuous two- to the three-layered tissue between the endodermis and the phloem with cells in radial disposition (Figs. 4A-C).

Figure 4 Trees/shrubs: Endodermis, pericycle, phloem and vascular cambium. A. endodermis and pericycle of Miconia jahnii Pittier; B. endodermis, pericycle, and phloem of Tibouchina lepidota (Bonpl.) Baill.; C. phloem of Miconia salicifolia Naudin; D. phloem and cambium of Graffenrieda emarginata (Ruiz & Pav.) Triana; E. phloem in radial section of Tibouchina lepidota (Bonpl.) Baill. (Bra, stone cell; Cam, vascular cambium; Dru, druse; End, endodermis; FF, phloem fiber; PC, sieve plate; Per, pericycle).
Phloem: Sieve-tube elements are arranged in radial disposition in Meriania nobilis, Miconia jahnii and Miconia salicifolia, scattered in Tibouchina lepidota, and Graffenrieda emarginata. Sieve plates are transverse, except for Tibouchina lepidota, where they are inclined. Fibers are only present in Miconia jahnii and Miconia salicifolia; in the former, grouped in tangential bands; in the latter, forming clusters of six to eight cells located on the distal part of phloem. Axial parenchyma is composed of strands of short cells containing druses, except for Graffenrieda emarginata (Figs. 4B-E).
Vascular cambium: The vascular cambium is a one- to five-layered tissue without discontinuities, variations or irregularities; xylem and phloem form continuous tissues with constant width (Figs. 4B-D).
Xylem vessels: The vessel arrangement has radial to diagonal patterns and the grouping is in multiples of two to four with a variable proportion of solitary vessels. The outline of solitary vessels is rounded in Meriania nobilis and Miconia jahnii, and angular in Tibouchina lepidota, Graffenrieda emarginata, and Miconia salicifolia. Perforation plates are simple and inclined. Intervessel pits are rounded and bordered, have an opposite arrangement in Meriania nobilis and alternate in the other species (Fig. 5).

Figure 5 Trees/shrubs: Xylem. A. xylem of Miconia jahnii Pittier; B. xylem of Graffenrieda emarginata (Ruiz & Pav.) Triana; C. vessel of Meriania nobilis Triana in tangential section; D. protoxylem in radial section of Tibouchina lepidota (Bonpl.) Baill.; E. xylem in tangential section of Meriania nobilis Triana; F. xylem in tangential section of Miconia salicifolia Naudin; G. rays in radial section of Miconia salicifolia Naudin. (DF, fiber dimorphism; Rad, ray; ST, fiber transversal septum).
Xylem fibers: The fibers are of the libriform type and have transversal septa. The xylem has fiber dimorphism with two morphotypes of libriform fibers. The difference between morphotypes is in the lumen diameter and the thickness of the secondary wall. Dimorphic fibers are grouped in two to five layers formed by units that can range from short clusters to long tangential bands. The distance between dimorphic fibers is larger than the distance between rays (Figs. 5A, B, E, F).
Xylem rays: Rays are uniserial, but Tibouchina lepidota shows multiseriate portions. The cellular composition is upright and square cells. Vessel-ray pits are rounded, oval or scalariform and half-bordered, with border only in the vessel lumen (Figs. 5A, B, E-G).
Pith: The pith has internal primary phloem towards the periphery; in the center, it has parenchymatous cells with isodiametric shape and diverse materials and accumulation of druses. Vascular bundles scattered throughout the pith are concentric amphicribal of primary growth where the phloem is predominant in Miconia salicifolia, being the xylem very reduced in Graffenrieda emarginata and Miconia jahnii, and non-existent in Tibouchina lepidota and Meriania nobilis. The Miconia jahnii pith is formed largely by stone cells with a wide lumen and simple and ramiform pits. The piths of Graffenrieda emarginata are common idioblasts that are axially elongated and related to the phloem, distributed around and within the internal phloem and vascular bundles (Fig. 6).

Figure 6 Trees/shrubs: Pith. A. pith of Miconia salicifolia Naudin; B. pith of Graffenrieda emarginata (Ruiz & Pav.) Triana; C. stone cells in pith of Miconia jahnii Pittier. (Dru, druse; HVM, medullary vascular bundle; Idi; idioblast).
Herbs
Epidermis: The trichomes are pluricellular; simple unbranched in Heterocentron subtriplinervium (Link & Otto) A. Braun & C.D. Bouché and Arthrostemma ciliatum Pav. ex D. Don, dendritic in Tibouchina kingii Wurdack and Chaetolepis microphylla. Trichomes of Arthrostemma ciliatum are scarce and longer than in the other species. The epidermis is absent in the fifth internode of Tibouchina kingii. The epidermis is single-layered and has an epicuticular wax with the form of granules in Heterocentron subtriplinervium and Arthrostemma ciliatum, whereas in Chaetolepis microphylla the epicuticular wax consists of short and thin filaments in perpendicular direction to the epidermis (Figs. 7A-D).

Figure 7 Herbs: Epidermis. A. dendritic hairs of Tibouchina kingii Wurdack; B. unbranched hair and epidermis of Arthrostemma ciliatum Pav. ex D. Don; C. epidermis and cortex of Chaetolepis microphylla (Bonpl.) Miq.; D. epidermis of Arthrostemma ciliatum Pav. ex D. Don; E. periderm, endodermis, pericycle, and phloem of Tibouchina kingii Wurdack; F. periderm and cortex of Chaetolepis microphylla (Bonpl.) Miq. (Col, angular collenchyma; Cut, cuticle; Dru, druse; End, endodermis; Epi, epidermis; Fed, phelloderm; Fel, phellogen; Per, pericycle; Sub, phellem).
Periderm: Periderm is present only in Tibouchina kingii and Chaetolepis microphylla; in this latter species the transition where the periderm is forming below the epidermis is observed. Scaly bark is present in Tibouchina kingii only. The periderm is superficial, single-layered and the formation is complete around the stem. The phellem is two- to four-layered with cells in radial disposition. The phellogen is one-layered. The phelloderm is four- to six-layered with cells in radial disposition (Figs. 7E, F).
Cortex: The cortex is absent in the fifth internode of Tibouchina kingii, just below the phelloderm is the endodermis. In other species, the cortex is mainly formed by parenchymatous cells with isodiametric shape, with diverse materials and accumulation of druses in Heterocentron subtriplinervium and Arthrostemma ciliatum, and presence of chloroplasts in Arthrostemma ciliatum. The collenchyma is of the angular type, forming a multi-layered tissue below the epidermis in Heterocentron subtriplivernium and Chaetolepis microphylla, and in the middle of the cortex in Arthrostemma ciliatum. In Heterocentron subtriplinervium and Arthrostemma ciliatum, vascular bundles of primary growth are concentric amphicribral and those located in the stem angles are quadrangular (Figs. 7C-F, 8).

Figure 8 Herbs: Cortex. A. transverse section of Arthrostemma ciliatum Pav. ex D. Don; B. cortex and cortical vascular bundle of Heterocentron subtriplinervium (Link & Otto) A. Braun & C.D. Bouché; C. cortex, phloem, and vascular cambium of Chaetolepis microphylla (Bonpl.) Miq. (Cam, vascular cambium; Col, angular collenchyma; Dru, druse; End, endodermis; Epi, epidermis; Flo, phloem; HVC, cortical vascular bundle).
Endodermis and pericycle: The endodermis is below the cortex, it is one- to three-layered and the tangential walls have more contact area. The pericycle is a five-layered continuous tissue between the endodermis and phloem, only present in Heterocentron subtriplinervium (Figs. 8A, 9A,B).

Figure 9 Herbs: Endodermis, pericycle, phloem, and vascular cambium. A. transverse section of Arthrostemma ciliatum Pav. ex D. Don; B. transverse section of Heterocentron subtriplinervium (Link & Otto) A. Braun & C.D. Bouché; C. phloem in radial section of Tibouchina kingii Wurdack. (Cam, vascular cambium; End, endodermis; Flo, phloem; Per, pericycle; PC, sieve plate; Pro, procambium; Xil, xylem).
Phloem: Sieve-tube elements are scattered in Tibouchina kingii, Heterocentron subtriplinervium, and Chaetolepis microphylla, and in radial disposition in Arthrostemma ciliatum. Sieve plates are transverse, but inclined in Tibouchina kingii. Axial parenchyma is organized in fusiform cells in Heterocentron subtriplinervium and Chaetolepis microphylla, and in a strand of short cells containing druses in Tibouchina kingii (Figs. 7E, 8B, C, 9).
Vascular cambium (procambium in Arthrostemma ciliatum): The vascular cambium is not observed in the fifth internode of Arthrostemma ciliatum, the procambium is a one-layered and simple tissue, thus the phloem and xylem are primary. In the other species, the vascular cambium is a one- to four-layered tissue without discontinuities, variations or irregularities (Figs. 8B, C, 9A-C).
Xylem vessels: Vessel arrangement has a radial to diagonal pattern, mainly solitary vessels with a little proportion of radial multiples, more common in Heterocentron subtriplinervium. The outline of solitary vessels is rounded in T. kingii and Chaetolepis microphylla, the outline is angular in Heterocentron subtriplinervium. The perforation plates are simple and inclined. Intervessel pits are rounded and bordered and have an alternate arrangement (Figs. 10A-D).

Figure 10 Herbs: Xylem. A. xylem of Heterocentron subtriplinervium (Link & Otto) A. Braun & C.D. Bouché; B. xylem of Tibouchina kingii Wurdack; C. vessel in tangential section of Tibouchina kingii Wurdack; D. protoxylem in radial section of Heterocentron subtriplinervium (Link & Otto) A. Braun & C.D. Bouché; E. xylem fibers in radial section of Arthrostemma ciliatum Pav. ex D. Don; F. xylem in tangential section of Tibouchina kingii Wurdack; G. rays in radial section of Tibouchina kingii Wurdack. (DF, fiber dimorphism; PP, perforation plate; PS, fiber simple pit; Rad, ray; ST, fiber transversal septum).
Xylem fibers: The fibers are of the libriform type and have transversal septa only in Heterocentron subtriplinervium and Arthrostemma ciliatum. Fiber dimorphism only in Tibouchina kingii and Chaetolepis microphylla. Dimorphic fibers in Tibouchina kingii are grouped in four- to six-layered tangential bands and the distance between the bands is bigger than the distance between rays. In Chaetolepis microphylla the dimorphic fibers form one tangential band of five to seven layers (Figs. 10B, E).
Xylem rays: Rays uniserial, formed by upright and square cells. Vessel-ray pits are rounded to oval and half-bordered, with a border in the vessel lumen only (Figs. 10B, F, G).
Pith: The pith has internal primary phloem towards the periphery, which is continuous in Chaetolepis microphylla and discontinuous in other species. The parenchyma is formed by cells with isodiametric shape and diverse materials and accumulation of druses. In Chaetolepis microphylla the pith is reduced and without intercellular spaces between parenchymatous cells. Vascular bundles scattered throughout the pith are concentric amphicribral of primary growth where the phloem is predominant, being the xylem very reduced in Arthrostemma ciliatum and non-existent in Tibouchina kingii. In the pith of Chaetolepis microphylla the vascular bundles are absent (Fig. 11).
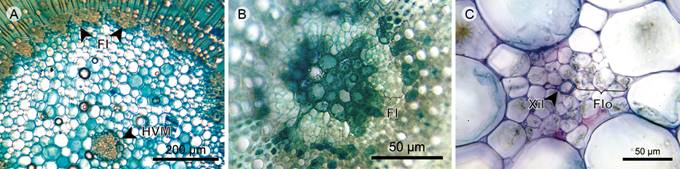
Figure 11 Herbs: Pith. A. pith of Tibouchina kingii Wurdack; B. pith of Chaetolepis microphylla (Bonpl.) Miq.; C. medullary vascular bundle of Arthrostemma ciliatum Pav. ex D. Don (FI, internal phloem; Flo, Phloem; HVM, medullary vascular bundle; Xil, xylem).
Lianas
Epidermis: Trichomes have a dendritic form in Monochaetum multiflorum (Bonpl.) Naudin and Blakea fasciculata Gleason, and are absent in Blakea quadrangularis. The epidermis is single-layered, the transition with the periderm is formed below the epidermis in Blakeaquadrangularis and Blakea fasciculata. The epidermis is absent in the fifth internode of Monochaetum multiflorum. The epicuticular wax has the shape of granules (Figs. 12A-D).

Figure 12 Lianas: Epidermis. A. dendritic hair of Monochaetum multiflorum (Bonpl.) Naudin; B. dendritic hair of Blakea fasciculata Gleason; C. epidermis and periderm beginning of Blakea quadrangularis Triana; D. epidermis, periderm and cortex of Blakea fasciculata Gleason; E. periderm, endodermis, and pericycle of Monochaetum multiflorum (Bonpl.) Naudin; F. periderm of Blakea quadrangularis Triana. (Bra, stone cell; Col, angular collenchyma; Cut, cuticle; Dru, druse; End, endodermis; Epi, epidermis; Fed, phelloderm; Fel, phellogen; Per, pericycle; Sub, phellem).
Periderm: Scaly bark is present in Monochaetum multiflorum only. The periderm is superficial and single-layered. The phellem is two- to ten-layered with cells in radial disposition, without intercellular spaces and with pits (except for Blakea fasciculata). The phellogen is one- to two-layered. The phelloderm is one- to three-layered with cells in radial disposition, and is absent in Blakea fasciculata (Figs. 12D-F, 13A, 14B).

Figure 13 Lianas: Cortex. A. transverse section of Blakea quadrangularis Triana; B. cortex of Blakea fasciculata Gleason. (Bra, stone cell; Col, angular collenchyma; Dru, druse; End, endodermis; Fed, Pheloderm; Fib, fiber; Par, parenchyma; Per, pericycle; Sub, phellem).

Figure 14 Lianas: Endodermis, pericycle, phloem, and vascular cambium. A. endodermis, pericycle, and phloem of Monochaetum multiflorum (Bonpl.) Naudin; B. transverse section of Blakea fasciculata Gleason; C. phloem in radial section of Blakea quadrangularis Triana. (Bra, stone cell; Cam, vascular cambium; Col, angular collenchyma; End, endodermis; FF, phloem fiber; PC, sieve plate; Per, pericycle; Perd, Periderm; PF, phloem parenchyma; Rad, ray).
Cortex: The cortex in the fifth internode of Monochaetum multiflorum is absent, the endodermis is just below the phelloderm. In other species, the cortex is mainly formed by parenchymatous cells with isodiametric shape, diverse materials, and accumulation of druses, with chloroplasts present only in Blakea fasciculata. Collenchyma is only present in Blakea fasciculata and of the angular type, forming a five- to six-layered continuous tissue below the phelloderm, the latter containing chloroplasts and druses. The stone cells form a four-layered discontinuous tissue in the center of cortex, with simple and ramiform pits (Figs. 12D, E; 13; 14A, B).
Endodermis and pericycle: The endodermis is a continuous tissue between the cortex and the phloem. In Monochaetum multiflorum the endodermis cells show a Casparian strip with unequal thickening of cell walls and reduced lumen. In Blakea quadrangularis and Blakea fasciculata the endodermis is subtle, formed by one layer of cells larger than the cortex parenchyma cells, with thin cellular walls and without cytoplasmic accumulations. The pericycle is one- to three-layered, only present in Monochaetum multiflorum and Blakea quadrangularis; in the latter the presence of solitary fibers with thick walls, reduced lumen and simple pits is common (Figs. 12E, 13A, 14A, B; 15A).

Figure 15 Liana: Xylem. A. transverse section of Blakea quadrangularis Triana; B. and C. two types of fiber dimorphism of Blakea quadrangularis Triana; D. xylem of Blakea fasciculata Gleason; E. vessels in radial section of Blakea fasciculata Gleason; F. protoxylem in radial section of Blakea fasciculata Gleason; G. xylem in tangential section of Blakea fasciculata Gleason; H. rays in radial section of Monochaetum multiflorum (Bonpl.) Naudin; I. xylem in tangential section of Blakea fasciculata Gleason. (AR, rays aggregates; Cam, vascular cambium; DF, fiber dimorphism; End, endodermis; FI, internal phloem; Flo, phloem; PA, axial parenchyma; Per, pericycle; PP, perforation plate; Rad, ray; ST, fiber transversal septum).
Phloem: Sieve-tube elements scattered, with simple and transverse sieve plates. Phloem fibers present only in Blakea fasciculata, where they are scattered, mainly solitary, or rarely in clusters. Axial parenchyma in a strand of short cells with accumulation of druses, scattered in Monochaetum multiflorum and Blakea quadrangularis, and grouped in clusters in Blakea fasciculata (Figs. 13B, 14, 15A).
Vascular cambium: In the same way as trees, shrubs, and herbs, liana species show a vascular cambium lacking discontinuities, variations or irregularities that may change the vascular system conformation. The vascular cambium of Blakea fasciculata, with eight layers of cells, is the thickest among the species studied here (Figs. 14, B, 15A).
Xylem vessels: The vessel arrangement has a radial to diagonal pattern, mainly solitary in Monochaetum multiflorum and Blakea fasciculata, and more common in multiples of two to three vessels in Blakea quadrangularis. The outline of solitary vessels is rounded in Monochaetum multiflorum and angular in Blakea quadrangularis and Blakea fasciculata. Perforation plates are simple and inclined. Intervessel pits are bordered, from scalariform to opposite rounded in Monochaetum multiflorum, and alternate in Blakea quadrangularis and Blakea fasciculata(Figs. 15A, D-F).
Xylem fibers: The fibers are libriform and have transversal septa. Dimorphic fibers grouped in two- to eight-layered tangential bands, with a wider lumen and thinner walls allowing intercellular spaces. Additionally, Blakea quadrangularis xylem shows a second type of dimorphic fiber, with a narrower lumen and thicker walls, grouped in two- to six-layered tangential bands (Figs. 15A-D, G).
Xylem axial parenchyma: The axial parenchyma is of the apotracheal type, grouped in three- to six-layered clusters close to vascular cambium. This tissue is absent in Monochaetum multiflorum (Fig. 15D).
Xylem rays: Rays are uniserial, but xylem of Blakea fasciculata also shows aggregates of two to four rays, which are formed by upright and square cells. Vessel-ray pits are rounded to oval and half-bordered, with border only in vessel lumen (Figs. 15G-I).
Pith: The periphery of the pith has internal primary phloem, forming a continuous band in Blakea quadrangularis and a discontinuous one in Monochaetum multiflorum and Blakea fasciculata. In the center, the pith is formed by parenchymatous cells with isodiametric shape, diverse materials, and accumulation of druses. Vascular bundles, which are scattered throughout the pith of primary growth, are concentric amphicribral in Monochaetum multiflorum and Blakea quadrangularis, and collateral in Blakea fasciculata. The pith of Blakea quadrangularis and Blakea fasciculata also shows fibers with a narrow lumen, thick walls, and simple pits; these fibers are located around the internal phloem and the vascular bundles (Fig. 16).

Figure 16 Liana: Pith. A. pith of Blakea quadrangularis Triana; B. pith of Blakea fasciculata Gleason; C. medullary vascular bundle of Monochaetum multiflorum (Bonpl.) Naudin. (Dru, druse; FI, internal phloem; Fib, fiber; Flo, phloem; HVM, medullary vascular bundle; Xil, xylem).
Vines
Epidermis: The trichomes in Clidemia epiphytica (Triana) Cogn. are simple unbranched, whereas they are simple unbranched and dendritic in Adelobotrys adscendens Triana. The epidermis is absent in the fifth internode of Blakea granatensis Naudin. The epidermis in other species is single-layered and has an epicuticular wax in form of granules (Figs. 17A, B).

Figure 17 Vines: Epidermis and periderm. A. unbranched hair of Clidemia epiphytica (Triana) Cogn.; B. epidermis and cortex of Adelobotrys adscendens Triana; C. periderm and cortex of Blakea granatensis Naudin. (Bra, stone cell; Col, angular collenchyma; Cut, cuticle; Epi, epidermis; Fed, phelloderm; Fel, phellogen; Sub, phellem).
Periderm: The periderm is absent in the fifth internode of Adelobotrys adscendens, whereas in Clidemia epiphytica the transition where the periderm is forming below the epidermis is observed. The periderm is single-layered, in Blakea granatensis it is a superficial and a continuous tissue, whereas in Clidemia epiphytica it is discontinuous. The phellem is one- to eight-layered with cells in radial disposition. The phellogen is one- to two-layered. The phelloderm is one- to five-layered, with cells in radial disposition only in Clidemia epiphytica (Figs. 17C, 18A, B).

Figure 18 Vines: Cortex. A. transverse section of Blakea granatensis Naudin; B. adventitious root of Adelobotrys adscendens Triana; C. cortical parenchyma of Clidemia epiphytica (Triana) Cogn. (Bra, stone cell; Col, angular collenchyma; End, endodermis; Flo, phloem, Perd, periderm; PA, axial parenchyma).
Cortex: The cortex is formed by parenchymatous cells with isodiametric shape, diverse materials, and accumulations of druses, with chloroplasts present in Adelobotrys adscendens. The collenchyma is of the angular type, forming a three- to four-layered continuous tissue below the epidermis in Adelobotrys adscendens. The stone cells form a four- to six-layered continuous tissue below the periderm in Blakea granatensis, and these have simple pits and tangential inner walls (in the direction of the pith) that are thicker than other walls (Figs. 18, 19A-C).

Figure 19 Vines: Endodermis, pericycle, phloem, and vascular cambium. A. transverse section of Blakea granatensis Naudin; B. transverse section of Adelobotrys adscendens Triana; C. phloem of Clidemia epiphytica (Triana) Cogn.; D. phloem in radial section of Clidemia epiphytica (Triana) Cogn. (Cam, vascular cambium; Dru, druse; End, endodermis; FF, phloem fiber; Fib, fiber; Flo, phloem; PA, axial parenchyma; Per, pericycle; PC, sieve plate; Rad, ray).
Endodermis and pericycle: The endodermis is a one- to two-layered continuous tissue; in Adelobotrys adscendens it is only formed by stone cells with simple and ramiform pits. The pericycle is a two- to three-layered continuous tissue; in Blakea granatensis it also consists of solitary fibers with simple pits and a narrow lumen. In Clidemia epiphytica the cells between the cortex and phloem have no variation or specialization, except that they are smaller than cortical parenchyma cells and have no cytoplasmic accumulations (Figs. 17C, 18, 19A-C).
Phloem: Sieve-tube elements are scattered in Blakea granatensis and Adelobotrys adscendens, and in clusters in Clidemia epiphytica. Sieve plates are simple and inclined, transverse in Clidemia epiphytica. Fibers are scattered in the distal part of phloem in Blakea granatensis, and form a four- to six-layered continuous tissue on the distal part of phloem in Clidemia epiphytica. Axial parenchyma is formed by a strand of short cells containing druses (Figs. 18A, 19).
Vascular cambium: The vascular cambium is a two- to four-layered tissue. In this group of species, the vascular cambium has a sinuous form that is more prominent than in other species (Figs. 18A, B, 19).
Xylem vessels: The vessel arrangement is radial to diagonal and the grouping is in multiples of two to four, with a variable proportion of solitary vessels. Vessel diameters in Blakea granatensis show a marked increase on the newly formed xylem. The outline of solitary vessels is angular. Perforation plates are simple and inclined. Intervessel pits are rounded, bordered and have an alternate arrangement (Fig. 20).

Figure 20 Vines: Xylem. A. Transverse section of Clidemia epiphytica (Triana) Cogn.; B. xylem of Adelobotrys adscendens Triana; C. xylem in radial section of Clidemia epiphytica (Triana) Cogn.; D. protoxylem in radial section of Blakea granatensis Naudin; E. xylem in tangential section of Adelobotrys adscendens Triana; F. axial parenchyma in tangential section of Blakea granatensis Naudin; G. rays in radial section of Clidemia epiphytica (Triana) Cogn. (Cam, vascular cambium; DF, fiber dimorphism; Dru, druse; FI, internal phloem; Flo, phloem; Med, pith; PP, perforation plate; PS, fiber simple pit; Rad, ray; ST, fiber transversal septum).
Xylem fibers: The fibers are of the libriform type and have transversal septa. The dimorphic fibers are grouped in two- to five-layered tangential bands and the distance between the bands is larger than the distance between the rays. Like Blakea quadrangularis, Adelobotrys adscendens shows two different types of dimorphic fibers (Figs. 20A-C).
Xylem axial parenchyma: Axial parenchyma is present in Blakea granatensis only, where it is the apotracheal type, grouped in four- to seven-layered tangential bands, more frequently close to the vascular cambium. The cells can be fusiform and short in their strand, showing an accumulation of druses (Figs. 18A, 19A, 20F).
Xylem rays: Rays uniserial, formed by upright and square cells. The vessel-ray pits are rounded to oval and half-bordered, with a border in the vessel lumen only (Figs. 20E-G).
Pith: The internal phloem is reduced in Adelobotrys adscendens and discontinuous in Blakea granatensis. The parenchyma cells have isodiametric shape and diverse materials and druses accumulation. Vascular bundles, which are scattered throughout the pith, are concentric amphicribal in Adelobotrys adscendens and Clidemia epiphytica (this one with a very reduced xylem), and just amphicribral in Blakeagranatensis. Blakea granatensis pith has fibers with a narrow lumen, thick walls, and simple pits; these fibers are found around the internal phloem and vascular bundles (Fig. 21).

Figure 21 Vines: Pith. A. pith of Adelobotrys adscendens Triana; B. pith of Clidemia epiphytica (Triana) Cogn.; C. medullary vascular bundle of Blakea granatensis Naudin. (Dru, druse; FI, internal phloem; Fib, fiber; Flo, phloem; HVM, medullary vascular bundle; Xil, xylem).
Hemiepiphytes
Epidermis: The epidermis is absent in the fifth internode of Blakea rosea (Ruiz & Pav.) D. Don. In Blakea watsonii (Cogn.) Penneys & Almeda, the epidermis is single-layered and shows small cells. The developing periderm is located just below the epidermis, and trichomes one absent (Fig. 22A).

Figure 22: Hemiepiphytes: Epidermis and periderm. A. transverse section of Blakea watsonii (Cogn.) Penneys & Almeda; B. periderm of Blakea rosea (Ruiz & Pav.) D. Don. (Dru, druse; Epi, epidermis; Fed, phelloderm; Fel, phellogen; PE, sclerotic parenchyma; Sub, phellem).
Periderm: The periderm is superficial, a single layer of continuous tissue. The phellem is one-layered in Blakea rosea, and two- to five-layered with scattered stone cells in Blakea watsonii. The phellogen is a one- to three-layered tissue of thin cells. The phelloderm cells have radial disposition; in Blakea watsonii it has one to two layers that accumulate druses; in Blakea rosea the number of layers varies from three to 15 layers, across the different regions of the transverse section (Figs. 22, 23).

Figure 23 Hemiepiphytes: Cortex. A. transverse section of Blakea rosea (Ruiz & Pav.) D. Don.; B. transverse section of B. watsonii (Cogn.) Penneys & Almeda (Cam, vascular cambium; Dru, druse; Epi, epidermis; FF, phloem fiber; Fib, fiber; PE, sclerotic parenchyma; Perd, periderm).
Cortex: The cortex is mainly formed by parenchymatous cells with isodiametric shape, druses are accumulated only in Blakea rosea in a layer below the phelloderm. Only in these two species, a sclerotic parenchyma forms a two- to four-layered continuous tissue below the phelloderm (Figs. 22, 23).
Endodermis: In these two species, the pericycle was not observed. The limit between the cortex and the phloem is only taken up by the endodermis, having two- to six-layered tissues with cells smaller than cortical parenchyma and without cytoplasmic accumulations. Scattered fibers with thick walls, narrow lumen and simple pits are present (Figs. 22A, 23).
Phloem: Sieve-tube elements have a radial disposition in Blakea rosea, and are scattered in Blakea watsonii. Sieve plates are simple and transverse. Fibers in Blakea rosea form a one- to four-layered continuous tissue close to the endodermis, in Blakea watsonii the presence is rare. Axial parenchyma is present in a strand of short cells, some containing druses, more commonly close to the endodermis (Figs. 23A, 24).
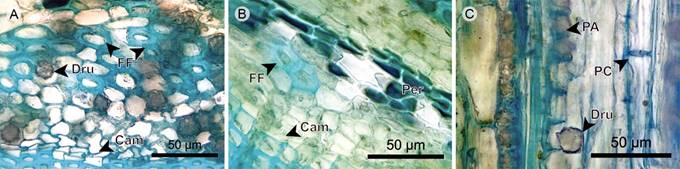
Figure 24 Hemiepiphyte: Endodermis, phloem, and vascular cambium. A. phloem of Blakea rosea (Ruiz & Pav.) D. Don.; B. phloem of Blakea watsonii (Cogn.) Penneys & Almeda; C. phloem in radial section of Blakea rosea (Ruiz & Pav.) D. Don. (Cam, vascular cambium; Dru, druse; FF, phloem fiber; PA, axial parenchyma; PC, sieve plate).
Vascular cambium: The vascular cambium is a one- to three-layered continuous and uniform tissue of small cells that have walls with irregular shape (Fig. 24A, B).
Xylem vessels: The arrangement of vessels has a radial to diagonal pattern and the grouping is in multiples of two to three, or more commonly solitary. The outline of solitary vessels is angular. Perforation plates are simple and inclined. Intervessel pits are rounded, bordered and have an alternate arrangement (Figs. 25A-D).

Figure 25 Hemiepiphyte: Xylem. A. xylem of Blakea watsonii (Cogn.) Penneys & Almeda; B. xylem of Blakea rosea (Ruiz & Pav.) D. Don.; C. xylem in tangential section of Blakea watsonii (Cogn.) Penneys & Almeda; D. protoxylem in radial section of Blakea watsonii Cogn.; E. xylem in radial section of Blakea rosea (Ruiz & Pav.) D. Don.; F. xylem in tangential section of Blakea watsonii (Cogn.) Penneys & Almeda (CP, prismatic crystal; DF, fiber dimorphism; PA, axial parenchyma; PP, perforation plate; PS, fiber simple pit; Rad, ray; ST, fiber transversal septum).
Xylem fibers: The fibers are of the libriform type and have transversal septa. Blakea watsonii xylem shows just one type of dimorphic fibers, and Blakea rosea show two, in the same way that occurs in Blakea quadrangularis and Adelobotrys adscendens, respectively. The tangential bands of dimorphic fibers have two to three layers and the distance between bands is larger than the distance between rays (Figs. 25A-C, E).
Xylem axial parenchyma: The axial parenchyma is of the apotracheal type and formed by strands of short cells. In the xylem of Blakea watsonii, the axial parenchyma forms a tangential five-layered band of cells that contain druses and have irregular and wide lumens. In Blakea rosea the axial parenchyma cells are scarce, isolated, and containing prismatic crystals (Figs. 25A, E).
Xylem rays: The rays are uniserial, formed by upright and square cells. Vessel-ray pits are rounded to oval and half-bordered, with border only in the vessel lumen (Figs. 25E, F).
Pith: The pith periphery incorporates a continuous tissue of internal phloem. In the center, it is formed by parenchymatous cells that show isodiametric shape and accumulation of druses. Vascular bundles are concentric and amphicribral, with primary growth, and are scattered throughout the pith. Fibers show a narrow lumen, thick walls, and simple pits, and are located around the internal phloem and the vascular bundles (Fig. 26).

Figure 26 Hemiepiphytes: Pith. A. pith of Blakea rosea (Ruiz & Pav.) D. Don.; B. pith of Blakea watsonii (Cogn.) Penneys & Almeda (Fib, fiber; HVM, medullary vascular bundles).
Epiphytes
This group only contains Monolena primuliflora Hook. f. The stem is composed by a wide periderm that gives it external support. Below the periderm, the stem is almost exclusively formed by parenchyma and a few narrow vessels from the adventitious roots. Therefore, for this species, it was not possible to delimit a cortex, vascular system, or pith in the inner area (Fig. 27A).

Figure 27 Epiphytes: Transverse section of Monolena primuliflora Hook. f. A. periderm; B. adventitious root; C. and D. xylem in longitudinal section; E. xylem in transverse section. (Dru, druse; Fed, phelloderm; Fel, phellogen; Sub, phellem; Vas, vessel; Xil, xylem).
Periderm: The periderm is superficial and single-layered with lenticels. The phellem is five- to eight-layered with cells in radial disposition. Phellogen is one-layered with thin cells. The phelloderm is eight to 12-layered with cells containing druses, without radial disposition (Figs. 27B, C).
Parenchyma: Composed by large isodiametric cells that show little accumulation of material and lack intercellular spaces. In this inner tissue, there is neither growth direction, nor determined cellular arrangement (e.g. an endodermis). In the parenchyma, except for the xylem, no other cellular type or tissue is present (Figs. 27B-F).
Xylem: The xylem has primary growth and is formed by few narrow and short vessels. These vessels show helical thickenings that sometimes are reticulated, and reach from the adventitious roots base to the center of the stem and along the periphery below the periderm. The vessels split along their trajectory (Figs. 27C-F).
Summary of anatomical differences among growth habits
The stem anatomy characters that distinguished Melastomataceae growth habits are summarized here:
A higher proportion of vessels in radial multiples than single ones among trees/shrub (Figs. 5A, B) and vine species (Fig. 20A).
A higher proportion of single vessels than grouped ones among herbs (Figs. 10A, B), lianas (Fig. 15A-D), and hemiepiphytes (Figs. 25A, B).
Xylem with shorter vessels and without rays (except for Tibouchina kingii) among herb species (Fig. 10).
A higher density of xylem vessels and vascular cambium in bands with sinuous form among vine species (Figs. 19A-C; 20A-C, F).
More rows in bands of dimorphic xylem fibers and adventitious roots among liana (Figs. 15A-D) and vine species (Figs. 18B, 20A, B).
Discontinuous intraxylary phloem among herb (Figs. 8A, 10A, 11A) and vine species (Figs. 18B, 20A, 21A).
Sclerotic parenchyma acting like support elements in the cortex of hemiepiphyte species (Figs. 22, 23).
Angular collenchyma acting like support elements in the cortex of species without periderm (trees: Meriania nobilis, Graffenrieda emarginata, Figs. 2C, D, 3A-C; herbs: Arthrostemma ciliatum, Heterocentron subtriplinervium,Figs. 8A, 9B; vines: Adelobotrys adscendens, Figs. 17B, 18B, 19B).
Quantitative differences among growth habits
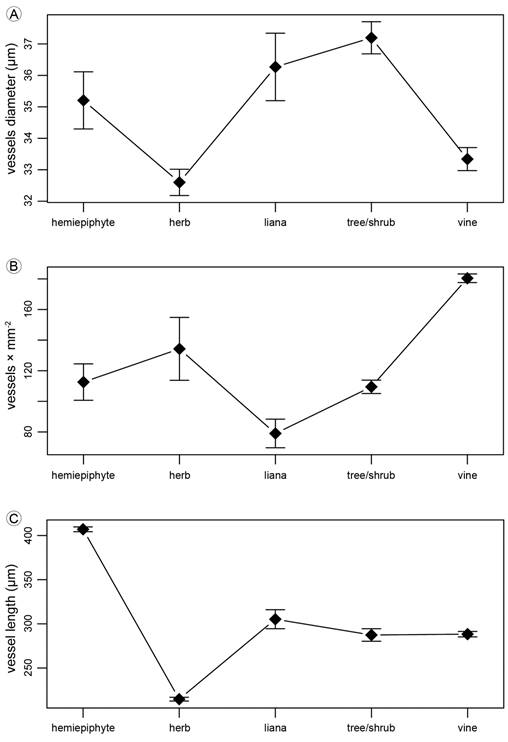
Growth habits differed in the tangential diameter of solitary vessels (p<0.001; tree/shrub>herbs=vines; Fig. 28A), the number of vessels per mm2 varied among growth habits (p<0.001; vines>hemiepiphyte=tree/shrub=herb>liana; Fig. 28B) and the vessel axial length (p<0.001; hemiepiphyte>three/shrub=liana=vine>herb; Fig. 28C). Species was a significant factor in the three ANOVAs (p<0.001).
Discussion
Botany practitioners continuously describe specimens using a range of growth habit terms, and yet the application of these can sometimes obscure rather than clarify the portrayal of a specimen. If growth habits are a natural depiction of a morphological pattern in plants, it can be safely assumed that anatomy is one of the character types that can be used to support classification of any group of plants into different growth habits. We described here 18 species of Melastomataceae using 125 anatomical characters of their stems. These plants were classified using external morphology into an inicial classification of growth habits that we compiled from the literature. This preliminary classification was not very different from others previously proposed and we are not suggesting that it is better than any other; it was rather a tool for the further analysis. Our main finding was that stem anatomy did not support all the growth habits that we started with. Some stem anatomy characters were observed in species of some growth habits, but there were very few characteristics exclusively shared by species of one particular growth habit. However, the species analyzed here could be grouped in fewer growth habits, which we hereafter describe and discuss.
Based on the anatomical study of 18 species of Melastomataceae, we recognize only three growth habits: trees/shrubs, herbaceous species, and climbers. Trees and shrubs grow their entire life cycle rooted in the soil, with erect orientation, self-supporting their weight with stems that have secondary growth (Font-Quer, 2001; Simpson, 2006). Most species of Melastomataceae belong to this group. One source of confusion about this growth habit in general is its inherent gradient of size, from suffrutex (a tall herb with woody stem base; Mendoza and Ramírez, 2006), to shrubs (in range from 2 to 5 m of height), to treelets (“small stature trees”; Opler et al., 1980), to trees growing up to 40 m high, like some Tessmannianthus Markgr. species. We observed in Melastomataceae that the stems of plants with sizes across this gradient share a structural design and cannot be distinguished using stem anatomy. We suggest that species that in the initial classification were labeled as herbs (Tibouchina kingii, Arthrostemma ciliatum, Chaetolepis microphylla, and Heterocentron subtriplinervium) and hemiepiphytes (Blakea rosea and Blakea watsonii) should be considered members of the trees/shrubs growth habit. These species not only showed an anatomy that was not distinguishable from trees/shrubs, they also develop secondary xylem, a characteristic absent from aerial stems of herbs (Eiten 1991; Font-Quer 2001). Therefore, among the 18 species studied here, only Monolena primuliflora should be catalogued as a herb because its stem does not develop organized inner tissues such as cortex, vascular system, pith, or secondary xylem.
We found that hemiepiphytes cannot be distinguished from trees/shrubs using stem anatomy characters. Hemiepiphytic species can be seen as shrubs accidentally sprouting and growing on a phorophyte. The definition of epiphytes and hemiepiphytes depends mainly on habitat (position above ground), and not on their habit (Simpson, 2006). It is confusing that the aspect of the same plant can be described with one term if it reached the ground and with another if it did not. The inconsistency of this classification is clear in cases when an “epiphyte” is found growing on the ground (“facultative epiphytism”; Renner, 1993) or when a shrub is found thriving on the axial concavity of a tree branch (“accidental epiphytism” (Zotz, 2005)).
Climbers are plants that require holding on a phorophyte to grow vertically. Lianas are climbers that can grow early in life in a self-supported manner, whereas vines always need the support of phorophytes to ascend (Rowe and Speck, 2004; Isnard et al., 2012). Vines are thin and flexible climbers, showing a xylem with more area occupied by vessels and fewer fibers (Ter Welle and Koek-Noorman, 1981; Araque et al., 2000; Fig. 28B). On the other hand, lianas reach altitude through surrounding vegetation, but they can grow some distance self-supported as if they were shrubs. In comparison to vines, lianas show thicker stems, cortical support tissues with more layers, and a xylem occupied by more fibers and fewer vessels. However, these differences in their stem anatomy are not so pronounced as to allow a clear separation of these two habits in Melastomataceae. Instead, climber plants show a gradient of robustness, from thin vines as Clidemia epiphytica to the woody lianas of the genus Blakea P. Browne; a thicker stem diameter increases stem stiffness in climbers (Isnard et al., 2012).
There is more than one way to be a climber. We found here that the characteristics shared only by the climber species of Melastomataceae were the presence of adventitious roots and more rows in bands of dimorphic xylem fibers. Adventitious roots are a typical character of climbing plants (Putz, 1984). The dimorphic xylem fibers bands have a variable composition of ordinary libriform fibers, dimorphic fibers (with a thinner wall, wider lumen, and different cell length), fusiform parenchyma cells (axially extended) and parenchyma strands (two-eight cells high; Ter Welle and Koek-Noorman 1978). This band occurs in about half of the genera of Neotropical Melastomataceae (where it is called pseudoparenchyma; Ter Welle and Koek-Noorman, 1981) and in a large proportion of Paleotropical Melastomataceae (where it is called deviating fibers; Vliet, 1981). The presence of more rows in the bands of dimorphic fibers, an increment in axial parenchyma, is related to an increased flexibility characteristic of climber stems (Koek-Noorman et al., 1979; Ter Welle and Koek-Noorman, 1981; Vliet, 1981; Carlquist, 1985).
Other stem characteristics that have been reported as typical for climbers are the presence of auxiliary elements that prevent embolism (vessel dimorphism, narrow vessels, tracheids and vasicentric tracheids) and elements that allow mechanical flexibility (anomalous growth, cambial variants, paratracheal parenchyma, and wide rays; Carlquist, 1985; Angyalossy et al., 2011, 2014; Isnard et al., 2012; Quijano-Abril et al., 2013). However, climbers studied here did not share such traits. The diversity of functional strategies to climb has been recognized before by multiple authors (Putz 1984; Moffet, 2000; Rowe and Speck, 2004; Simpson, 2006). Climbers can have tendrils, which can be adhesive, they can twine with their main stem or with some branches, or they can sprawl leaning on rocks or trees without attaching to them (Putz, 1984). All these different morphological adaptations to climb could be translated into growth habit names, but that would be an unnecessary proliferation of terms. For the concept of growth habits to be useful it should be avoided to elevate specific functional morphology adaptations to the generalized category of growth habit.
Our study has two caveats that should be addressed in future analyses. Our definition of growth habits in Melastomataceae could change if more characters are used to compare species from apparently different growth habits. That is, a more accurate distinction of growth habits could be obtained with the use of additional characters that describe plant morphology, architecture, or biomechanics (Rowe and Speck, 2004; Isnard et al., 2012). The second issue is that we did not take into account the underlying evolutionary hierarchy when comparing the species of this study. Some characteristics have a phylogenetic component that we did not address here. The species of Blakea have characteristic longer vessels (Figs. 15E, F, I, 20F, 25C, F), tangential bands exclusively composed by apotracheal parenchyma (Figs. 15A, D, 18A, 25A, E, F), prismatic crystals and druses in xylem parenchyma (Figs. 20F, 25E), and presence of fibers with very lignified walls and reduced lumen being part of pericycle, surrounding the intraxylary phloem and medullary vascular bundles (Figs. 13A, 14B, 15A, 18A, 19A, 21C, 23, 26). The species of the tribe Melastomeae (Tibouchina lepidota, Tibouchina kingii, Arthrostemma ciliatum, Heterocentron subtriplinervium, Chaetolepis microphylla and Monochaetum multiflorum) have cortical vascular bundles (Figs. 3B, 8A, B) and thin vascular cambium with few rows of little cells (Figs. 3B, 4B, 8A, C, 9A, B, 10A, 14A). The species of Tibouchina (and Monochaetum multiflorum) have scaly bark and have a very simplified cortex or even lack it (Figs. 3B, 7E, 12E). The species of the Merianieae tribe (Meriania nobilis, Graffenrieda emarginata, and Adelobotrys adscendens) show angular collenchyma (Figs. 2C, D, 3A, C, 17B, 18B, 19B) and sclereids (Figs. 3A, 18B, 19B) that act like support elements in their cortex. The species of Blakea and Miconia Ruiz & Pav. (and Clidemia epiphytica) have fibers in their phloem (Figs. 4C, 13A, 14B, 15A, 18A, 19A, C, 20A, 23, 24A, B). These characters may have taxonomic value (Ter Welle and Koek-Noorman, 1978; Koek-Noorman et al., 1979; Ter Welle and Koek-Noorman, 1981; Vliet, 1981; Vliet et al., 1981), to identify some lineages of the family, independently of their growth habit.
Author contributions: JP and MQA contributed to the conception of this work. JP performed fieldwork and anatomical descriptions. JP, MT, and MQA made analyses and wrote the manuscript. All authors contributed to the discussion, review, and approval of the final manuscript.
Financing: This work was supported by the Faculty of Engineering and the Management of Research Universidad Católica de Oriente, Colombia.











 nueva página del texto (beta)
nueva página del texto (beta)