Introduction
The name Humaria (Fr.) Boud. was first used by Fries (1822) in the rank of tribe of his broad genus Peziza Dill. ex Fr. Cooke (1879), following Fries´ definition, used the rank of subgenus; then Boudier (1885) erected it at the rank of genus. Clements and Shear (1931) selected Humaria leucoloma (Hedw.) Boud. as lectotype of this genus. With such a typification Humaria becomes an obligate synonym of Octospora Hedw. Fuckel (1870) used the name Humaria with his own definition, encompassing several species of the series Lachnea defined by Fries (1822) or the tribe Lachnea (Fr.) Boud. Its type has been designated by Denison (1959) with Humaria hemisphaerica (F.H. Wigg.) Fuckel (Peziza hemisphaerica F.H. Wigg.) and must be followed. Humaria became the type of family Humariaceae proposed by Velenovský (1934), then accepted by subsequent authors such Le Gal (1947), Dennis (1960), Moser (1963), Berthet (1964), Rifai (1968), until Eckblad (1968) emended the family Pyronemataceae to widen its definition, including Humariaceae. This has been followed by Korf (1972) and subsequent authors. The modern classification based on molecular phylogeny confirmed the position of Humaria inside the family Pyronemataceae inside its own linage (Perry et al., 2007; Hansen et al., 2013; Van Vooren et al., 2021). Fuckel (1870) characterized this genus as having terrestrial ascomata, cupuliform apothecia when young, discoid when mature, gregarious, sessile, with tomentose hairs; asci cylindrical, elongated, containing 8 spores; ascospores oval to oblong-oval, containing 1-3 guttules, hyaline; filiform paraphyses. Among the 512 names listed in the Index Fungorum (2022) database under Humaria as genus, many of them are now combined in other genera, but 66 names are “accepted” in this repository, although many of them are old names, hard to interpret in a modern sense. Humaria represents an ectomycorrhizal genus of fungi, associated with different deciduous trees like Quercus spp., Fagus sylvatica L., Tilia cordata Mill. (Tedersoo et al., 2006; Erős-Honti et al., 2008), Carya spp. (Rudawska et al., 2018), as well as conifers like Pinus spp. (Tedersoo et al., 2006). On the American continent, only two species have been described: H. cazaresii (M.E. Sm. & Trappe) M.E. Sm., Healy & P. Alvarado and H. hemisphaerica. In Mexico, H. hemisphaerica has been cited from Durango, Guerrero, Hidalgo, Jalisco, Mexico City, Mexico State, Michoacán, Morelos, Oaxaca, Sonora and Tamaulipas, frequently in Pinus-Quercus and Quercus forests, montane cloud forests, and in coniferous forests (Chacón and Guzmán, 1983; Frutis and Guzmán, 1983; Bautista et al., 1986; Díaz-Barriga et al., 1988; Heredia, 1989; Pompa-González and Cifuentes, 1991; Esqueda et al., 1992; García Jiménez and Guevara Guerrero, 2005; García Jiménez and Valenzuela, 2005; Villarruel-Ordaz and Cifuentes, 2007; Raymundo et al., 2012, 2013; Gándara et al., 2014; García et al., 2014; Rodríguez-Alcántar et al., 2018, 2019). However, only Bautista et al. (1986) and Ortega López (2015) have given a detailed description. The present study aims to describe a new Humaria species in oak forest from Tamaulipas, Mexico based on morphological, ecological and molecular data.
Materials and Methods
Study material
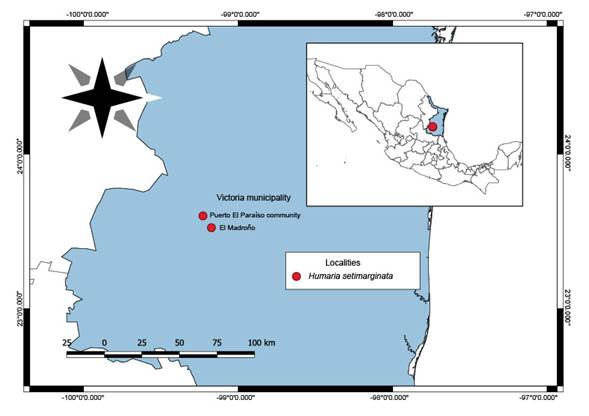
Specimens were collected in 2019 in the Victoria municipality, Tamaulipas, located in the Sierra Madre Oriental (Fig. 1). The specimens were deposited in the José Castillo Tovar Mycological Herbarium of the Instituto Tecnológico de Ciudad Victoria (ITCV) and the Escuela Nacional de Ciencias Biológicas Herbarium (ENCB) of the Instituto Politécnico Nacional.
Morphological analyses
The collected material was examined following the traditional techniques proposed by Cifuentes et al. (1986). The specimens were described when fresh, macroscopic characters, like size, shape, color, and possible hosts were recorded. The terminology used herein refers to the Nuevo Diccionario Ilustrado de Micología (Ulloa and Hanlin, 2006). Colors are indicated according to the color table of Kornerup and Wanscher (1978). Longitudinal sections of dried apothecia were rehydrated with 70% alcohol, observed in 5% KOH, and in water. Ornamentation of ascospores, and other structures were observed using Melzer´s staining reagent, as well as cotton blue in lactophenol.
The microscopic structures were observed using an optical microscope (OM) (Axiostar Plus, Zeiss, Jena, Germany). Photographs were taken with a Rebel T-1i camera and a 100 mm macro lens (Canon, Tokyo, Japan). Scanning electron microscopy (SEM; SU1510, Hitachi High Technologies, Tokyo, Japan) was used to observe the ornamentation of the ascospores in detail. The works of Dennis (1981), Fuckel (1870) and Beug et al. (2014) were used to differentiate the species.
DNA extraction, amplification and sequencing
Total DNA was extracted from dried herbarium specimens using a modified version of the protocol of Martínez-González et al. (2017) protocol. PCR amplification, based on Mullis and Faloona (1987), included 35 cycles with an annealing temperature of 54 ºC, and was carried out with the ITS5 and ITS4 primers (White et al., 1990; Gardes and Bruns, 1993) for the ITS nrDNA region, and LR0R and LR5 primers (Vilgalys and Hester, 1990; Cubeta et al., 1991) for the 28S nrDNA region (LSU).
PCR products were verified by agarose gel electrophoresis. The gels were run for 1 h at 95 V cm⁻³ in 1.5% agarose and 1× TAE buffer (Tris Acetate-EDTA). The gel was stained with GelRed (Biotium, USA) and the bands were visualized in an Infinity 3000 transilluminator (Vilber Lourmat, Germany).
The amplified products were purified with the ExoSAP Purification kit (Affymetrix, USA), following the manufacturer’s instructions. They were quantified and prepared for the sequence reaction using a BigDye Terminator v. 3.1 (Applied Biosystems, USA). These products were sequenced in both directions with an Applied Biosystem model 3730XL (Applied BioSystems, USA), at the Instituto de Biología of the Universidad Nacional Autónoma de México (UNAM). The obtained sequences were compared with the original chromatograms to detect and correct possible reading errors.
Phylogenetic analysis
To explore the phylogenetic relationship of the new species, an alignment was made based on the taxonomic sampling employed by Erős-Honti et al. (2008), Healy et al. (2022) and sequences deposited in the Gen Bank NCBI database (GenBank, 2022).
Each gene region was independently aligned using the online version of MAFFT v. 7 (Katoh et al., 2002, 2017; Katoh and Standley, 2013). Alignments were reviewed in PhyDE v. 10.0 (Müller et al., 2005), followed by minor manual adjustments to ensure character homology between taxa.
The matrices consisted of 31 taxa for ITS (700 characters) and 18 taxa for LSU (962 characters) (table 1). The aligned matrices were concatenated into a single matrix (34 taxa, 1662 characters). Phylogenetic inferences were estimated with Maximum Likelihood in RAxML v. 8.2.10 (Stamatakis, 2014) with a GTR + G model of nucleotide substitution. To assess branch support, 1000 bootstrap replicates were run with the GTRGAMMA model. For Bayesian posterior probability, the best evolutionary model for alignment was sought using PartitionFinder v. 2.0 (Lanfear et al., 2014; 2016; Frandsen et al., 2015).
Table 1: GenBank, 2022 accession numbers corresponding to the sequences used in the phylogenetic analyses. The accession numbers of the new species are in bold.
| Species name | Voucher Number | GenBank Accession | ||
| ITS | LSU | |||
| Genea gardneri Gilkey | SOC 690 | AY830857 | ----- | |
| Genea gardneri Gilkey | src831 | DQ206850 | ----- | |
| Genea gardneri Gilkey | src867 | DQ206851 | ----- | |
| Genea harknessii Gilkey | Trappe 13313 | DQ220334 | ----- | |
| Genea harknessii Gilkey | Trappe 11775 | DQ220335 | ----- | |
| Genea verrucosa Vittad. | AH44208 | KJ938935 | ----- | |
| Genea verrucosa Vittad. | BP104856 | KJ938936 | ----- | |
| Humaria cazaresii (M.E. Sm. & Trappe) M.E. Sm., Healy & P. Alvarado | Trappe18044 | DQ206863 | ---- | |
| Humaria hemisphaerica (F.H. Wigg.) Fuckel | Andy 10/15/03 | ----- | AY789389 | |
| Humaria hemisphaerica (F.H. Wigg.) Fuckel | BAP 320 (FH) | ----- | DQ220352 | |
| Humaria hemisphaerica (F.H. Wigg.) Fuckel | HKAS 82077 | MG871304 | MG871339 | |
| Humaria hemisphaerica (F.H. Wigg.) Fuckel | FHKH03100 | DQ200832 | DQ220353 | |
| Humaria hemisphaerica (F.H. Wigg.) Fuckel | JMP0104 | EU819470 | ----- | |
| Humaria hemisphaerica (F.H. Wigg.) Fuckel | K (M) 187356 | MZ159485 | ----- | |
| Humaria hemisphaerica (F.H. Wigg.) Fuckel | GO-2009-385 | KC152113 | ----- | |
| Humaria setimarginata Sánchez-Flores, Raymundo, Van Vooren & García-Jiménez | Type ITCV | OP521892 | OP529804 | |
| Humaria sp. | FLAS-F-61555 | MG019762 | MG019797 | |
| Humaria sp. | FLAS-F-61554 | MG019761 | MG019796 | |
| Humaria sp. | LY NV2012.10.16 | MG019765 | MG019800 | |
| Humaria sp. | LY NV2014.08.35 | MG019768 | ----- | |
| Humaria sp. | LY NV2014.07.19 | MG019767 | ----- | |
| Humaria sp. | LY NV2012.10.16 | MG019765 | MG019800 | |
| Humaria sp. | ISC 443646 | MG019764 | MG019799 | |
| Humaria sp. | ISC 437685 | MG019763 | MG019798 | |
| Humaria sp. | FLAS F-61548 | MG019759 | MG019794 | |
| Humaria sp. | FLAS F-61555 | MG019762 | MG019797 | |
| Humaria sp. | FLAS F-61554 | MG019761 | MG019796 | |
| Humaria sp. | FLAS F-61552 | MG019760 | MG019795 | |
| Humaria sp. | FH 823753 | MG019757 | MG019792 | |
| Humaria sp. | FH 00304592 | MT505219 | MT505178 | |
| Humaria sp. | FLAS F-66262 | MN653025 | MZ018863 | |
| Humaria sp. | GEN 4 | OP177902 | ----- | |
| Humaria sp. | FLAS-F-68407 | OM672766 | ----- | |
Phylogenetic analyses were also performed using MrBayes v. 3.2.6 x64 (Huelsenbeck and Ronquist, 2001). The information block for the matrix included two simultaneous runs, four Montecarlo chains, temperature set to 0.2 and sampling 10 million generations (standard deviation ≤0.1) with trees sampled every 1000 generations. The first 25% of samples were discarded as burn-in, and stationarity was checked in Tracer v. 1 (Rambaut et al., 2014). The two simultaneous Bayesian runs continued until the convergence parameters were met, and the standard deviation fell below 0.0001 after 10 million generations. No significant changes in tree topology trace or cumulative split frequencies of selected nodes were observed after about 0.37 million generations, so the first 2,500,000 sampled trees (25%) were discarded as burn-in. Trees were visualized and optimized in FigTree v. 1.4.4 (Rambaut et al., 2014).
Results
Molecular analyses
We successfully amplified and sequenced the ITS and LSU region from the holotype of our Humaria collection. Both the Bayesian and Maximum Likelihood analyses (Fig. 2) recovered Humaria setimarginata, supporting the existence of a new taxon distinctive from related species of Humaria (1 Bayesian Posterior Probability (PP) and 100% bootstrap values (BP) for Maximum Likelihood). Thus, Humaria setimarginata Sánchez-Flores, Raymundo, Van Vooren & García-Jiménez is proposed as a new species for science (see Taxonomy).
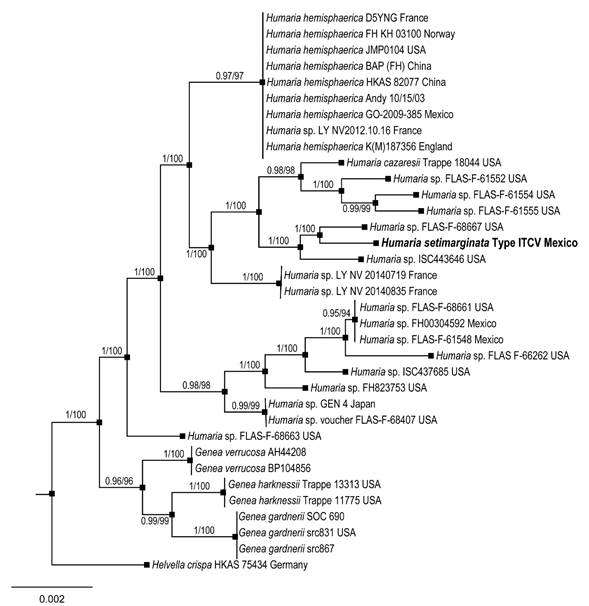
Figure 2: Phylogram of Bayesian inference (BI) tree from the ITS and LSU sequence data of 34 specimens. The values above branches represent Bayesian posterior probabilities (PP) and bootstrap values (LP) for Maximum Likelihood, respectively. The scale bar represents the expected number of nucleotide substitutions per site. Sequences obtained from this study are in bold.
Taxonomy
Ascomycota
Pezizomycetes
Pezizales
Pyronemataceae
Humaria setimarginata Sánchez-Flores, Raymundo, Van Vooren & García-Jiménez, sp. nov. Figs. 3, 4, 5, 6, 7. MycoBank no. MB 841440.

Figure 3: Humaria setimarginata Sánchez-Flores, Raymundo, Van Vooren & García-Jiménez. A., B. apothecia; C. longitudinal section of the apothecium.
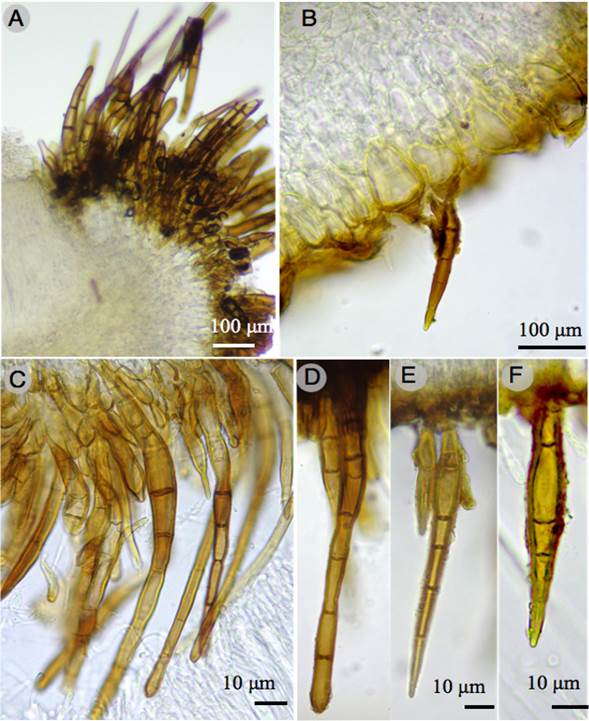
Figure 4: Humaria setimarginata Sánchez-Flores, Raymundo, Van Vooren & García-Jiménez. A-F. hairs of the apothecium.
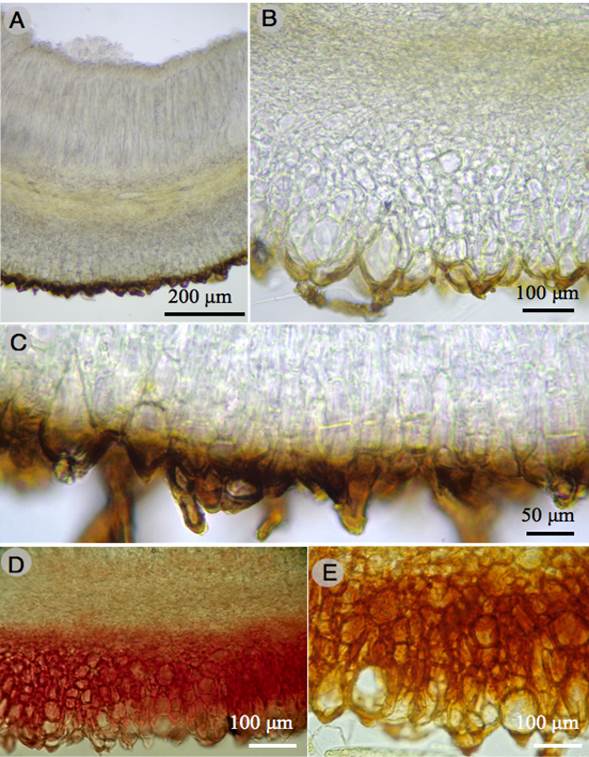
Figure 5: Humaria setimarginata Sánchez-Flores, Raymundo, Van Vooren & García-Jiménez. A. longitudinal section of the apothecium; B., C. ectal excipulum; D., E. dextrinoid reaction in Melzer´s reagent of the ectal excipulum.
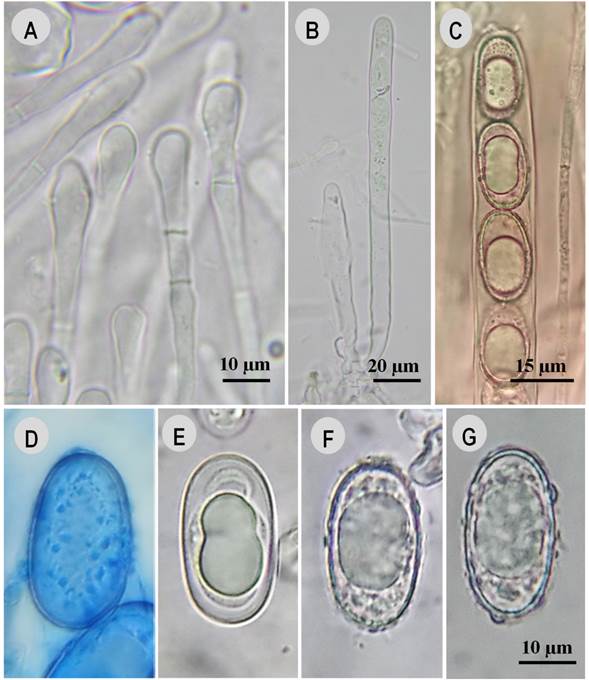
Figure 6: Humaria setimarginata Sánchez-Flores, Raymundo, Van Vooren & García-Jiménez. A. paraphyses; B. asci; C. asci and ascospores; D. ascospore in cotton blue; E. ascospore in 5% KOH, ornamentation having dissolved; F., G. ascospores in water.

Figure 7: Humaria setimarginata Sánchez-Flores, Raymundo, Van Vooren & García-Jiménez. A., B. ascospores with SEM.
TYPE: MEXICO. Tamaulipas, Victoria municipality, El Madroño, 1443 m a.s.l., 23°36'16.32"N, 99°13'45.2"W, 11.XI.2019, M. Sánchez 1889 (holotype: ITCV!, isotype: ENCB!).
Humaria setimarginata is characterized by the size, 60-353 × 13-20 µm, and position of marginal hairs, by a dextrinoid reaction in the ectal excipulum with Melzer´s reagent, and ascospores 19-25 × 10-15 µm, ellipsoid to oblong-ellipsoid.
Apothecial ascomata solitary or gregarious, 10-15 mm diameter, sessile, deeply cupulate, with a greyish white (1B1) hymenium when fresh, light orange (5A4) when dry, outer surface dark brown (6F8), covered with scattered short hairs, margin entire, with dense dark brown hairs; marginal hairs, 60-353 × 13-20 µm, wall 2-5 µm thick, with 1-14 septa, brown, superficial, having a single napiform base, with slightly rounded apex; excipular hairs similar, scattered, with a sharp apex; ectal excipulum 138-225 µm thick, of textura angularis, composed of angular to subglobose cells, 15-55 × 10-30 µm, walls of cells brown or hyaline, tissues turning greyish ruby (12C7) to reddish in Melzer´s reagent (dextrinoid reaction); medullary excipulum 80-130 µm thick, of textura intricata, made of hyphae 4-8 µm diameter, hyaline, without reaction in Melzer´s reagent; paraphyses hyaline, septate, filiform, 5-7 µm diameter at the apex, widened, some widened or having a bulge in the top cell; asci 217-237 × 12-15 µm, cylindrical, with croziers, hyaline, containing 8 uniseriate spores, inamyloid; ascospores 19-25 × 10-15 µm (X=21.7 × 12.5 µm, n=60), Q=1.5-2, Qm=1.7, ellipsoid to oblong-ellipsoid, hyaline, ornamented with cyanophilous warts, 1 µm high (side view), disappearing with 5% KOH.
Habitat: on soil, under Quercus rysophylla Weath. and Q. polymorpha Schltdl. & Cham.
Distribution: only known from the type locality.
Etymology: from Latin seta, meaning “hair”, and margo, meaning “edge, margin”, referring to the abundant hairs in the margin of the apothecium.
Additional material examined: MEXICO. Tamaulipas, Victoria municipality, Puerto El Paraíso community, 1650 m a.s.l., 23°31'38.99"N, 99°12'20.04"W, 01.XI.2019, M. Sánchez 1847 (ITCV), 1852 (ITCV).
Notes: Humaria setimarginata is characterized by its abundant, short marginal hairs, contrary to H. hemisphaerica which has longer hairs (sometimes reaching more than 1000 µm, see table 2), mostly concentrated on the edge of the margin. This species also differs from H. hemisphaerica by the dextrinoid reaction of the ectal excipulum with Melzer's reagent and by slightly smaller ascospores (24 × 14 µm). It also differs from H. cazaresii, which is a hypogeous species which has smaller ascospores 18-21 × (12)14-16 µm (Smith et al., 2006). Finally, H. setimarginata is considered to be associated with Q. rysophylla and Q. polymorpha.
Table 2: Comparison of species of Humaria (Fr.) Boud, in the American continent with species of the Humaria hemisphaerica (F.H. Wigg.) Fuckel complex.
| Species | Distribution | Apothecia | Hymenium | Marginal hairs | Asci | Ascospores | Paraphyses | Reference |
| H. cazaresii (M.E. Sm. & Trappe) M.E. Sm., Healy & P. Alvarado | USA | 10 mm diameter, hypogeous | No data | Without hairs | 170-220 × 10-13 µm | 18-21 × (12) 14- 16 µm | 2.5- 5 µm | Smith et al., 2006 |
| H. hemisphaerica (F.H. Wigg.) Fuckel | England | 30 mm diameter | White or whitish | 500-1000 × 20 µm | 350 × 20 µm | 20-24 × 10-12 µm | 7-8 µm | Dennis, 1981 |
| H. hemisphaerica (F.H. Wigg.) Fuckel | Europe and USA | 20-30 mm diameter, | White or whitish | 400-500 × 15-20 µm | 325 × 15-18 µm | 25-27 × 12-15 µm | 7-8 µm | Seaver, 1928 |
| H. hemisphaerica (F.H. Wigg.) Fuckel | France | 6-30 mm diameter | Dull white to pale grey | 120-1300 ×17-28 µm | 205-270 × 16-25 µm | 20-24 × 11.5-14 µm | 5-9 µm | Van Vooren 2014 |
| H. hemisphaerica (F.H. Wigg.) Fuckel | Germany | No data | White | No data | 168 × 16 µm | 24 × 14 µm | No data | Fuckel 1870 |
| Humaria hemisphaerica (F.H. Wigg.) Fuckel | Mexico | 10-30 mm diameter | Whitish | 770-850 × 20-22 µm | 250-300 × 13.2- 16.5 µm | 19.8-20.9 × 11- 12.1 µm | 5.5-7.7 µm | Bautista et al., 1986 |
| Humaria hemisphaerica (F.H. Wigg.) Fuckel | Mexico | 15-30 mm diameter | Whitish | 400-830 × 13-19 µm | 190-230 × 10-13 µm | 13-19 × 6-8 µm | 3-4 µm | Ortega-López (2015) |
| H. hemisphaerica (F.H. Wigg.) Fuckel | Turkey | 10-30 mm diameter, 5-15 mm high | Whitish or greyish | No data | No data | 22-27 × 12-15 µm | No data | Sesli, (1998) |
| H. hemisphaerica (F.H. Wigg.) Fuckel | USA | 10-30 mm diameter | Whitish to pale grey | No data | 230-270 (350) × 19-23 µm | 22-27 × 10-13 µm | No data | Beug et al. (2014) |
| H. setimarginata Sánchez-Flores, Raymundo, Van Vooren & García- Jiménez | Mexico | 10-15 mm diameter | greyish white | 60-353 × 13-20 µm | 217-237 × 12-14 µm | 19-25 × 10-15 µm | 5-7 µm | This study |
Discussion
Humaria setimarginata sp. nov. only known from the type specimen in Quercus forests in Mexico, is proposed based on the combination of morphological, ecological, and molecular characters. One of the main characters that differentiates this species is the dextrinoid reaction of the ectal excipulum in Melzer’s reagent, a feature that has not been seen in other specimens or reported in current descriptions of H. hemisphaerica or any other species of Humaria. In addition, H. hemisphaerica differs from H. setimarginata in the size of ascospores and the size of the marginal hairs (see table 2). One of the problems in differentiating H. hemisphaerica is the wide range of macro and microscopic characters that have been provided in the literature. Dennis (1981) described the latter with slightly narrower ascospores 20-24 × 10-12 µm, wider asci 350 × 20 µm and wider paraphyses 7-8 µm. Fuckel (1870) gives ascospores measuring 24 × 14 µm. Sesli (1998) described larger ascospores, 22-27 × 12-15 µm. Van Vooren (2014) indicated 20-24(-25) × (11-)11.5-14 µm for ascospore dimensions. In Mexico, Bautista et al. (1986) described this species as having longer hairs, 770-850 × 20-22 µm, and smaller and narrower ascospores 19-21 × 11-12 µm. Ortega López (2015), in his master's thesis, described it with larger and narrower hairs, 400-830 × 10-13 µm, and with smaller and narrower ascospores, 13-19 × 6-8 µm.
All these variations in spore size and hair dimensions suggest the existence of several potential distinct species named under H. hemisphaerica. Our phylogeny (Fig. 2) shows that such a diversity exists in the genus Humaria. A detailed revision of the H. hemisphaerica species complex is required, including type study, but this is beyond the aim of our article.
Reports of Humaria species in Mexico are scarce, as are their descriptions; this is probably due to the morphological similarity of the collections made in the country. However, the macro and microscopic differences could cause them to be classified as different taxa. Considering that Humaria is an ectomychorrizal genus (Tedersoo et al., 2006; Erős-Honti et al., 2008) and the country has a high diversity of ecosystems, being the home of 161 species of Quercus (Valencia-A, 2004) and 50 of Pinus (Gernandt and Pérez-de la Rosa, 2014; Pérez-de la Rosa and Gernandt, 2017), we believe that a comprehensive review of the genus is needed. It is imperative that morphological and phylogenetic studies of other collections identified as Humaria hemisphaerica be carried out, as well as their ecology.











 nueva página del texto (beta)
nueva página del texto (beta)