Introduction
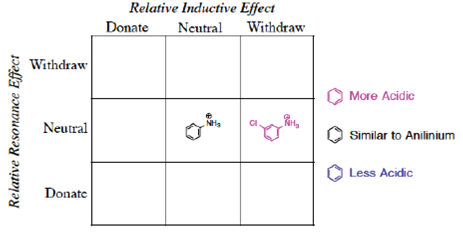
In a previous paper (Lamoureux & Arias-Álvarez, 2020), we showed the utility of using grids to organize the reactivity of electrophilic aromatic substitution and to separate the inductive and resonance effects in quadrants (Figure 1). These effects are also important in the determination of acid/base chemistry, especially in the case of aromatic acids and bases. For example, there is a reasonable relationship between the rates of electrophilic aromatic substitution of Ph-X and the pKa of the corresponding X-substituted benzoic acids (Rossi, 2013).
This organizational tool is just one part of the teaching process for undergraduate chemistry. As Walters (Walters, 1994) indicated, the learning process, as well as the scientific method (Quack, 2014), proceeds through a four-step refinement:
Data: a series of facts, such as one may acquire on the Internet
Information: organized data in a way to help solve problems
Knowledge: organized information that is internalized and, hopefully, permanent
Wisdom: fully integrated knowledge that is useful
This process in the twenty first century begs the question: which level do we want the students to achieve? Walters (Walters, 1994) lamented that undergraduate students in chemistry focus on only level 1) and 2). However, with infinite data soon to be available 24/7 to everyone through Internet sources, the focus should be on the organization and retention, levels 2) and 3). Wisdom, level 4), might require too much time and effort to expect undergraduates to master.
In this article, we provide a logical presentation of substituted aromatic systems to clarify the reactivity. Students can use this organization to take known data about the acidity of an aromatic compound, create a framework of knowledge, and use this knowledge to predict an unknown acid. We report first on a new process to induce students to the level of knowledge, level 3), using our grids as part of the process to organize information.
New system for inducing knowledge
We propose a new system for the acquisition of knowledge via a three-stage procedure: Search-Organize-Predict (SOP). In this system, the undergraduate students are presented with a problem to solve that requires the Search of known data (from reliable sources), the Organization of these data in useful form and ultimately the use of these organized information to Predict unknown or hard-to-find data. We should be providing students the skills that professionals in science jobs require: working with reliable data, organizing the information according to the problem to solve, and often using back-of-the-envelope calculations as a prediction (Coso et al., 2014).
The Search for data has become difficult by the total volume available. Unfortunately, most students are more familiar with Wikipedia (Méndez & Cerdá, 2016) or Google (Shultz & Zemke, 2019), which are less reliable as sources of data than the scientific literature. The first step in the undergraduate’s science training should be a reappraisal of their information literacy skills (Shorish & Reisner, 2016).
Once the information from reliable sources is obtained, the next step is to Organize the information to provide a framework of understanding or knowledge. One of the best pedagogical tools is a ‘graphic organizer’, a visual aid to relate information in an easily remembered format (Dye, 2000). These organizers have also proven useful for problem solving and prediction (Drăghicescu, et al., 2014).
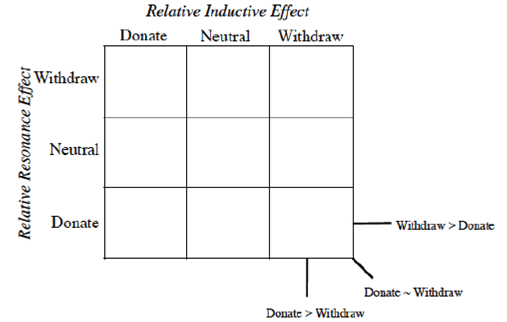
More graphic organizers are necessary in undergraduate chemistry to provide successful learning strategies. One of the most useful types of organizer for the sciences is the ‘frame or grid’ which presents information in columns and rows (Trowbridge & Wandersee, 2005). Filling in a two-dimensional grid provides spatial separation of concepts and is better than a list to memorize. These grids also show where information is lacking or limited, if the students cannot complete the grid (Trowbridge & Wandersee, 2005). The Resonance/Induction grid introduced in a previous paper (Lamoureux & Arias-Álvarez, 2020) is a new type of these grids for organic chemistry.
Finally, after organizing the Information, one of the best ways to show knowledge is the Prediction of the solution to a new problem. Repeating known problems, such as those given in a textbook, may allow students to solve the same problems without understanding the concepts behind the problems (Zheng & Campbell, 2018).
The SOP system can be used in any class (flipped or lecture), is student centered active learning and, once mastered, may be used to evaluate progress during the semester or on written evaluations, such as a final exam.
Simplification of acid/base chemistry
Even though acid/base chemistry is one of the most basic reactions taught in General and Organic Chemistry, the reaction fundamentals are surprising complex. One aspect that is incongruous to the textbook presentation is that the thermodynamic acidity in water, on which the pKa scale is based, depends mostly on entropic effects in solution (Allen & Wright, 1964), not the intrinsic enthalpic effects that are shown in this article. The interaction of solvation, steric effects, the three-dimensional orientation of all groups, concentration and temperature on both the experimental (Wilson, Gore, Sawbridge, & Cardenas-Cruz, 1967) and calculational (Huang, Liu, Liu, Liu, & Liu, 2011) absolute ionization constants of substituted benzoic acids shows this complexity. Also, the basicity of aromatic amines depends on both the enthalpic and entropic effects in the aniline substrate and the conjugate acid, anilinium ion (Pankratov, Uchaeva, Doronin, & Chernova, 2001), as every thermodynamic process depends. Are all these details necessary to show students how to use structures to predict relative acidity or basicity?
The use of only electronic effects on the substrate as a guide to acidity or basicity is a gross simplification that still works for teaching undergraduates today. The use of these effects “proved remarkably successful, and which is still adequate, 50 years later, for most purposes of the practicing organic chemist…the naive electronic theory survives as an effective guide because very large substituent effects persist, even if much attenuated, in solution. (Edward, 1982)” We strongly recommend this simplification in teaching undergraduates because it falls within the “90% rule” (Schultz, 2010): most (>90%) of acidity data can be understood using these electronic effects and the exceptions and further details can be dealt with in another, more advanced, course if the student is interested. It is important to indicate that, whereas the textbook may deal with these exceptions, we do not test students on data outside the standard cases.
There also seems to arise a lore in organic chemistry circles that to predict relative acidity among a series of acids one must analyze the stability of their conjugate bases, and then use the reciprocal strength relationship between acids and bases. This generalization does not hold in all cases. In reality, the acidity depends on all the structural effects in the initial (acid) and final (conjugate base) states; in practice it is more common (>90% of the time) to look at the electronic effects in the structure of the acid (or conjugate acid) substrates for relative acidity. After all, values of pKa (measured for the acidic substrate) provide evidence of the relative experimental acidity (Rossi, 2013). In this article, we will only focus on the inductive and resonance effects in the acid and show how it can be extrapolated to compare the relative base strength.
Effect of substituents on reactivity of benzoic acids
There are seven electronic effects that affect the reactivity of substituted acids (Stock, 1972). Most of these are ‘pillars’ of organic chemistry and are considered essential in the teaching of structure/property relations (Mullins, 2008).These effects are listed as:
Electronegativity (of atom or group directly connected to acidic or basic site)
Polarizability (of atom or group directly connected to acidic or basic site)
Geometry (bond angles around atom connected to acidic or basic site) (Lamoureux & Ogilvie, 2019a; Lamoureux & Ogilvie, 2019b)
Delocalization (conjugated pi system in substrate or conjugate base)
Aromaticity (presence of aromaticity or anti-aromaticity in substrate or conjugate base)
Inductive effects (donation, withdrawal, or neutral effects of atom or group connected via sigma system to acidic or basic site)
Resonance effects (donation, withdrawal, or neutral effects of atom or group connected via pi system to acidic or basic site)
Whereas most of these effects are shown in Introductory Chemistry, effects 5) to 7) are usually not seen by undergraduate students until the chapters on aromaticity in the Organic Chemistry textbooks. This delay in the presentation of fundamental principles of reactivity also delays the ability to apply these effects and limits the knowledge of the students. Our recommendation is that all these effects should be mentioned, with examples, as soon as concept of how the acidity/basicity of compounds are related to structure is discussed.
Aromatic carboxylic acids
The origin of the Hammett constants began with the study of how the structure of substituents affect the acidity of benzoic acids in water at 25 °C (Equation 1):
The substituent constant, σx, measures the electronic effects of a substituent X (in the meta- or para-position) relative to hydrogen H and is, in principle, independent of the nature of the reaction. K a (X) is the ionization constant of the substituted benzoic acid and K a (H) that of benzoic acid itself. Inductive (F) and resonance effects (R) are quantified and collected in databases according to the substrate. For substituted benzoic acids, we will use in this article the σp values, and the corresponding F and R parameters according to Hansch, Leo and Taft (Hansch, et al., 1991).
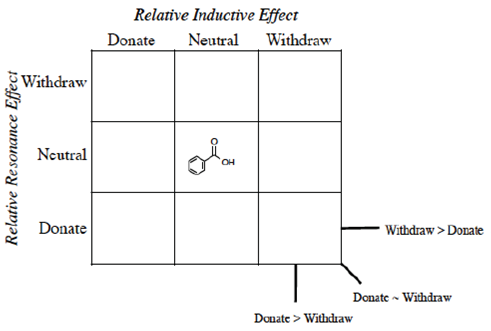
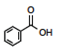
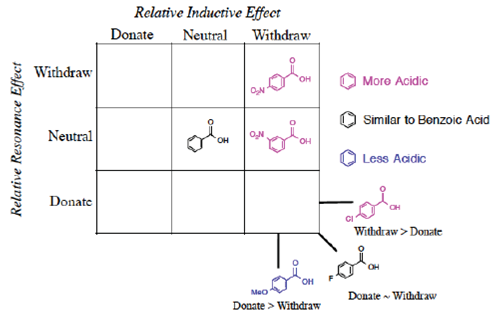
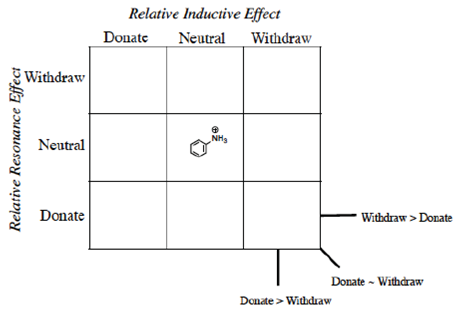
Instead of Ka values indicated in the Hammett equation, most tabulated data provide pKa values for substituted benzoic acids. One such tabulation is provided in the CRC Handbook of Chemistry and Physics (Lide, Ed., 2006), long considered a reliable resource for data. The pKa of unsubstituted benzoic acid of 4.20 is generally considered the standard. It should be emphasized that this number is not the ‘correct’ value (Is there such a thing as a ‘correct’ or ‘true’ value in chemistry?) but rather the consensus experimental number determined under specific conditions (25 °C, dilute solution in water, precision limited) and published under constant review. As every other substituted benzoic acid will be measured according to this standard, we place this molecule in the center of the 3 x 3 grid (Figure 2) grid and will organize the other substituents in relation to this standard.
If students are provided with a list of substituted benzoic acids, one ability expected of them is to Search for the corresponding pKa values using the name or Lewis structure of the compound. They should be able to prepare a chart such as Table 1 (structure vs. pKa) using the reliable values.
They should also observe that some of the pKa values are higher (less acidic), some values are lower (more acidic) and some values can be very similar to the standard (benzoic acid, pKa = 4.20). How to organize these substituents to provide conceptual knowledge of the structure/reactivity relationship?
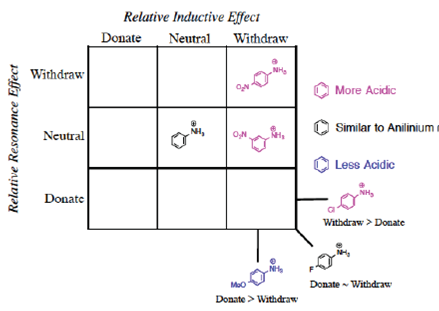
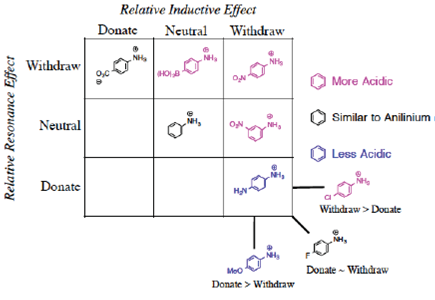
We propose once the grid system (Figure 3) is used, the Organization is obvious.
The first realization from this organizational grid is that the acidity relates to the position in the grid (Figure 4).
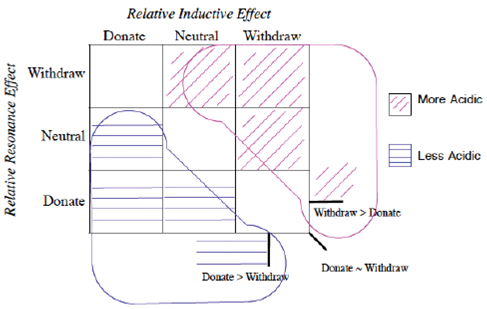
Figure 4 Visual separation of more acidic/less acidic related to induction/resonance position in grid.
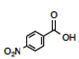
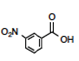
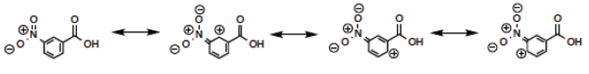
It might not be clear why the-meta-nitro compound was placed (Figure 3) in the square of [Neutral by resonance and Withdrawal by induction] if it is known that the nitro group is a group [Withdrawing by resonance and induction] in the electrophilic aromatic substitution grid. Indeed, for the para-nitrobenzoic acid, both withdrawing effects are present. However, in the situation of meta-substitution, the relative ability of the nitro substituent to extract by resonance is limited because there is no direct resonance interaction between the nitro and carboxyl groups, usually rationalized by showing the extended Lewis structures of the resonance forms (Figure 5), yet the inductive effect continues through the sigma framework. Thus, a general rule can be formed: if the substituent is in the meta-position, the relative resonance effect is neutral, without need to draw the complete resonance structures for each substituent. This supposition is supported by the quantitative data for the nitro substituent (Hansch et al., 1991): σ p = +0.78, σ m = +0.71, F = 0.65, and R = +0.13. The electron-density withdrawal from the para-position is only slightly more than in the meta-position, thus the values of pKa for the para- and meta-compounds are similar.
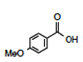
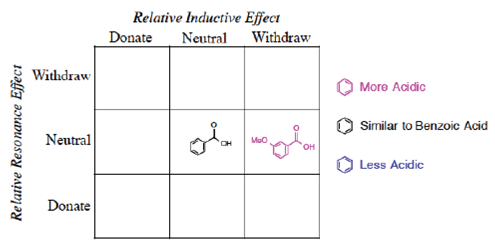
Now let us apply the Prediction phase of the process. If a new molecule is provided, with a substituent less common or with an unknown pKa, can we use organized data (Information) to create Knowledge? Note it is not necessary to provide an absolute numerical prediction but rather a relative prediction of pKa. One test case is m-methoxybenzoic acid, Figure 6.
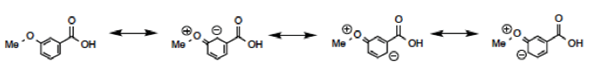
Using our organization in a grid, we can easily apply the general rules and place the molecule (Figure 7) in the right-hand side of the chart [Neutral by resonance and Withdrawal by induction]. Furthermore, the position directly relates to a prediction of a pKa less than benzoic acid. This result may be surprising to some students who memorize “Resonance effects always win” (Grossman, 2003) and remember that the methoxy group donates by resonance. Resonance does not always win. It is possible to write a complete set of Lewis resonance structures (Figure 8) that show no delocalized contact between the two functional groups, yet it would be advantageous to predict the acidity without resorting to this long process. A complete search of the databases shows that m-methoxybenzoic acid has a pKa of 4.10 (less than 4.20), as predicted.
Continuing the Prediction process, let us apply the grid system to another test case, o-methoxybenzoic acid (Figure 9).
In this case, we predict the [Donation by resonance, Withdrawal by induction, Donate > Withdraw] effects should provide an acid in the lower right-hand corner (Figure 10). This prediction would lead one to assign the o-isomer with a pKa greater than benzoic acid.
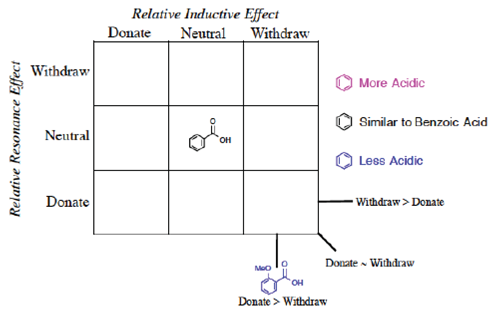
Figure 10 (Incorrect) Predicted placement of o-methoxybenzoic acid in grid relative to benzoic acid.
However, this prediction of acidity would be wrong, even if the electronic effects are correctly predicted. The experimental pKa, 4.08, is less than benzoic acid and the other isomeric methoxybenzoic acids. When simplistic predictions and experimental results disagree, this discrepancy shows a problem with our models. Further research is necessary and, in this case the experimental results show (Exner, Fiedler, Buděšínský, & Kulhánek, 1999) a strong steric effect between the methoxy- and the carboxyl-group that cannot be predicted using our simple electronic model. The grid cannot and should not be used for all ortho-substituted isomers because we cannot simplify the interactions into a two-effect system. It is worthwhile to teach students that organic chemistry is complex, with many interacting effects; the examples in the textbook are usually simplified versions of real-world examples. In the professional world, our initial predictions may not be valid. Chemistry is still an experimental science that requires the Search of reliable data and the Organization of this data to Predict results, but in the end the experiment decides.
Aromatic bases
The basicity of the nitrogen lone pair in substituted anilines is determined by the substituents connected to the aromatic ring (vide supra). This basicity is in inverse proportion to the acidity of the conjugate acid, the anilinium ion. “This concept is derived from the reciprocal strength relationship that exists for a conjugate acid−base pair according to Brønsted−Lowry theory; that is, the stronger the conjugate acid, the weaker the conjugate base and vice versa (Rossi, 2013)”. We will separate the effects in the substituted anilinium ion into inductive and resonance effects. The unsubstituted standard, as in the case of benzoic acid, is placed in the center of the grid (Figure 11).
The most extensive quantitative data available for substituted anilines are not the values for basicity but rather the values of pKa of the acids. The anilinium ion has an experimental pKa of 4.87 (Table 2). Other pKa values for anilinium ions can be found in the CRC Handbook (Lide, Ed., 2006).
Using these structures of the substituted anilinium ions, one can then organize each into the following induction/resonance assignments (Figure 12). This grid presents the least acidic in the lower left and the most acidic in the upper right. Hence, in a reciprocal relation, the most basic aniline corresponds to the structure of the conjugate base of the lower left and the least basic aniline to the upper right.
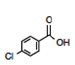
Proceeding to the Predict stage of the process, how should one classify m-chloroaniline as a base (Figure 13)? Remember the general rule that in the meta-position, the resonance effects are neutral.
The first step is to form the conjugate acid and determine the electronic effects present in the structure. An experienced student would place the structure of m-chloroanilinium in the [Withdraw by Induction, Neutral by Resonance] position (Figure 14). This prediction can be confirmed by appraisal of the pKa value for the corresponding chloroanilinium ion, 3.52. To respond to the question as presented, the m-chloroaniline would be a weaker base than aniline. The overall effect of the meta-chloro would be to withdraw electron density from the basic site and reduce the basicity relative to aniline.
We finish this discussion with another prediction exercise. The test molecule in this case is cyclohexylamine (Figure 15), which to the untrained eye appears similar to aniline. There are no substituents connected to the ring, and naively there should not be any substituent effects.
In the grid system, the cyclohexylammonium ion might be placed in the same position as the anilinium ion (Figure 16). However, this molecule cannot and should not be compared in the simple Inductive/Resonance framework. This grid system only works for aromatic systems that relay the inductive and resonance effects throughout the ring, not for aliphatic systems. Moreover, there are electronic effects beyond only Induction/Resonance that might be important for the determination of acidity.
Cyclohexylamine is a much stronger base (pKa of cyclohexylammonium ion = 10.64) than aniline. This value is not surprising because the structure of cyclohexylamine has completely different delocalization, aromaticity, and geometry effects from aniline. One must observe in the substrate structure the sum of all the electronic effects present. With practice, students can accept the limitations of the grid system while using it efficiently for the prediction of almost all substituted analogs.
In trying to complete the grid (Figure 17), we cannot find examples to include in the three blank spaces shown. The number of substituted anilines known is limited; most of the anilines with known values of pKa contain substituents that are clearly withdrawing or a combination of [Withdraw by Induction-Donate by Resonance]. It is often incompatible for the same phenyl ring to contain strongly donating substituent groups in the presence of the acidic NH3 + group, thus limiting the options. Perhaps this lack of data will spur future chemists to investigate, probably by computational chemistry, these anomalous cases.
Conclusion
Using the sequence of Search-Organize-Predict, a general new procedure to induce students to think is proposed. The Organize step to relate structure to Induction/Resonance effects benefits from graphic organizers, which the 3x3 grid of is a prime example. The advantages and limitations of this grid system are highlighted. In the case of aromatic acids, all the data cannot be neatly organized into simple electronic effects; there are always exceptions to the rules and these exceptions provide another learning opportunity to the students. The aromatic amines have their own reach and scope and show the reciprocal relationship to the acids that can be predicted using the grid graphic organizer.











 nueva página del texto (beta)
nueva página del texto (beta)