Introduction
Misconceptions are a severe problem (Özden, 2009; Papaphotis & Tsaparlis, 2008). The misconception has harmful properties in chemistry learning. Characteristics of misconceptions are: (1) resistance to change; (2) hard to be reduced (Schmidt,1997); and (3) need a particular strategy to overcome it (Demircioglu et al., 2005; Yakmaci-Guzel, 2013); (4) negatively impacts the following concept; (5) for one concept has some different misconceptions; (6) makes sense to students; (7) is ingrained in the students’ mind; (8) is hidden; and (9) like a snowball, is becoming more significant if not corrected (Yakmaci-Guzel, 2013). Students’ misconceptions of chemistry are the most concerning issue of world chemical education (Regan et al., 2011). Chemical education research on conceptual change and understanding concepts owned by teachers and students ranked first in a decade of study, 2003-2013 (Teo et al., 2014). Conceptual change is a strategy for transforming misconceptions into correct concepts (scientifically acceptable concepts).
Research on misconceptions is divided into three categories: identification, elimination, and prevention. Misconceptions occur in almost all chemical topics. Among the misconceptions identified and reported are: acid-base titration and argentometry (Widarti et al., 2017); chemical bonding (Vrabec & Proksa, 2016); particle position during physical change (Smith & Villarreal, 2015); solution colligative properties (Luoga et al., 2013); osmosis (Kramer & Myers, 2012); ionic compounds dissolving (Naah & Sanger, 2012); atomic structure, chemical bonding, equilibrium, electrochemistry, oxidation-reduction (Al-balushi et al., 2012); reaction rate (Kolomuç & Tekin, 2011); boiling point elevation and freezing point decreases (Pinarbasi et al., 2009); solution, dissolving, and diffusion (Akgun, 2009); solution (Özden, 2009); aromaticity (Topal et al., 2008); buffer solution (Orgill & Sutherland, 2008); nuclear chemistry (Nakibog’lu & Tekin, 2006); material changes (Kikas, 2004); electrification, bonding, geometry, and microscopic representations (Nicoll, 2001); molecule isomerism and neutralization (Schmidt, 1997); chemical changes, dissolving of solids, atomic conservation, periodic systems, and phase changes (Abraham et al., 1994); atoms and molecules (Griffiths & Preston, 1992); covalent bonding and structures (Peterson et al., 1986); and chemical equilibrium (Wheelert & Kass, 1978).
The misconceptions research reported relating to several things: (1) the poor understanding of prerequisite concepts; (2) the lack of formal thinking ability; (3) chemical representation; and (4) positive attitudes in chemistry. According to Gurcay and Gulbas (2018), the higher the significance of a students’ orientation rote learning score, the more misconceptions they experience. Akgun (2009) found that 40% of prospective teachers who experienced misconceptions about dissolving had a poor understanding of substance change concepts. Substance change is a prerequisite for the dissolving topic. Nakiboglu and Tekin (2006) found that the misconceptions experienced by students of nuclear chemistry. The misconceptions are partly related to the prerequisite concepts of nuclear chemistry: atomic numbers, atomic mass, elements, atoms, radioisotopes, nuclides, and isotopes. Nakiboglu (2003) found that some misconceptions of hybridized material resulted from students’ initial concept of atomic orbitals. The atomic orbitals are underlying hybridized material.
Some researchers have reported misconceptions and their relation to formal thinking abilities. Tsitsipis et al. (2012) reported that misconceptions about material structure are related to logical thinking abilities. They found that the more significant the level of logical thinking ability, the fewer misconceptions. The highest level of logical thinking based on Piaget’s intellect development theory is the formal thinking ability. According to Lawson and Thompson (1988), formal thinking ability associate with misconceptions of genetics and natural selection materials, with a correlation of r = 0.41 based on Pearson tests. They found that, on average, students with concrete thinking ability had 1.69 misconceptions, while students with formal thinking ability had 0.75 misconceptions. Wheelert & Kass (1978) found that students who did not achieve formal thinking skills experienced more misconceptions of equilibrium material than students who achieved formal thinking skills.
Some researchers have reported misconceptions and their relation to understanding chemical representation. Kelly (2014) found misconceptions caused by students’ mistakes in identifying objects or symbols of representation. From the results of his research, a student experienced a misconception that NaCl solution does not conduct electricity because, based on animation, gold atoms of gold metal as electrodes do not move. Vrabec and Prokša (2016) reported that some students (48.6%) had held a misconception at the level of symbolic representation of chemical bonding: the placement of electron points between different atoms that bond to the same distance. Naah & Sanger (2012) found that more than 40% of students experienced a misconception of dissolving ionic compounds in water. The most popular answer is the reaction between water and ionic compounds produced by metal oxides and acids. Irsyad & Linuwih (2018) reports that learning linked to multiple representations effectively reduces misconceptions in the hot topic.
Some researchers have reported misconceptions and positive attitudes towards chemistry. Okinoglu & Tandogan (2007) reported that problem-based active learning effectively eliminates misconceptions of force and energy of motion material and improves positive attitudes towards science than traditional learning. Ozmen (2008) reports that computer-assisted learning can reduce chemical bonding misconceptions and improve positive attitudes towards chemistry than traditional learning. Sesen & Tarsan (2010) report that active learning can prevent misconceptions of an acid-base topic and improve positive attitudes towards chemistry than teacher-centered learning.
Misconceptions have been overcome about several chemical topics. The moduleassisted intervention programs have been used to eliminate misconceptions about substance properties, atomic structures, chemical reactions, and stoichiometry (Regan et al., 2011). Elimination of acid-base misconceptions has been conducted through a conceptual conflict strategy (Demircioglu et al., 2005). Chemical equilibrium misconceptions have been eliminated through constructivist approaches with conceptual changes (Akkus et al., 2003). In a solution animated and concept change strategy, the flow of electrons has overcome misconceptions (Sanger & Greenbowe, 2000). The misconception of the microscopic depiction of substance changes has been eliminated by analogy activity (Tsai, 1999). The analogy methods have been used to prevent misconceptions about equilibrium topics (Pekmez, 2010). The historical perspective and philosophy of science have been performed to prevent misconceptions about acid-base equilibrium topics (Kousathana et al., 2005). According to the studies mentioned above, identifying and eliminating misconceptions occur more frequently than preventing misconceptions.
Learning aimed at eliminating misconceptions by the above researchers generally effectively reduces misconceptions. However, the elimination of misconceptions has the disadvantage of spending time. All elimination of misconceptions always begins with the identification of misconceptions. Regan et al. (2011) identified misconceptions with 15 diagnostic questions before and after eliminating module-assisted program intervention. If each diagnostic question takes an average of 3 minutes, then misconception identification takes 90 minutes. Tsai (1999) identified misconceptions by having students describe substances in three phases microscopically in pre-tests, post-tests, and delay tests. Tsai performs elimination of misconceptions after pre-tests and before post-tests. If each pre-test, post-test, and delay test takes an average of 21 minutes, misconception identification takes 63 minutes. Sanger and Greenbowe (2000) identified misconceptions with nine questions: algorithmic questions, visual, conceptual questions, verbal, conceptual questions in pretests, post-tests, and test delays. If each pre-test, post-test, and delay test takes an average of 27 minutes, misconception identification takes 81 minutes. Demircioglu et al. (2005) identified misconceptions with pre-tests and interviews before carrying out elimination.
Another drawback of elimination is the resistant nature of misconception itself. Chemical misconceptions tend to re-occur even though they have been eliminated. Smith and Villarreal (2015), Nicoll (2001), and Bodner (1991) have proven that misconceptions are resistant. Therefore, the prevention of misconceptions is a better option than elimination. The learning of the misconception prevention reported above is still limited to particular concepts. As a result, constructing an effective chemical learning strategy to avoid misconceptions is essential.
Characteristics of Chemical Concepts and The Abilities Needed to Understand Them
Concepts are specific parts of an object or phenomenon grouped by common properties and indicated by names or symbols (Herron, 1996). According to Gagné, some concepts categorized are concrete concepts and defined concepts. Gagné gives an example of the concrete concept of chairs and seating as a defined concept. Based on the division of concepts by Gagné (Ertmer et al., 2003), chemical concepts fall into the category of defined concepts. A defined concept is a concept that can have attributes both physical and nonphysical. Chemical concepts generally involve nonphysical or abstract attributes such as ions, atoms, and molecules. An abstract concept is characteristic of chemistry (Henriksen, & Neppl, 2014; Yakmaci-Guzel, 2013; Stroud & Schwartz, 2010; Ebenezer, 2005).
The chemical concept consists of complex and straightforward concepts based on the number and type of attributes. The concept of vapor pressure is relatively more complex than the evaporation concept and relatively simple of the vapor pressure decreasing concept. The following is a chart showing the relationship between the concept of evaporation, vapor pressure, and vapor pressure decreases.
Figure 1 indicates that to understand vapor pressure, students must first understand the concept of evaporation, whereas to understand the concept of vapor pressure decreasing, students need to understand evaporation and vapor pressure. The relationship between the concept of evaporation, vapor pressure, and vapor pressure decreasing indicates that chemical concepts are interconnected, mutually underlying, and form a hierarchy. Characteristics of chemical concepts other than abstract are (1) mutually underlying, one concept is usually built by the underlying concepts (Seery, 2009; O’Connor, 2015); and (2) tiered or forming hierarchy (Chittleborough & Treagust, 2007; Jensen, 1998; Gultepe et al., 2013). Propositions or statements of knowledge can demonstrate the relationship between chemical concepts. A series of concepts form a statement of knowledge or proposition (Novak, 2002; Wonorahardjo, 2011).
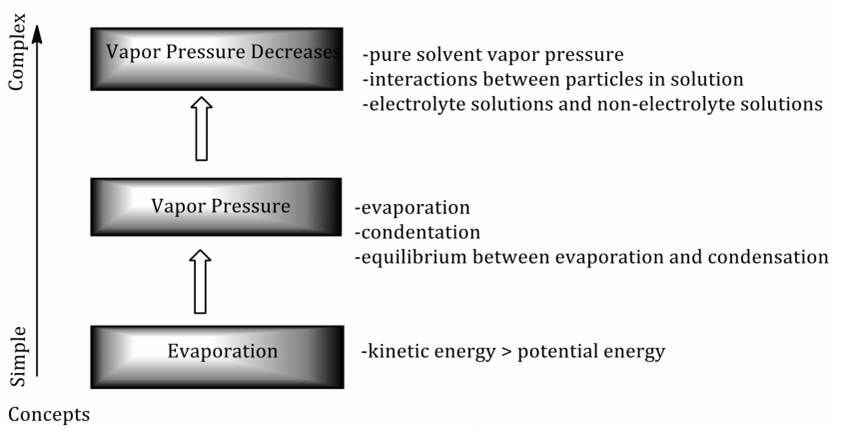
Figure 1 The relationship between the concept of evaporation, vapor pressure, and vapor pressure decreases.
Abstract concepts can be well understood by students who have reached formal thinking based on Piaget’s Theory of Intellectual Development. Based on Piaget’s Theory of Intellectual Development, individuals aged 12 years have achieved formal thinking ability. However, some research results show that not all students who have aged over 12 years have developed formal thinking skills (Bakir & Öztekin-Biçer, 2015; Igaz & Proks, 2012; Budiasih, 2011; Valanides, 1999; Abraham et al., 1994; Good et al., 1979; Chiappetta, 1976). Some students who have not achieved formal thinking skills make the concept of chemistry potentially tricky to understand.
The concepts of chemistry that are mutually underlying and hierarchy require an understanding prerequisite. However, some studies show that not all students can understand the fundamental concepts. Limited and improper prior knowledge leads to meaningless learning (Taber, 2001).
Some students learned chemistry by understanding the wrong concepts and minimal prerequisite concepts (Sirhan, 2007). The number of students who cannot understand the prerequisite concepts makes connecting the introductory concepts and the new topic hard.
Chemical Phenomena and Abilities Needed to Understand Them
The chemical phenomenon involves three levels of representation, macroscopic, submicroscopic, and symbolic (Russell et al., 1997; Gabel, 1999; Johnstone, 2000). Chemistry experiments are always accompanied by changes that can be depicted by the three levels of representation (Milenković et al., 2014). Macroscopic representation is an overview to understand chemical phenomena observed with the five senses (Cook et al., 2008). For example, a burning magnesium ribbon phenomenon. The presence of white ash, which is magnesium oxide, can be seen as a macroscopic representation after the magnesium band has been burned. The microscopic representation provides an overview of chemical phenomena at the particle level related to interactions at the atomic, ion, electron, and molecular levels (Devetak et al., 2010; Cook et al., 2008). A particle-level representation of magnesium oxide formation in the form of a microscopic representation of magnesium ribbon combustion. Symbolic representation is an expression of the properties of macroscopic and microscopic levels of chemical symbols, reaction equations, and data in the form of graphs or tables (Devetak et al., 2010; Wu & Shah, 2004). The symbolic representation of the combustion of a magnesium ribbon is a reaction equation.
Sim et al. (2014) found that high school students in Malaysia, including the upper, middle, and lower classes, cannot understand chemical representation. The students’ low ability to understand chemical representation makes chemistry potentially hard for students to understand. Naah & Sanger (2012) found misconceptions in writing down the dissolving reaction of ionic compounds in water by showing solution components through images at the particle level.
Understanding the Concept of Chemistry
Chemistry is related to conceptual understanding and algorithmic understanding (Herron,1996; Gultepe et al., 2013). However, some studies have suggested that the emphasis on algorithmic understanding is more dominant than learning that emphasizes conceptual understanding (Niaz, 2005). As a result, students’ conceptual understanding generally lags behind their algorithmic comprehension (Cracolice & Deming, 2008). Applying conceptual understanding and algorithmic understanding that is inadequate makes chemistry potentially tricky for students to understand. Difficulty in understanding the concept of chemistry will cause students to have a wrong understanding.
Positive Attitude towards Chemistry in Chemistry Learning Design
The teachings with an inquiry approach emphasize the development of concepts and include attitudes as the main objectives in learning science education (El-Gosbi, 1982). Herron (1996) stated that the teacher needs to write and teach about the value or positive attitude. He also emphasized that curiosity is the fundamental value of science.
Curiosity towards the concepts he learned in this learning is reinforced at the exploration and concept construction stages. At the exploration stage, students are stimulated to connect the concepts already owned with the concepts learned. At the construction stage, the student concept is stimulated to construct the concept. Stimulation is done aimed at bringing up ideas that show curiosity. Curiosity can be improved with different types of questions/problems (Priede & Kurmina, 2012).
The character of unyielding students is developed at the stage of concept validation. Students who do not have the right concept are directed to make modifications to the concept. The ability of students to change concepts towards a valid concept shows an unyielding attitude.
The confident character of students can be developed at the concept application stage. Students are then asked to apply valid concepts that have been constructed to solve conceptual and algorithmic questions. The ability to solve conceptual and algorithmic problems makes students confident that the concepts they construct are valuable and worth maintaining to be stored in long-term memory. The confident attitude of the benefits of the concept he constructed shows confidence. Tekkaya (2003) states that learning makes students confident that the concepts they learn meaningfully can increase confidence.
Herron (1996) states that learning involving problem-solving has many opportunities to develop values or characters because in class discussions will find several different solutions, which requires resolution. Pro problem solver characters can be developed at the problem-solving stage. Students are asked to take a stand as part of a solution to everyday problems at this stage. Students are stimulated to find strategies and solutions based on the concepts they construct to solve the problem. Confidence and unyielding can be improved at the problem-solving stage conducted in the group (Tingle & Good, 1990).
A positive attitude can be known based on observation when learning occurs (Kırbulut & Beeth, 2013) and special instruments in the attitude scale (Akgun, 2009). The cognitive conflict strategies can reduce chemical misconceptions and improve positive attitudes in students (Demircioglu et al., 2005). Because chemical misconceptions occur in almost all concepts, it can be suspected that not all students who study chemistry have the necessary positive attitude in understanding chemistry. Therefore, it is necessary to learn that can improve the positive attitude of science to prevent misconceptions.
Some Obstacles that Are the Source of the Cause of Misconceptions Chemical Concepts
Based on the study of the characteristics of chemical concepts, chemical phenomena, chemical understanding, and good character of chemistry that has been written above, several things have been obtained, including the fact that some students:
Have not achieved formal thinking ability;
Have a weak understanding of the concept of prerequisites;
Have the low ability in connecting chemical representations,
Lagging of conceptual understanding compared to algorithmic understanding; and
Have a low positive attitude towards chemistry.
These five conditions make students have difficulty understanding the concept of chemistry correctly. Difficulty in understanding the concept of chemistry will cause students to have a wrong understanding. Consistent errors in understanding the concept are one indicator of students experiencing misconceptions. Misconceptions are the people’s understanding of things, which is different from scientific understanding (Herron, 1996; Nakhleh, 1992).
Method
Based on the four steps for constructing CAS, a chemistry learning idea to prevent misconceptions. The following is a table showing the process of constructing CAS.
Table 1 Process constructing CAS..
| The four steps for constructing CAS | The results of each step |
|---|---|
| 1. The first step is to conduct a literature review to identify sources of chemistry misconceptions by examining the following: (1) characteristics of chemical concepts and the abilities needed to understand them; (2) chemical phenomena and the abilities required to comprehend them; and; (3) an understanding of the concept of chemistry; and (4) a positive attitude toward chemistry in the design of chemistry learning. |
The five sources of misconceptions were found: (1) not all students have mastered formal thinking (2) some students enroll in classes with low prerequisite abilities; (3) a lack of understanding of chemical representations; (4) some students use algorithmic strategies to solve chemical problems without understanding the concept; and (5) low positive attitude to chemistry |
| 1. The second step is to search for research articles that aim to overcome misconceptions based on the first step results. | The five articles: (1) Igaz & Proksa (2012). Effectiveness of chemistry learning in overcoming the achievement of formal thinking ability. (2) Rivet and Krajcik (2008) examined the effectiveness of science learning in overcoming the low quality of prerequisite concepts. (3) Effectiveness of chemical learning in overcoming low understanding of chemical representation by Stieff (2011) (4) Gultepe et al. (2013). Effectiveness of chemical learning in overcoming the lagging of conceptual understanding compared to algorithmic understanding. (5) Demircioglu et al. (2005) investigated the effectiveness of chemistry learning in improving positive attitudes toward chemistry. |
| 1. The third step is to review the five articles that have been described in order to address the five misconception sources. | The factors influencing the effectiveness of chemical learning include: the ability to think; the truth of the concept; chemical representation; and a positive attitude toward chemistry. |
| 1. The fourth step is to construct a learning strategy to prevent misconceptions in chemistry by deciding the following; (1) a chemical learning approach estimated to be effective in preventing misconceptions based on the learning that has been applied; (2) learning measures that are thought to be adequate to prevent chemical misconceptions; (3) terminology; (4) learning support system that can prevent misconceptions; and (5) indicators of learning strategy success. | the learning stages designed to prevent misconceptions are: (1) exploration; (2) concept construction; (3) concept validation; (4) concept application; and (5) problem-solving. |
Result and discussion
Effective chemistry learning is learning to achieve the objectives of chemistry learning. The following five research results were analyzed and considered representative to identify effective learning to address the five sources of misconceptions that have been identified above accordingly. Several studies that address the five sources of misconceptions are analyzed in Table 2-Table 6.
Table 2 Effectiveness of chemistry learning in overcoming the achievement of formal thinking ability by Igaz & Proksa (2012).
| Constraints | Not all students have achieved formal thinking, so some students are unable to solve conceptual questions. |
| Learning | Cognitive Acceleration through Science Education, CASE. Use four additional questions to answer/solve conceptual questions. Additional questions include two questions involving concrete properties, one question aimed at building metacognition, and one question that causes cognitive conflict. |
| Emphasis | Conceptual understanding, concrete representation (macroscopic), metacognition knowledge, and cognitive conflict strategies, assessment of formal thinking abilities |
| Goals achieved (Effective) | Cognitive Acceleration through Science Education, CASE, accelerates the increase of formal thinking ability. Some were previously unable to answer conceptual questions related to daily life; abstract concepts and concrete concepts with CASE could solve conceptual questions. |
Table 3 Effectiveness of science learning in overcoming the low quality of prerequisite concepts by Rivet & Krajcik (2008).
| Constraints | Some students attend classes with low prerequisite abilities. |
| Learning | It is contextualizing instruction. The learning applies the concept of prerequisites and the daily experience of students. |
| Emphasis | Conceptual understanding, representations of images, graphs, everyday experiences |
| Achieved Goals (Effective) | Contextual learning effectively facilitates the ability of prior knowledge to understand everyday phenomena. |
Table 4 Effectiveness of chemical learning in overcoming low understanding of chemical representation by Stieff (2011).
| Constraints | Low ability to understand chemical representation |
| Learning | Inquiry Learning with Molecular Simulation |
| Emphasis | Contextual local, chemistry representation as a medium of learning |
| Goals achieved (Effective), | Effectively improves chemical understanding and representational capabilities. |
Table 5 The effectiveness of chemical learning in overcoming the lagging of conceptual understanding compared to algorithmic understanding by Gultepe et al. (2013).
| Constraints | Some students solve chemical problems through algorithmic strategies without understanding the concept. |
| Learning | Exploration of conceptual understanding to solve algorithmic problems |
| Emphasis | Conceptual understanding, chemical representation |
| Goals achieved (Effective) | Conceptual understanding is more effective at solving chemical algorithmic problems than mathematical process skills. |
Table 6 Effectiveness of chemistry learning in improving positive attitude towards chemistry by Demircioglu et al. (2005).
| Constraints | Acid-base misconceptions, low positive attitude to chemistry |
| Learning | New learning program acid-base material (conceptual change) |
| Emphasis | Cognitive conflict, prerequisite concept, conceptual understanding |
| Goals achieved | Effectively improves chemical understanding, improves positive attitudes towards chemistry, and reduces misconceptions experienced by students. |
Analysis of practical chemistry and science learning to address the source of misconceptions based on (1) obstacles overcome; (2) learning used; (3) things emphasized; and (4) goals achieved (practical).
Learning to overcome formal thinking’s low ability emphasizes conceptual understanding, chemical representation, metacognition knowledge, and cognitive conflict (Igaz & Proksa, 2012). According to Igaz & Proksa, formal thinking ability can be quickly improved, as evidenced by students’ ability to solve conceptual problems involving metacognition, representation chemistry, and cognitive conflict.
Learning to overcome the poor understanding of prerequisite concepts emphasizes conceptual understanding, involving representation, and daily experience (Rivet & Krajcik, 2008). According to Rivet & Krajcik, contextual learning effectively facilitates prior knowledge in understanding everyday phenomena.
Learning to overcome the poor understanding of chemical representation emphasizes chemical representational local context (Stieff, 2011). According to Stieff, inquiry learning with molecular simulations effectively improves chemical understanding and representational capabilities.
Learning that aims to overcome the lagging conceptual understanding emphasizes the exploration of conceptual chemical representation (Gultepe et al., 2013). According to Gultepe et al., exploration of conceptual understanding involving effective chemical representation in solving algorithmic problems.
Learning improving positive attitudes towards chemistry emphasizes prerequisites, conceptual understanding, and cognitive conflict (Demircioglu et al. 2005). According to Demircioglu et al. (2005), learning conceptual changes eliminates misconceptions while improving positive attitudes towards chemistry.
Practical learning in addressing misconceptions based on misconception resources, including (1) involving formal thinking ability, 20%; (2) paying attention to the truth of the concept: prerequisite concept, 40%, conceptual understanding, 80%; concept change by conditioning cognitive conflict strategies, 40%; conceptual problem-solving involving metacognition, 20% (3) using chemical representation, 80%; and (4) develop a positive attitude towards chemistry, 20%. Based on the findings of the analysis, which revealed some research findings aimed at addressing the sources of misconceptions, it is possible to conclude that factors influencing the effectiveness of chemical learning include: (1) the ability to think; (2) the truth of the concept; (3) chemical representation; and (4) a positive attitude toward chemistry. Thus, the suspected learning to prevent chemical misconceptions is the exploration that accommodates these four factors.
A Chemical Learning Approach that Is Estimated to Be Effective in Preventing Misconceptions Based on the Learning that Has Been Applied
Chemistry is overgrowing in line with the findings of the study. Data is one of the results of research communicated. Based on data, the dominant chemical study conducted so far is the approach of verification and inquiry. The verification approach establishes the explanation of the concept as evidenced by the data, while the inquiry approach emphasizes understanding the data to build the concept (Pavelich & Abraham, 1979). The data in question can be observation data, calculation results, or in the form of images. Learning with an outline verification approach is learning with concept explanations from the teacher, then students verify through various data, methods, and appropriate media. In general, learning with an inquiry approach allows the student to understand data to build concepts. Students can analyze the data to construct new concepts with theories (Vikstrōm et al., 2013) and concepts previously owned.
Prilliman (2014) states that the core of inquiry learning is three stages of exploration, concept discovery, and application. According to the National Research Council (NRC, 2000), stages in the study of inquiry include, among other things, (1) orientation; (2) concept construction; (3) evaluation; and (4) communication. The approach that is not expected to cause misconceptions is learning-oriented towards inquiry learning. The advantage of the inquiry model is that students have the opportunity to find ideas in building concepts. However, in the learning phase of the inquiry, no stage explicitly claims that the concept constructed by the student is valid or follows the concept received scientifically, and the problem-solving stage aims at training children as problem solvers. Problem-solving skills assist students in interaction in their society (Valdez & Bungihan, 2019) and increase self-efficacy to solve the following problem (Ozgur, 2021). Therefore, it is necessary to additionally validate the concept before the application stage of concept and problem-solving. Concept validation is when students show the concept constructed based on evidence in the form of concept explanation. The evidence used in concept validation can be test data or calculation results, or logical arguments. The teacher validates the construction concept by the student at the concept validation stage.
Learning Measures that Are Thought to Be Adequate to Prevent Chemical Misconceptions
The first stage of learning aimed at preventing misconceptions is exploration. The exploration stage is a series of activities to investigate the concept of prerequisites (Warfa, 2013). Some of the activities at the exploration stage include:
The prerequisite concept that students have activated.
Students who experience a misconception of the concept of prerequisite are led to modify the concept of accommodation through a condition of cognitive conflict.
Students who have the correct prerequisite concept will perform the assimilation process.
The learning associates the prerequisite concepts to new ideas.
The method presented at the exploration stage is a question and answer involving chemical representation, demonstration, and experimentation.
Rational of exploration stage
Students who experience chemical misconceptions, in general, have a poor understanding of the concept of prerequisites (Yakmaci-Guzel, 2013). The poor understanding or even misconceptions over prerequisite concepts need to be addressed. That is because the concept of prerequisites is one factor that influences the success of understanding interconnected chemical concepts (Crippen & Brooks, 2009; Seery, 2009; Tsaparlis et al., 2010; Pekmez, 2010). Understanding the prerequisite concept is necessary for new concepts to be understood and stored in long-term memory (Gabel, 1999; and Johnstone, 2006).
Some of the activities presented at the exploration stage are questions and answers involving chemical representations, demonstrations, and experiments. Scaffolding accompanies activities at the exploration stage. The teacher gives the minimum possibility of scaffolding to invoke students’ ability and is used as a bridge in building new concepts.
Identifying the concept of prerequisite and its misconceptions is necessary to determine learning methods to overcome misconceptions (Pekmez, 2010). If misconception, in this case, the concept of prerequisites has been identified, the cognitive conflict strategy is carried out by presenting a combination of macroscopic, submicroscopic, and symbolic representations (Rahayu & Kita, 2010).
The second stage of learning aimed at preventing misconceptions is the construction of a concept based on data. The concept construction stage provides activities to build concepts based on data (Pavelich & Abraham,1979). Several activities at the concept construction stage include:
Rational of the concept construction stage
Learning with a constructivist approach can eliminate misconceptions (Metin,2011). Concept construction by students is a hallmark of learning with a constructive approach. Herron (1996) states that the construction of knowledge is based on knowledge previously possessed through assimilation and accommodation on learning that presents problems. Therefore, the concept construction stage is carried out after the exploration stage.
In order for students to construct concepts, teachers give questions as scaffolding. Scaffolding can be done by using small steps to build a new understanding based on a previous understanding of prerequisite concepts that have been possessed by students (Han, 2013).
The third stage of learning aimed at preventing misconceptions is concept validation. The concept validation stage is a set of activities that aim to validate the students’ concept valid before applying the concept. Some of the activities at the concept validation stage include:
Rational of the concept validation stage
Learning that links several concepts can make students validate the concepts they construct (Osborne & Wittrock, 1983). Students can validate the concepts they construct by presenting evidence in experimental data (Dale, 1970). Students validate the concept during the learning process according to its building concept (Bodner, 1991). Based on Osborne & Wittrock, Dale, and Bodner, it can be stated that students can validate the concepts constructed during the learning process. Concept validation is the ability of students to measure/assess the concepts they build based on scientifically accepted concepts. During the learning process, the teacher guides students to validate the concepts they are constructing.
The fourth stage of learning aimed at preventing misconceptions is concept applications. The concept application stage is a set of activities for students to apply concepts to conceptual and algorithmic questions. Some of the activities that can be done at the concept application stage include:
Students apply concepts to solve conceptual and algorithmic problems collaboratively.
Teachers facilitate students to apply concepts collaboratively.
Students are asked to develop strategies for answering questions during the application stage. That is done to train students to develop their metacognition skills.
Rational stage application concept
The concept application stage needs to be done to ensure students can apply the correct concept to the correct situation. Lazarowitz & Lieb (2006) found some students applying the correct concept to the wrong situation. The concept application stage also needs to store the concepts constructed by students in long-term memory (Devetak et al., 2010). Students become convinced that the concept they are constructing helps solve problems. That indicates attitudes as part of the solution based on the core competencies of chemistry based on the 2013 educational curriculum.
The fifth stage of learning aimed at preventing misconceptions is problem-solving. The problem-solving stage is a set of activities that aims to make the concept owned by students to solve everyday problems by finding solutions to the problems. Some activities that can be done at the problem-solving stage include:
Students apply concepts to find solutions to problems related to daily life collaboratively.
Teachers facilitate students to implement problem-solving concepts collaboratively.
At the problem-solving stage, students are asked to find strategies for finding solutions.
That is done to train students to develop their metacognition skills.
Rational of problem-solving stage
The question can be expressed as a problem requiring a particular solving method (Bodner & Mcmillen, 1986). At this stage, students are given problems related to daily life and need a particular strategy to find a solution. A problem-solving process requiring a strategy to solve the problem is a feature of metacognition construction (Igaz & Proks, 2012). So, the problem-solving stage shows the application of metacognitive knowledge. Gultepe et al. (2013) state that problem-solving is the main thing to understanding chemical concepts.
This study aims to prevent chemical misconceptions. Learning and developing formal thinking ability can prevent misconceptions. The ability to solve problems is an indicator of a student’s ability to formalize their thinking. Kavanaugh & Moomaw (1981) states that the approach used in problem-solving is an indicator of formal thinking.
The learning measures that are estimated to be adequate to prevent misconceptions are (1) Exploration; (2) Concept construction; (3) Concept Validation; (4) Concept Application; and (5) Problem-solving. The learning stage that is an essential feature in learning to prevent misconceptions is the concept validation stage. At the concept validation stage, the teacher only approves the valid concept to be applied. Therefore, this learning is Concept Approval Strategy (CAS).
Terminology Concept Approval Strategy
In this learning, students construct the concept. When students construct concepts, they may be correct or incorrect. As a result, before students can apply the constructed concept, it must be validated. The teacher was recognized as an expert in the classroom during the concept validation stage. Approval is also an abbreviation for Application and Problem Solving with Validated Concepts.
Learning Support System that Can Prevent Misconceptions
This learning support system includes tools and materials, media, resources, environment, skills, and knowledge. The following is an example of a learning support system.
Learning tools and materials: experimental tools and materials
Learning media: whiteboard, stationery, Liquid Crystal Display (LCD)
Learning resources: student’s workbooks, teacher manuals, textbooks, and learning videos
Learning environment: classrooms and laboratory
Skills and knowledge: understanding the concept of prerequisites and thinking skills
Learning Strategy Success Indicators
CAS is declared successful if it can prevent misconceptions. CAS can prevent misconceptions for an effectiveness indicator if students who experienced chemical misconception per non-proposition < 20%. The reason based on misconceptions is significant if experienced by at least 20% of students for each misconception (Peterson et al., 1986; Al-balushi et al., 2012). Another indicator used is misconceptions held by students who learned with CAS less than the control group. The reason is based on the statement that misconceptions could be prevented if students in the experimental group experienced fewer misconceptions than the control group (Sesen & Tarhan, 2010; Pekmez, 2010).
Conclusions
CAS is learning constructed to prevent misconceptions. CAS improves formal thinking skills, understanding representation, emphasizing concepts constructed by students, and developing students’ positive attitudes toward chemistry. CAS consists of five stages: Exploration, concept construction, concept validation, concept applying, and problemsolving. CAS is oriented toward guided inquiry equipped with concept validation and problem-solving stages. Through the validation stage, the concept embedded in the student’s cognition is the right concept. The CAS needs to be developed and applied to prevent misconceptions.











 nueva página del texto (beta)
nueva página del texto (beta)


