INTRODUCTION
The industrial production of paper, textiles, rubber, plastic, paint, printing and leather requires large quantities of water and produces enormous volumes of effluents that can cause pollution in the receiving water. Rhodamine B, a synthetically prepared xanthine dye, is widely used as a colorant in textile dyeing, leather and paint industries, and also as a biological stain in the laboratory. Human or animal exposure to rhodamine B has caused skin, eye and respiratory tract irritation, and has induced phototoxic and photoallergic reactions. Literature has reported carcinogenic, genotoxic and chronic effects caused by this dye (Lewis et al. 1981, Huang et al. 2016). Thereby, to avoid environmental pollution and health risk, it is necessary to treat dye wastewater correctly before discharge. The treatment or removal of pollutants from wastewater can be done with techniques such as coagulation, ion exchange, aerobic and anaerobic processes, electrolysis, advanced oxidative processes, activated sludge or biosorption. Biological materials, called biosorbents, have been employed to remove pollutants in waters or effluents. Barks, leaves, stalks and other low-cost forest or agricultural by-products have been used in biosorption processes (Gadd 2009, Bhatnagar and Sillampää 2010, Ali et al. 2012).
In the south of Brazil there are Araucaria forests, the main species being Araucaria angustifolia. This tree is an angiosperm that produces a cone with edible seeds covered by a lignocellulosic structure, called bract. Sterile bracts do not have seeds inside and could mean more than 80 % of the lignocellulosic material in the cone (Michelon et al. 2012). Sterile bracts are mainly composed of lignin, hemicellulose and cellulose. All of them are biopolymers which have polar and nonpolar chemical groups that are able to interact with several different compounds. This feature makes A. angustifolia sterile bracts a potential biosorbent to remedy industrial effluents containing dyes, including rhodamine B.
This work aims to evaluate the use of A. angustifolia sterile bracts in the removal of rhodamine B from an aqueous solution by biosorption and to use those bracts for the removal of this dye from wastewater.
EXPERIMENTAL PROCEDURE
Biosorbent preparation and characterization
The sterile bracts of Araucaria cones were collected from sales points around the town of Curitibanos, located in upland areas of Santa Catarina state in the south of Brazil. The material was milled by a Wiley mill and sieved to granulometry between 20 to 60 mesh. The composition of the sterile bract raw (SR) was characterized in order to determine ash, total extractives and lignin contents (Sjöström and Alén 1999). The moisture was determined in an oven at temperature 103-105 ºC until constant weight; the ash content was made in an oven at 600 ºC for 2.5 h. The total extractive was obtained using a Soxhlet apparatus. Sequential extractions steps were of 6 h each with ethanol:toluene (1:2, v/v), ethanol and dichloromethane, and washed with warm water at the end. Lignin was quantified by the Klason method (Nicholson et al. 2014). Briefly, the extractive-free sample was treated with 72 % sulphuric acid and subsequently heated with dilute acid to hydrolyze the polysaccharides; after that, the solid residue was washed, dried and weighed. These experiments were made in duplicate. In order to remove soluble phenolic compounds present in the SR, it was boiled in distilled water for 2 h, dried in an oven and put in a desiccator until its use; this material was henceforward called SB. The boiling step was needed to prevent soluble compounds from moving into the solution during the bath experiments (Michelon et al. 2012). The most important chemical groups present in the SR and SB were analyzed by Fourier transform-infrared spectroscopy (FTIR) (ABB, FTLA 2000) and thermogravimetric analysis was used to explore the behavior of thermal biosorbent (Shimadzu, TGA-50). The point of zero charge (PZC) was determined by the salt addition method (Dahri et al. 2016) using 0.1 mol/L NaCl. The pH of each salt solution (pHi) was adjusted to a range of 3-10 using 0.1 mol/L HCl and 01 mol/L NaOH. These solutions (50 mL) were mixed with biosorbent (1 g) and agitated until pH stabilization (pHf). The initial pH (pHi) and final pH (pHf) were measured and a diagram was created with the pHi versus pHi-pHf values. The PZC value was obtained from this diagram at the point where the data curve intercepts the abscissa and the value of dpH becomes equal to zero.
Batch biosorption and models simulation
Both experiments were carried out by mixing 0.50 g of biosorbent with 50 mL of rhodamine B (Sigma) aqueous solution in 150 mL borosilicate Erlenmeyer flasks (Matias et al. 2013). Solutions were agitated using an orbital shaker at 150 rpm (Cientec, CT-712RTN) and a controlled temperature of 20 ºC. All samples were prepared in duplicate. The effects of contact time (Ci = 10 mg/L), pH (3 to 10; Ci = 10 mg/L) and initial dye concentration (2.5 to 220 mg/L) on the biosorption process were investigated. The amount of dye removed by the biosorbent at equilibrium (q e [mg/g]) was calculated (equation 1) by analyzed filtrate mixture on 553 nm using visible spectroscopy (Bel, SP2000).
where C i and C e are the initial and equilibrium dye concentrations (mg/L), respectively, V is the dye solution used (L) and m is the mass of biosorbent used (g).
To investigate the kinetic behavior of the biosorption, experimental data obtained for the contact time was applied to the kinetic models of pseudo first order (equation 2) and pseudo second order (equation 3) (Lagergren 1898, Ho and McKay 1998):
If q t is the amount of dye removed in the time considered (mg/g), q e is the amount of dye removed by the biosorbent at equilibrium (mg/g), t is the time (min) and k 1 and k 2 the constants of speed for the kinetics of pseudo first order (min-1) and pseudo second order (g/mg × min), respectively. The data of the equilibrium experiments for initial concentration were tested in Langmuir (IV) and Freundlich (V) isotherm models (Freundlich 1906, Langmuir 1916):
where q e represents the mass removed by biosorbent at chemical equilibrium (mg/g), Q the maximal biosorption capacity (mg/g), C e the dye concentration at equilibrium (mg/L), K L (L/mg), and K F (mg1-β /g.Lβ) the Langmuir and Freundlich equilibrium constants, respectively. β is a thermodynamical parameter related to mutual interaction among molecules of dye absorbed.
The experimental data to kinetics and biosorption isotherm models were simulated by a non-linear method using tools provided in the Microcal Origin 5.0 software. Simulated q e (q e, sil ) were generated from formulae of various kinetics or isotherm models. Both q e, sil and the experimental data (q e ) were fitted into a chi-square test (χ2 ) and average relative error equations (ARE) for error analysis (equation 6). The smallest value indicates the least error model into to the experimental data (Kooh et al. 2016).
Wastewater treatment and toxicity assay
In order to evaluate the biosorbent application to the removal of dye from wastewater, a sample of commercial rhodamine B was obtained from a local timber industry site. A commercial rhodamine B solution was prepared in laboratory as a synthetic wastewater and characterized by visible spectroscopy scanning (Bel, SP2000). The treatment was made using biosorbent in a bath system (10 g/L) and its effectiveness was evaluated by toxicity bioassays. The inhibition of root growth in Allium cepa (onion) was observed to examine the sub-chronic toxicity in untreated and treated synthetic wastewater according to Fiskesjö (1993). Individuals of A. cepa (n = 6) were exposed to 50 mL of untreated and treated synthetic wastewater. Commercial mineral water was used as the negative control. After 7 days of exposure, the relative inhibition of root growth was calculated and expressed as a percentage. Statistical analysis of the sub-chronic toxicity in A. cepa was performed using analysis of variance (ANOVA) complemented with the Student-Newman-Keuls (SNK) and Dunnet tests. The results were expressed as mean ± SD.
RESULTS AND DISCUSSION
Biosorbent characterization
The composition of SR was as follows: ash 1.88 %, total extractives 6.06 %, lignin 45.38 % and holocellulose (hemicellulose + cellulose) 46.68 % (calculated by the difference). These results are recorded in literature in relation to the estimated composition of the bark of conifers (Rowell 2013). Regarding the infrared spectroscopy analysis, both SR and SB spectrums (Fig. 1) presented strong signal bands to OH groups intermolecular interaction (3415 cm-1) and C-H bond stretching (2923 cm-1). The peaks at 1510 and 1610 cm-1 correspond to the C=C aromatic ring skeleton stretching vibration and the peak at 1054 cm-1 corresponds to the C-O-C pyranose stretching. The peaks obtained are features to groups on carbohydrates and lignin, the major components in bract composition (Silverstein et al. 2005, Kumar et al. 2014, Liu et al. 2014).
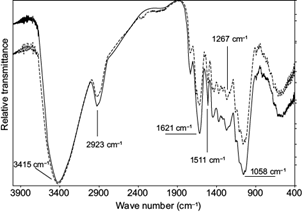
Fig. 1 Fourier transform-infrared spectra of sterile bract raw (solid line) and sterile bract boiled (dashed line)
These signals show the presence of important polar groups in the biosorbent chemical composition. The polar groups can strongly interact by hydrogen-bonding and/or dipole-dipole interactions with compounds that possess, for example, O-H and N-H, in their chemical structures. The SR spectrum is similar to SB, indicating that the main lignocellulosic groups of biosorbent were not modified by the boiled treatment. Differential thermogravimetric analysis (DTGA) showed that SR has a lower thermal stability (182 ºC) than SB (204 ºC) (Fig. 2). This higher degradation temperature for SB can be attributed to boiled treatment that might remove or degrade the phenolic compounds. In fact, when SR is put in water, the solution becomes colored after a few minutes. This happens because its soluble phenolic compounds move to the aqueous solution. Boiling removed these compounds from SR and reduced its mass nearly by 14 %. Because of these facts, only SB has been used in rhodamine B biosorption studies.
Rhodamine B biosorption studies
The chemical balance of biosorption between the biosorbent and the dye was reached after 2 h of contact. This time was used for all the equilibrium experiments performed in this work. The effect of pH on biosorption of rhodamine B by SB is showed in figure 3. The removal capacity of rhodamine B by SB was greater than 80 % at pH lower than 5. The minimum removal occurred at pH 10 with approximately 30 %.
It is evident from the above that pH has a great influence on the biosorption behavior of naturally occurring biosorbents as well as on some adsorvates. The protonation or deprotonation of functional groups present in the biosorbent and in the dye can be pH-dependent and influence the attraction effects between charges involved in the biosorption process. For SB, the PZC was obtained at pH 6. This indicates that the biosorbent has its surface charged positively in a medium in which the pH is below 6. Above pH 6, its surface has predominantly negative electric charge. Rhodamine B is described as a cationic dye; however, the carboxyl group of its structure has pKa around 3.7 which favors interactions of charge with the biosorbent in acidic pH. For a medium in which the pH is greater than 3.7, the zwitterionic form of the dye causes the dimerization of rhodamine B. The morphology of the sterile bract has been recently described as highly porous and with a rough surface (Carmo et al. 2017), and a dye dimerization can reduce biosorption by limiting its access to the sites of biosorbent interaction located within the pores (Huang et al. 2016). Indeed, several studies have described rhodamine B as having a higher biosorption capacity in acid media and at pH below the PZC of the biosorbent (Hii et al. 2009, Dahri et al. 2016, Kooh et al. 2016).
Based on this result, we studied the kinetics of dye biosorption by SB at pH 4 and 8. Data were applied to the kinetic models of pseudo first and pseudo second orders using non-linear regression (Fig. 4). The results confirmed a higher biosorption capacity at equilibrium for acidic pH (q e = 0.564 mg/g) to the detriment of the alkaline medium (q e = 0.450 mg/g). In both pH values the best data fit was obtained for the kinetic model of pseudo second order, in which the lowest χ2 and ARE values were obtained (Table I). This indicated that the rate-controlling step of the biosorption process may be governed by chemical processes, which involve valency forces through the sharing or exchange of electrons between the biosorbent and adsorbate as covalent forces, and ion exchange (Ho 2006).
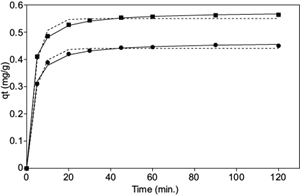
Fig. 4 Effect of contact time and comparison of different kinetic models for rhodamine B onto sterile bract boiled (C i = 10 mg/L; 20 ºC) in pH 4 (■) and 8 (●) (pseudo first order in dash and pseudo second order in plain)
TABLE I PARAMETERS FOR THE KINETICS MODEL
| Pseudo first order | Pseudo second order | ||
| pH 4 | |||
| q e, sil (mg/g) | 0.550 (± 0.00598) | q e, sil (mg/g) | 0.576 (± 0.00144) |
| k1 (min-1 ) | 0.255 (± 0.0184) | k 2 (g/mg.min) | 0.883 (± 0.0228) |
| χ2 (10-5) | 21.0 | χ2 (10-5) | 0.703 |
| ARE | 0.0328 | ARE | 0.0111 |
| pH 8 | |||
| q e, sil (mg/g) | 0.440 (± 0.00425) | q e, sil (mg/g) | 0.464 (± 0.00352) |
| k 1 (min-1 ) | 0.233 (± 0.0141) | k 2 (g/mg.min) | 0.951 (± 0.0688) |
| χ2 (10-5) | 11.0 | χ2 (10-5) | 4.00 |
| ARE | 0.193 | ARE | 0.00753 |
q e, sil : amount of dye removed by the biosorbent at equilibrium simulation (mg/g), k 1 : pseudo-first-order rate constant, k 2 : pseudo-second-order rate constant, χ2: chi-square test, ARE: average relative error
The equilibrium of dye biosorption by SB was studied at pH 4 and 8. Data were fitted to the Langmuir and Freundlich equations using non-linear regression (Figs. 5 and 6). For both pH values, the best fit was obtained by the Freundlich model, which achieved the smallest χ2 and ARE values as well (Table II). The disagreement with the Langmuir model might occur due to the fact that the hypothesis of constant energy biosorption is not performed, because the biosorbent surface is heterogeneous. The K F constant calculated for the experiment at pH 4 had a higher value than that obtained for the biosorption of the dye at pH 8. This confirms a higher removal capacity of rhodamine B by the biosorbent under acidic conditions. Other studies have also described the removal of dye by biosorbents as heterogeneous and a greater capacity for acid media. The removal of rhodamine B by mango endocarp (Inyinbor et al. 2015) and lignin from sugarcane bagasse (Abou-Gamra and Medien 2013) showed a biosorption intensity (denoted by parameter β) of a very similar value to that obtained in the present study (0.618 ± 0.0151). This parameter is also used to estimate the biosorbent surface heterogeneity. The higher the β value, the greater the surface heterogeneity (Vázquez et al. 2007, Lacerda et al. 2015).

Fig. 5 Isotherm of rhodamine B biosorption on sterile bract boiled in pH 4 (C i = 2.5 to 220 mg/L; 20 ºC). Non-linearized Langmuir isotherm (dashed line) and Freundlich (solid line) isotherm

Fig. 6 Isotherm of rhodamine B biosorption on sterile bract boiled in pH 8 (C i = 2.5 to 220 mg/L; 20 ºC). Non-linearized Langmuir isotherm (dashed line) and Freundlich (solid line) isotherms
TABLE II CALCULATED PARAMETERS FOR LANGMUIR AND FREUNDLICH ISOTHERMS
| Langmuir | Freundlich | ||
| pH 4 | |||
| K L (L/mg) | 0.0350 (± 0.00523) | K F (mg1-β /g.Lβ) | 2.25 (± 0.110) |
| Q (mg/g) | 36.7 (± 3.07) | β | 0.618 (± 0.0151) |
| χ2 | 0.516 | χ2 | 0.136 |
| ARE | 19.6 | ARE | 5.39 |
| pH 8 | |||
| K L (L/mg) | 0.0384 (± 0.00930) | K F (mg1-β /g.Lβ) | 1.71 (± 0.127) |
| Q (mg/g)) | 24.6 (± 2.77) | β | 0.588 (± 0.0211) |
| χ2 | 0.583 | χ2 | 0.133 |
| ARE | 12.3 | ARE | 6.46 |
K L :Langmuir isotherm constant, K F : Freundlich isotherm constant, Q: Langmuir biosorption capacity, β: Freundlich thermodynamical parameter, χ2: chi-square test, ARE: average relative error
Evaluation of wastewater treatment
The evaluation of SB to treat wastewater was made using a synthetic wastewater containing commercial rhodamine B obtained from a local timber industry where the dye is dissolved in water (125 mg/L) and is stored in a big tank, which is used to dye plywood by dipping. Plywood is a sheet material manufactured from thin layers of softwood and it is dyed to identify its commercial application. The rhodamine B commercial sample was investigated by spectroscopy and showed similar characteristic bands as the analytical grade dye, which confirm its chemical identity. Treatment of synthetic wastewater containing rhodamine B within SB removed 96 % in the mass of dye (q e = 4.95 mg/L). After this, the treatment performance was evaluated by toxicity bioassays. Untreated wastewater containing the dye caused significant growth inhibition on roots of A. cepa (Fig. 7). This phytotoxic effect could be related to bioaccumulation of rhodamine B in plants and interactions with specific areas in the meristematic cell nucleus. Due to this, different kinds of physiological, biochemical and molecular effects can be initiated, including mitotic activity change, nuclear aberration (e.g., micronucleus, nuclear buds and bridged nuclei) and oxidative stress (Tan et al. 2014).
CONCLUSIONS
Boiling of sterile bract of A. angustifolia seems to preserve chemical groups that could be acting on rhodamine B biosorption. However, this boiled bract has a higher thermal stability than the unboiled one. Biosorption equilibrium studies identified the acid medium as the best condition to dye removal. The rate-controlling step of the biosorption process was found to be governed by chemical processes and shown to be heterogeneous, based on the fit to the Freundlich equation model. The treatment of commercial rhodamine B by SB was successful in reducing toxicity in the A. cepa root. The results showed that bract may be considered as a low cost alternative biosorbent to be applied in the removal of water pollutants and the treatment of potentially toxic effluents.











 nueva página del texto (beta)
nueva página del texto (beta)





