INTRODUCTION
As a technological material, alumina represents a great opportunity, because its properties allow very diverse applications that can range from the food and pharma industry to the chemical and electronic industry. Alumina can undergo different polymorphic transformations depending on the conditions in which these transformations are carried out (West 2014): thermal and via precursors, which allow obtaining different crystalline phases called transition aluminas (Levin and Brandon 1998). Three large types of alumina are known industrially: metallurgical grade, ceramic grade and transition aluminas. This last group, due to its multiple crystallographic and allotropic forms, presents the greatest number of uses and applications (West 2014). These aluminas can be used as catalyst support in combination with other activated transition aluminas (De Souza et al. 2000), as well as cementitious material of interest for its use as high temperature refractory cement (Lee and Moore 1998).
The aim of this work is to determine a technological path that, starting with recycled aluminium in Venezuela (Vitalis 2015), allows obtaining transitional aluminas (Mahinroosta and Allahverdi 2018). An important point is the handling of the raw material (Gonzalo 2008) to be used in this process, which follows the global recycling trend instead of continuing with the degradation of the different ecological systems from which the different precursors can be obtained (Schlesinger 2007).
We propose the use of soft-drink cans (Adans et al. 2016), which have a high potential in terms of reducing, recycling and reusing (RRR). In general, the vast majority of materials, especially those of metallic type, can be recycled once their useful life is over, thus giving way to materials of the same nature or with some variation to satisfy the needs of the market without having to resort to the sources of the metal. Such is the case of aluminium for soft-drink cans, whose post-consumer deposits (used beverage cans, UBC) are of particular interest for the present study. According to the Venezuelan regulation COVENIN 2352-86, the aluminium content in these deposits should be in the order of 97 % (Iesmat 2015). These products represent a source of aluminium of relatively high purity with a high potential for recycling or reuse (COVENIN 1986). In general, the vast majority of materials, especially those of metallic type, can be recycled once their useful life is over, giving way to materials of the same nature or with some variation to meet the needs of the market without resorting to metal sources (Meshram and Kumar 2018).However, since the production levels of transition aluminas are very limited (if not inexistent) in Venezuela, obtaining materials of this nature may represent an alternative both for the development of new technologies and for companies that seek to stock up on this type of material or any other that may act as a precursor. In summary, we propose a method of reusing metallic aluminium from waste to obtain ceramics material with added value.
MATERIALS AND METHODS
The experimental procedure included four steps (Fig. 1 ):
Synthesis of the precursor through precipitation in aqueous media at low temperature (25 ºC, ambient temperature); heating of cans at 500 ºC for 45 min (López et al. 2018); dissolution in HCl; pH adjustment with ammonium hydroxide (Sharma 2003), and aging precipitates (De Souza 1990, Ahmedzeki et al. 2017).
Characterization of the precursor by X-ray diffraction (XRD): 10º-90º (0.02º/seg, CuKα), 35 KV, 25 mA; scanning electron microscopy/energy dispersive spectroscopy (SEM/EDS): secondary electrons, and particle size distribution (PSD): 0.02-2000 µm.
Thermogravimetric analysis (TGA) and thermogravimetric differential analysis (TDA), thermal treatment of the precursor in order to achieve transition aluminas.
Characterization of the resulting products.
RESULTS AND DISCUSSION
Chemical composition analyses of the raw material (aluminium cans) and the precursor (precipitated material) were performed by SEM/EDS. The results (Table I) were as expected for both materials. Chlorine (Cl) was detected in the precipitated precursor due to acid media dissolution with HCl and pH adjustment with ammonium hydroxide (NH4OH), as well as some reaction products according to the following formula:
TABLE I CHEMICAL COMPOSITION OF ALUMINUM SCRAP AND PRECIPITATED PRECURSOR BY SCANNING ELECTRON MICROSCOPY/ENERGY DISPERSIVE SPECTROSCOPY.
| Element (wt %) | Raw material | Precursor |
| Mg | 2.33 | - |
| Al | 96.24 | 87.76 |
| Mn | 0.87 | - |
| Fe | 0.57 | - |
| Cl | - | 12.24 |
It is relevant to note that no metallic elements (like Fe or Mn) were observed in the precursor, indicating that the controlled precipitation process could allow the synthesis of materials free of contaminants.
The precursor was characterized using different techniques: XRD, PSD and morphological assessment with SEM. Boehmite (γ-AlOOH) and bayerite (α-Al(OH)3) were detected by XRD (Fig. 2). Both compounds are precursors phases of transition aluminas according to the sequences of phase transformations toward the stable α-phase reported in literature (West 2014). Percentages of detected phases were estimated with the Rietveld technique and pseudo-Voigt adjustment (Young 2002).
The composition of the precipitated precursor was 55.4 % of bayerite, 41.2 % of boehmite and 3.4 % of ammonium salt. The PSD of the precursor (Fig. 3) indicates a bimodal distribution with two distinctive particle sizes, 68 and 500 µm, respectively (Iesmat 2015). The SEM evaluation (Fig. 4) revealed agglomerates with particle size ranging from 50 to 300 µm, which confirmed the PSD performed with laser granulometer. Also, particles with sizes around 1 µm were observed.
The thermal evolution of the precursor was studied through typical DTA/TGA methodologies (Smykatz-Kloss 1974). The dehydration appears to start at 106 ºC (Fig. 5a), indicated by the endotherm peak, and continues up to 145 ºC, probably because the small capillarity of the gelatinous precursor slowed down the water loss through the material. This step accounts for 8 % of the mass loss. A sharp exothermic peak appears at 225 ºC, which represents energy release during the transformation phase from bayerite to η-alumina (Sato 1962). Another step occurres between two endothermic peaks at 260 and 360 ºC, which may be attributed to two events: elimination of residual hydroxyls and ammonium chloride decomposition. The exotherm that appears at 490 ºC corresponds to the transformation of boehmite into γ-alumina (Alphonse and Courty 2005). Thermal evolution occurring between 190 and 750 ºC, approximately, account for 30 % of mass loss (Fig. 5b).
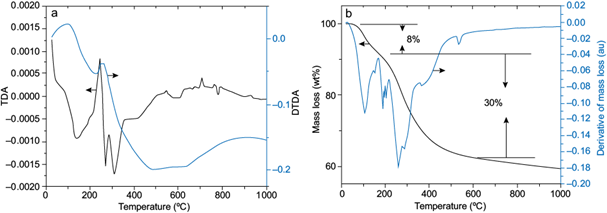
Fig. 5 Thermal evolution of the precursor (a) by thermogravimetric differential analysis (TDA) and derivate thermogravimetric differential analysis (DTDA); (b) by thermogravimetric analysis.
Discrepancies between TDA and TGA curves may indicate that the change in enthalpy is not directly proportional to the rate of mass loss, which is normally encountered in complex reactions. However, in the present study, all the events observed during thermal evolution analysis were confirmed with X-ray diffractograms at various temperatures of the precursor (Fig. 6).
Since transition aluminas could be prepared by calcining aluminium hydroxides, thermal treatment of the precursor was conducted at 350 and 750 ºC at a rate of 10 ºC/min for 1 h and 4 h (Dwivedi 1985). The obtained product was characterized by XRD. Different kinds of transition alumina were obtained after the thermal treatment of the precursor: ƞ-Al2O3 (Fig. 7a) and ɤ-Al2O3 Ɵ-Al2O3 (Fig. 7b).
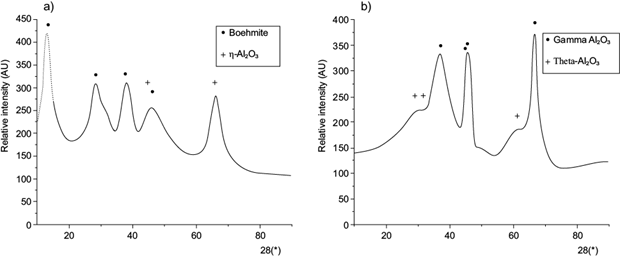
Fig. 7 X-ray diffractograms of synthetized transition alumina at (a) 350 ºC for 4 h; (b) 750 ºC for 4 h.
In order to detect possible hydraulic activity of the obtained transition aluminas, heat-treated samples of synthesized product (at 350 and 750 ºC) were subjected to hydration with distilled water for 168 h. Then the samples were washed with ethanol and prepared with gold for the observation in SEM model FEi Inspect F50. The comparison of morphological features (Fig. 8a, b, ) (heat-treated samples at 350 ºC) reveals morphological changes of the transition aluminas after hydration. These transformations are correlated with those studied by Lefévre et al. 2002 and Sato 2007, who characterized this type of transformation for periods of 4 days, observing a transient amorphous hydrated phase, followed by an increase in bayerite concentration, which stabilizes after about two months. Thermodynamical calculations predict the hydration reaction of γ-alumina leading to a more stable phase (bayerite, gibbsite, or boehmite), since the surface reactivity and sorption properties of solids are factors controlling the transport of elements in water. This could be an indication of potential hydraulic activity due to the formation of new phases. In contrast, comparison of heat-treated samples at 750 ºC and 168 h (Fig. 8c, d) with and without hydration, did not show any relevant difference in morphological features; thus, apparently this material does not present any hydraulic activity, at least under this conditions

Fig. 8 Photomicrographs of transition aluminas at different conditions: (a) heat-treated at 350 ºC without hydration, (b) heat-treated at 350 ºC and hydrated for 168 h, (c) heat-treated at 750ºC without hydration, (d) heat-treated at 750 ºC and hydrated for 168 h.
Nanometric particles smaller than 100 nm (Fig. 9) were observed in the synthesized transition aluminas heat-treated at 350 ºC. This shows that the synthesis technique could yield micro- and nanometric alumina particles with potential conditions to be used in the binding systems of monolithic refractories. However, some parameters needs to be adjusted in future studies in order to improve the results of this preliminary work.
CONCLUSIONS
It is possible to synthesize transition aluminas (γ, η, θ) from aluminium scrap by using controlled conditions of a wet-chemical route. The presence of the ρ-Al203 phase is not ruled out, due to the amorphous region observed in the diffraction pattern of the sample.
According to the synthesis method, it is possible to obtain transition aluminas in particularly interesting combinations for their application as catalyst supports, activated aluminas or pozzolanic additives for cement. In the near future, these materials could be potential candidates for binding systems in monolithic refractories. Further studies will be conducted to adjust variables in the methodology, for example, the application of washes to remove ammonium chloride.











 nueva página del texto (beta)
nueva página del texto (beta)








