INTRODUCTION
Crude oil and its derivatives have been used as a major source of energy for centuries. However, its use as a source of energy and raw material has had a negative effect on the environment, becoming an ever-growing problem across the world. The extraction and transport of crude oil can sometimes result in oil spills, wreaking havoc on local environments and ecosystems in general. Crude oil is composed of many different organic and inorganic compounds, including a mixture of aliphatic, alicyclic (saturated cycloaliphatic structures), aromatic hydrocarbons, and traces of heavy metals. A large percentile of these compounds are aromatic hydrocarbons (15-37%); these can vary in their structure, size, and conformation (Díaz-Ramírez et al. 2012, Cavazos Arroyo et al. 2014, Speight 2020). Polycyclic aromatic hydrocarbons (PAHs) are a class of xenobiotic and anthropogenic pollutants, mostly from the oil industry (Korniłłowicz-Kowalska et al. 2006, Byss et al. 2008). PAHs are lipophilic constituents that are released into the environment as a result of human and natural activities, such as incomplete combustion of fossil fuels, shale oil, volcanic eruptions, smoking cigarettes, accidental oil spills, use and disposal of petroleum products, and gasification and liquefaction of coal (Díaz-Ramírez et al. 2012, Cavazos Arroyo et al. 2014, Speight 2020). Due to high levels of oil contamination, several microorganisms have adapted in such a way that they are capable of metabolizing, mineralizing, or biodegrading different oil derivatives (Zheng et al. 2019). These microbial consortia can be isolated, studied, and preserved for future bioremediation studies (Owsianiak et al. 2009). Microorganisms play an important role in the elimination of PAHs in terrestrial and aquatic ecosystems; in fact, microbial degradation is the main natural decontamination process to eliminate oil and its derivatives (Singh 2006, Biache et al. 2017)2017; Singh, 2006.
Understanding and controlling this natural process is critical to aid bioremediation technologies (Rodríguez-Vázquez et al. 1999, Passarini et al. 2011, Pernía et al. 2012, Chen et al. 2015, Naranjo et al. 2015, Riskuwa-Shehu and Ijah 2016)as well as the biodeterioration of crude oil and its derivatives, is one of the major environmental, operational and economic problems in the Venezuelan oil industry. Fungal contaminants are able to produce large quantities of biomass and synthesize peroxides and organic acids, causing severe damage on metal surfaces and promoting the contamination and biodeterioration of fuels. No evidences regarding fungal strains have been reported to be associated to petroleum naphtha, widely used as a diluent of extra heavy crude oil (EHCO. Fungal degradation activity has been observed in various settings, in which different types of wood, stored paper, textiles, plastics, leather, among other have been disintegrated (Refugio 2016). Hitherto fungal metabolism of PAHs is still limited when compared to that of bacteria; however, fungi are as important as bacteria in the bioremediation of PAHs in aquatic systems and terrestrial environments (Cerniglia 1984, Godoy et al. 2016). For instance, bacteria cannot efficiently degrade PAHs that have more than four aromatic rings, while fungi can degrade and mineralize those PAHs (Singh 2006). In addition, oxidation of PAHs as a prelude to ring fusion and assimilation is known in bacteria, whereas fungi are known to achieve hydroxylation of PAHs as a prelude to detoxification (Botello et al. 1995, Sack et al. 1997, Gouma et al. 2014). Fungi have developed an important ecological role, as the polar and reactive metabolites can be mineralized or detoxified to harmless adduct (Oh and Chung 2006)CBMN assay and EROD-microbioassay. Fungal mycelium also has the ability to grow in soil and propagate itself to metabolize PAHs (Singh 2006), allowing fungi to form bound PAH residues in soil, thus reducing their toxicity (Tirado-Torres et al. 2015). Isolation of fungi from hydrocarbon contaminated sites (roads, pipes, tanks, soil, containers, etc.) represents a good alternative to discover new strains of fungi that are adapted to petroleum derivates under contaminated condition; this is an advantage over cultures derived from laboratory collections (Betancur-Corredor et al. 2013, Ma et al. 2018). In this research work, a fungal strain was isolated from an aged fuel oil plastic tank that has previously stored fuel oil, used to control apple orchards temperature during cold-winters in Chihuahua, Mexico. After isolation and characterization, the microorganism (Acremonium sp.) was evaluated as a PAH biodegrading agent using UHPLC as principal analytical method.
MATERIALS AND METHODS
Isolation of the fungal strain
Acremonium sp. was collected from an aged 25 000 liter tank using sterilized swabs. Specifically, the samples were taken from the internal walls of a plastic tank that was used to store fuel oil. Then, taken to a microbiology lab where they were inoculated in potato dextrose agar medium (PDA). Identification of the fungal species was carried out by macroscopic and microscopic studies of morphological characteristics (hyphae, conidia, conidiogenous cells, and conidiophores). These features were compiled and compared with known taxonomic keys.
Inoculum preparation
The inoculum was taken from an isolated pure culture and placed in 250 mL Erlenmeyer flasks containing potato dextrose agar (PDA). After incubating at 28 ºC for 10 days, the inoculum was filtered through a 0.22 µm pore size 50 mm diameter nylon membrane using 20 mL of deionized water as the washing solvent and 100 µL of Tween-80 as surfactant. Spore suspension was obtained in the filtrate, whereas the membrane retained the mycelium fragments. The spore (conidia) concentration was determined using Neubauer’s chamber technique.
Preparation of liquid medium M9
Three phenanthrene, anthracene, and pyrene (PAHs; Fig. 1) solutions were individually prepared at concentrations of 50 µg/mL in M9, a media composed of 3.0 g KH2PO4; 0.5 g NaCl; 1.0 g, NH4Cl; 1 mL of MgSO4×7H2O 1M; and 0.5 mL of CaCl2×2H2O 1M in 100 mL of distilled water. For each experiment, 100 mL of the M9 liquid medium was transferred to a 250 mL Erlenmeyer flask with the PAHs (as the unique carbon source) previously dissolved in 10 mL of acetone. To remove the acetone and to sterilize the solution and flask, the samples were incubated inside an autoclave at 121 ºC and at 15 psi. The inoculum was added to the M9 liquid medium at a concentration of 1 × 105 conidia/mL. This freshly prepared medium was used to perform all the reported experiments (replicated six times); with inoculum to study the PAHs degradation and without inoculum as an abiotic control.
Incubation conditions
Fungal cultures were incubated at 28 ºC with 150 rpm in a Labnet 211 DS incubator and degradation of PAHs was monitored by UHPLC. Samples were prepared by removing 800 µL of sample from the culture and filtering it with a syringe nylon filter with a pore size of 0.22 µm at 0, 7, 14, 21, and 30 days. Fungal growth was also monitored; dry weight was measured by collecting the slurry of organic matter with a filter (pore size of 0.22 µm), dried under sterile conditions at room temperature, and weighed in an Ohaus Adventurer model H-5276 analytic balance.
Extraction of PAHs
A 50 mL saturated solution of Na2SO4 was added to the aqueous mixture (filtrated from above) and to the resulting slurry was added ethyl acetate (3 × 15 mL × 2 min agitation) to extract the PAHs. The organic layer was concentrated to final volume of 10 mL under reduced pressure, at 40 ºC, using a Büchi R-210 rotatory evaporator.
Chromatographic analysis of polycyclic aromatic hydrocarbons
In a 10 mL volumetric flask, 1 mg of each analyte (phenanthrene, anthracene, and pyrene) was dissolved in a solution of acetonitrile and acidified (0.1% TFA) water (70-30). The standard solution was filtered through 0.22 μm nylon disks and injected into an Ultra High-Performance Liquid Chromatography (UHPLC) employing acetonitrile and acidified (0.1% TFA) water (70-30) v-v as mobile phase. For the calibration curve, 0.05, 0.1, 0.25, 0.5, 1, 2, 4, 8, 12, 24, and 36 μl of the standard solution were injected. Samples were prepared by diluting 100 µL of the extracted PAHs in 1 mL acetonitrile and acidified (0.1% TFA) water (70-30); quantification was completed with a Thermo Scientific Ultimate 3000 UHPLC equipment with diode array detector (DAD) at a wavelength of 254 nm. The UHPLC was equipped with a Thermo C18 Acclaim 120 4.6 x 150 mm column and the samples were ran at 40 ºC with an injection volume of 5µL, using the same mobile phase at 1 mL/min flow during 20 minutes of data acquisition.
All the experiments in this study were repeated at least three times. Individual biodegradation of the PAHs as well as combined biodegradation of the PAHs were analyzed with our UHPLC. Degradation was measured using concentration changes of phenanthrene, anthracene, and pyrene in intervals of 7, 14, 21 and 30 days, starting with and initial concentration of 50 µg of each analyte in both individual and combined experiments. An M9 without inoculum was ran and used as the abiotic analytical control.
RESULTS AND DISCUSSION
Morphological characterization of Acremoniumsp.
Comprehensive macro- and microscopic examination, as well as fungal structure comparation with known CBS keys (The Dutch Centraalbureau voor Schimmelcultures, Utretch Netherlands) (Samson 2004) allowed identification of the fungal microorganism as Acremonium sp. Observation of cultivated colonies presented mycelium with irregular dark brown edges as shown on figure 2; taxonomically, the strains matched to the known structures cited in the literature, characterized by the presence of grouped conidia simulating a head at the tip of the conidiophore (Samson 2004). The mycelium was constituted mainly by phialides, conidia, and conidiophores; the latter showed fusiform, hyalin, and unicellular characteristics.
Poly aromatic hydrocarbon toxicity
Toxicity concentration was determined by adding a mixture with molar equivalent amounts of phenanthrene, anthracene, and pyrene at 50, 100, 200, 400, 800, 1600, 3200, and 6400 mg/L concentrations. Each concentration showed viability, but 50 mg/L was selected as our experimental concentration due to the desired solubility of the PHAs in aqueous conditions.
Hydrocarbon degradation
Both negative controls studies using Acremonium sp. demonstrated that this fungus is capable of assimilating hydrocarbons as a carbon source. As described by Oh and Chung (2006), 25% of the total composition of fuel oil are small aromatic compounds (up to 15 carbons). The chemical characteristics of fuel oil allows the title fungus to quickly adapt its metabolism to degrade PAHs as carbon source instead of glucose; therefore, exhibiting a metabolism capable of breaking down phenanthrene, anthracene, and pyrene (Gangola et al. 2019). Herein, we demonstrated that degradation began at day 0 of incubation and only increased with time, with a high peak at day 30; however, it is important to note that efficiency didn’t increase with longer periods of times (> 30 days). Figure 3 shows an Erlenmeyer flask with high degradation activity (day 30), where it is possible to see mold and guttations formed by microorganism growth activity.
Since phenanthrene, anthracene, and pyrene were the only source of carbon present in the reaction flask, we can assume that the fungus is taking PAHs as its carbon source. Nonetheless, to quantify the degradation potential of our title microorganism, UHPLC was utilized. UHPLC quantitative measurements were calculated by using peak areas of the chromatograms; identification was completed using the retention times of phenanthrene (8.47 min), anthracene (9.19 min), and pyrene (12.33 min) analytical standards (Fig. 4).
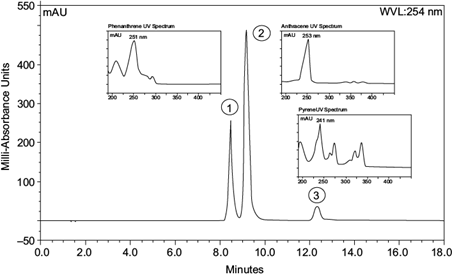
Fig. 4 Scan chromatogram identification of standard analytes with their respective ultraviolet spectrums. 1)Phenanthrene λ max 251 nm, tr = 8.47 min, 2) Anthracene λ max 253 nm, tr = 9.19 min and 3) Pyrene λ max 241 nm, tr = 12.33 min. (tr = time retention).
After 30 days of incubation at 28 ºC, 150 rpm, and using 50 µl/mL as initial PAH concentration, the Acremonium sp. biodegradation capacity was directly measured from PAHs concentration change in function of time. The dry weight was determined to be in average 2.38 times higher than the PAHs consumed by the microorganism, as shown on figure 5. This means that for every milligram of PAHs degraded, 2.38 mg of dry material was found. These results agree with the literature, where carbon content accounted for 35-55% Acremonium sp’s weight (Khan et al. 2021).
Individual PAHs experiments showed phenanthrene degradation of 92-95%, anthracene degradation of 89-94%, and pyrene degradation of 81-89%. Combined degradations (the three PAHs together) were 91-96% for phenanthrene, 89-93% for anthracene, and 83-89% for pyrene (Table I). As seen in figure 6, the area reduction in chromatogram signals of each analyte indicated a decreased concentration for PAH after fungal activity (see Y-axis scale of figure 4 vs. figure 6). The appearance of new signals between minutes 1.8 and 4.5 indicates the presence of oxidized, smaller, and more polar chemical compounds with lower molecular weight, accounting for its short retention time in the chromatograms. Those new signals indicate the formation of secondary metabolites derived from the fungi using PAHs as a substrate or carbon source.
TABLE I RESULTS OF DEGRADATION OF POLY AROMATIC HYDROCARBONS (PAHs), S.D. * STANDARD DEVIATION.
| PAH | Abiotic control residual amount µg/mL ± S.D* | Residual Amount 30 days µg/mL ± S.D.* | Biodegradation % |
| Individual PAHs Degradation | |||
| Phenanthrene | 49.34 ± 0.14 | 2.51 ± 1.73 | 94.98 ± 2.46 |
| Anthracene | 49.23 ± 0.28 | 3.02 ± 1.41 | 93.96 ± 1.66 |
| Pyrene | 49.16 ± 0.06 | 5.53 ± 1.33 | 88.94 ± 1.01 |
| Combined PAHs Degradation | |||
| Phenanthrene | 49.40 ± 0.09 | 3.26 ± 1.03 | 93.49 ± 2.06 |
| Anthracene | 49.33 ± 0.27 | 4.51 ± 0.91 | 90.98 ± 1.82 |
| Pyrene | 49.24 ± 0.26 | 7.27 ± 1.38 | 85.47 ± 2.76 |
All experiments were run with initial concentration of 50 µg/mL per each PAH.
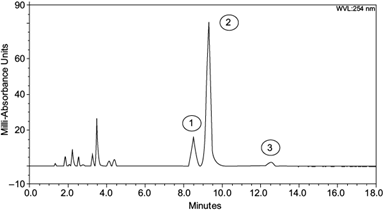
Fig. 6 Ultra high performance chromatography degradation chromatogram at 30 days, (1) phenanthrene, (2) anthracene and (3) pyrene; peak signals between minutes 1.8 to 4 indicate the presence of new organic compounds.
The results of this study corroborate previous research on fungi mineralization of organic compounds and their ability to degrade chemical compounds of high molecular weight, such as lignin (Jiao et al. 2019). Therefore, our data and earlier mentioned reports are significant in bioremediation, as they stand out when compared to bacteria (Samson 2004), whose metabolism is limited to a certain number of rings in PAHs, molecular weight, or conformation.
CONCLUSION
An aqueous, environmentally friendly reverse phase UHPLC method was successfully developed for the individual and combined resolution of phenanthrene, anthracene, and pyrene with retention times of 8.47, 9.19 and 12.33 min, respectively. The microorganism isolated from an apple orchards fuel reservoir tank was identified as Acremonium sp. This fungus showed an individual degradation capacity up to 95% for phenanthrene, 94% for anthracene, and 89% for pyrene and a combined degradation capacity up to 93%, 91% and 85% for phenanthrene, anthracene, pyrene, respectively. Trials were completed in a 30-day incubation period using an initial concentration of 50 μg/mL at 28 ºC, 150 rpm, and an M9 liquid medium. Dry weight experiments were matched with literature values. Literature records establish that dry fungi material contains 35% to 55% carbon, whereas this experiment had a maximum content of 39.5% carbon. Molecular size of the substrates played an important role on degradation rate, finding that the larger size pyrene degraded slightly slower than the other two analytes. The less aromatic of the three compounds, phenanthrene, was the most biodegradable in both individual and combined studies. Fortuitously, we discovered that Acremonium sp. was able to assimilate three different hydrocarbons as the only carbon source without the need for an adaptation period.











 nueva página del texto (beta)
nueva página del texto (beta)