Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.44 no.2 Ciudad de México abr./jun. 2000
Investigación
Sesqui- and Tri- Terpenoids from Esenbeckia species (Rutaceae)
Arturo Cano,* José Luis Bucio, Marina Espinoza and Alejandro Ruíz-Cancino†
Facultad de Estudios Superiores Zaragoza, Universidad Nacional Autónoma de México. Av. Guelatao No. 66 (Eje 7 Oriente). Col. Ejército de Oriente. Iztapalapa 09230, México D. F. Teléfono: 5623-0758. Email: cafa6205@prodigy.net.mx.
Recibido el 18 de marzo del 2000.
Aceptado el 16 de junio del 2000.
Abstract
Three Esenbeckia species (Rutaceae) were chemically analyzed by means of conventional chromatographic, spectroscopic and spectrometric techniques. From the aerial parts of E. berlandieri ssp berlandieri were obtained friedelin, β-sitosterol, and β-sitosteryl-β-D-glucoside. Limonin was characterized as the main constituent from the seeds of Esenbeckia ovata. From the aerial parts of E. velutinosa friedelin, caryophyllene β-oxide, lupeol, lupenone, cryptomeridiol and β-sitosterol were isolated and characterized.
Key Words: Esenbeckia berlandieri ssp. berlandieri, E. ovata, E. velutinosa, Rutaceae, sesquiterpenes, triterpenes, limonin.
Resumen
Fueron analizadas químicamente tres especies del género Esenbeckia (Rutaceae) por medio de técnicas cromatográficas, espectroscópicas y espectrométricas convencionales. De las partes aéreas de E. berlandieri ssp berlandieri se aisló fridelina, β-sitosterol y el β-D-glucósido de β-sitosterilo. La limonina fue caracterizada como el principal constituyente de las semillas de E. ovata. De las partes aéreas de E. velutinosa se aisló e identificó a la fridelina, β-óxido de cariofileno, lupeol, lupenona, criptomeridiol y β-sitosterol.
Palabras clave: Esenbeckia berlandieri ssp. berlandieri, E. ovata, E. velutinosa, Rutaceae, sesquiterpenos, triterpenos, limonina.
In memoriam to Dr. Jacobo Gómez-Lara
Introduction
Esenbeckia is an American genus of ca. 30 species, 17 of which are located mainly in the tropical regions of the states of Veracruz, Guerrero, Michoacán, Oaxaca and Chiapas in Mexico [1-2]. Previous chemical studies from the aerial parts of some species indicate that limonoids, acridone and furoquinoline alkaloids, and furanocoumarines are the main secondary metabolites [3-8]. In addition, phloroglucinols and polyprenols have been characterized from E. nesiotica [9], alkaloids from E. almawilla [10], indole alkaloids, triterpenes, lignanes and amides from the roots of E. leiocarpa [11-12], furanocoumarines and quinoline alkaloids from E. glandiflora [13], and terpenes and coumarins from E. stephani and E. ovata [14].
Results and Discussion
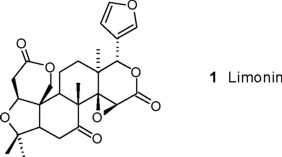
From the aerial parts of E. berlandieri ssp berlandieri were isolated and identified friedelin [15], β-sitosterol and β-sitosteryl-β-D-glucoside. From the seeds of E. ovata was isolated a white solid that exhibited positive reaction in the Ehrlich test for furanes [16], whose presence was according to the signals at δ 7.42 (2H, s, H-21, H-23) in the 1H NMR spectrum, and was confirmed by the fragment at m/z 95 in the EIMS. The presence of an AB system centered at δ 4.92 (1H, J = 13 Hz) and δ 4.48 (1H, J = 13 Hz) in the 1H NMR spectrum is characteristic for an oxygenated function at C-19 of polycyclic modified triterpenes, according to the molecular formula C26H30O8 determined by EIMS. The presence of an α, β-epoxy-δ-lactone in the D-ring was established by the signals at δ 5.47 (H-17) y δ 4.04 (H-15), suggesting a limonoid-type substance. COSY experiments allowed the assignments of the signals at δ 2.68 (dd, J = 16.8, 2.1) and δ 2.98 (dd, J = 16.8, 3.9 Hz) for the hydrogens α to the carbonyl group in the A-ring, while those at δ 2.86 (t, J = 15) and δ 2.47 (dd, J = 15, 3.5 Hz) corresponded to the hydrogens at C(6). The methyl signals at δ 1.30, 1.18, 1.17 and 1.08 were assigned to CH3-18, CH3-25, CH3-26 and CH3-24, respectively, confirming the structure of limonin A (1) for this secondary metabolite [17]. Friedelin, β-sitosterol, lupeol [18], cryptomeridiol [19], lupenone and caryophyllene epoxide [20] were isolated from the aerial parts of E. velutinosa and identified by direct comparison with authentic samples.
These results along with those previously reported [3-14] indicate that Esenbeckia species biosynthesize different structural types of secondary metabolites. Additional chemical studies of other species are necessary in order to establish the chemotaxonomic significance of the isolated substances.
Experimental
Esenbeckia berlandieri ssp berlandieri ex Hemsley was collected in the state of Veracruz. A voucher specimen is deposited at the National Herbarium, (MEXU, CHR-30). The organic extract was obtained by maceration with ethanol of the air-dried aerial plant material (1.9 kg). This extract was chromatographed at reduced pressure (VLC) [21] using n-hexane and mixtures of n-hexane-ethyl acetate as elution system. This procedure allowed to isolate friedelin (240 mg), β-sitosterol (100 mg), and β-sitosteryl-β-D-glucoside (390 mg). Friedelin. White crystals, mp 245-247°C [15]; IR (CHCl3) νmax 2943, 2867, 1703, 1455, 1389, 1361, 1192, 1139, 1110 cm−1; 1H NMR (CDCl3, 300 MHz): δ 0.72 (3H, s, CH3-27), 0.85 (3H, d, CH3-23), 0.87 (3H, s, CH3-29), 0.95 (3H, s, CH3-24), 1.0, 1.18 y 1.25 (9H, s, CH3-25, CH3-26, CH3-28), 2.25 (1H, c, J = 7 Hz, H-4), 2.42 (1H, dd, J = 5 Hz, H-2), 2.38 (1H, dd, J = 5, 2 Hz, H-2); EMIE m/z (int. rel.): 426 [M+] (29), 302 8169, 274 (22), 273 8379, 246 (25), 218 8299, 205 824), 191 830), 179 (32), 164 (36), 125 (59), 123 (60), 121 (40), 109 (77), 96 852), 95 (100), 81 (60), 69 (62), 55(93).
The seeds of E. ovata Brandegee (940 g; collected in the state of Veracruz, provided by Prof. C.H. Ramos, key: CHR 32) were extracted with n-hexane and then with CHCl3 at room temp. affording 63.4 g of residue. The crude CHCl3 was adsorbed onto silica gel and analyzed via vacuum liquid chromatography (VLC) [21], the cromatographic fractions were monitored using the Ehrlich´s test. Limonin (1 [17], 123 mg) was isolated after purification by recristallization from EtOH-iPrOH. White crystals, mp: 232-233°C, orange with Ehrlich's test; IR (CHCl3) νmax 3064, 2958, 2876, 1753, 1284, 1028 cm−1; NMR 1H (CDCl3, 300 MHz): δ1.30, 1.18, 1.78 and 1.08 (s, 12H), 2.23 (dd, J = 15.9 0, 3 Hz, 1H, H-5), 2.47 (dd, J = 14.7, 3.5 Hz, 1H, H-6a), 2.56(dd, J = 12.2, 3 Hz, 1H, H-9), 2.68 (dd, J = 16.7, 2.1 Hz, 1H, H-2a), 2.86 (t, J = 15.5 Hz, 1H, H-6b), 2.98 (dd, J = 16.8, 3.9 Hz, 1H, H-2b), 4.04 (s, 2H, H-1 and H-15), 4.47 (d, J = 13.2 Hz, 1H, H-19a), 4.77 (d, J = 13.2 Hz, 1H, H-19b), 5.47 (s, 1H, H-17), 6.34 (dd, J = 1.35, 0.9, 1H, H-22), 7.41 (s, 2H, H-21 and H-23). 1H NMR (DMSO-d6, 500 MHz): δ 1.18, 1.11, 1.03 and 1.00 (s, 12H), 2.28 (dd, J = 15, 3 Hz, 1H, H-5), 2.46 (dd, 15.8, 3.5 Hz, 1H, H-6a), 2.58 (dd, J = 19.5, 3 Hz, 1H, H-9), 2.62 (dd, J = 16.5 Hz, 1H, H-2a), 2.77 (t, J = 16 Hz, 1H, H-2b), 3.12 (dd, J = 15 Hz, 1H, H-6a), 4.11 (d, J = 15 Hz, 2H, H-1 and H-15), 4.92 (d, J = 13.2 Hz, 1H, H-19a), 4.48 (d, J = 13 Hz, 1H, H-19b), 5.48 (s, 1H, H-17), 6.51 (dd, J = 1.5, 1.0, 1H, H-22), 7.68 (t, J = 2 Hz, 2H, H-23), 7.72 (d, J=0.5 Hz, 1H. H-21). 13C NMR (CDCl3, 75 MHz, assignments by APT): δ77.8 (t, C-2), 36.4 (C-2), 166.6 (C-3), 80.3 (C-4), 53.9 (C-5), 35.7 (C-6), 206.1 (C-7), 45.9 (C-8), 48.1 (C-9), 51.3 (C-10), 18.9 (C-11), 30.8 (C-12), 37.9 (C-13), 65.7 (C-14), 60.6 (C-15), 169.1 (C-16), 79.2 (C-17), 21.4 (C-18), 65.3 (C-19), 119.9 (C-20), 141.1 (C-21), 109.6 (C-22), 143.2 (C-23), 17.6 (C-24), 20.7 (C-25), 30.2 (C-26). EMIE m/z (rel. int.). 348 (100), 347 (28.2), 329 (12.8), 147 (20.5), 121 (28.2), 119 (20.5), 97 (33.3), 95 (100), 93 (35.9), 91 (56.4), 69 (59), 43 (100), 41 (61).
After drying, the aerial parts of E. velutinosa C. H. Ramos (sp. nov.) (1.8 kg, provided by C. H. Ramos and deposited at the National Herbarium) were macerated with acetone. The crude extract (62 g) was adsorbed onto silica gel and carefully chromatographed on a silica gel column at reduced pressure (VLC) [21] eluting with n-hexane and n-hexane containing increasing proportions of EtOAc. This procedure allowed the isolation of friedelin (250 mg) [15], β-sitosterol (220 mg), lupeol (22 mg) [18]. Elution with n-hexane-EtOAc (3:2), afforded 3.45 g of a residue, which was rechromatographed at reduced pressure (VCC), and some fractions (TLC control) were further purified by prep. TLC (silica gel, n-hexane-AcOEt, 5:9), to give cryptomeridiol (19 mg) [19]. The mother liquors from the isolation of friedelin, were rechromatographed via VLC using n-hexane as eluent, to give lupenone (20 mg) and caryophyllene β-oxide (40 mg) [20]. All the substances were identifed by direct comparison with authentic samples.
Acknowledgements
The authors are grateful to M. Sc. Clara H. Ramos (Instituto de Biología, UNAM) for the supply and identification of plant material, to M. Sc. María Isabel Chávez, Beatriz Quiróz, Rocío Patiño, Luis Velasco and Francisco J. Pérez Flores from Instituto de Química de la UNAM for technical assistance, and Dr. Guillermo Delgado for stimulating interest and support to this work. We also thank to Carrera de Biología and Coordinación de Investigación de la FES-Zaragoza (UNAM) for partial financial support.
References
1. Kaastra, R. C. Acta Biot. Neerl. 1977, 26, 471-476. [ Links ]
2. Ramos, C. H. Revisión Taxonómica de Esenbeckia (Rutaceae) de México. Tesis de Maestría. Facultad de Ciencias. UNAM (en proceso).
3. Vitagliano, J. C.; Comin, J. Anales Asoc. Quím. Argentina 1970, 58, 273-275. [ Links ]
4. Vitagliano, J. C.; Comin, J. Anales Asoc. Quím. Argentina 1970, 58, 59-61. [ Links ]
5. Dreyer, D. L.; Peckering, M. V.; Cohen, P. Phytochemistry 1972, 11, 705-713. [ Links ]
6. Dreyer, D. L.; Phytochemistry 1980, 19, 941-944. [ Links ]
7. Bevalot, F.; Fournet, A.; Moretti, C. and Vaquette, J. Planta Medica 1984, 50, 522-523. [ Links ]
8. Rios, M. Y.; Delgado, G. J. Nat. Prod. 1992, 55, 1307-1309. [ Links ]
9. Rios, M. Y.; Delgado, G. Phytochemistry 1992, 31, 3491-3494. [ Links ]
10. Guilhon, G. M. P. S.; Baetas, A. C. S.; Maia, J. G. S.; Conserva, L. M. Phytochemistry 1994, 37, 1193-1195. [ Links ]
11. Delle Monache, F.; Delle Monache, G.; De Moraes E Souza, M. A. Gazz. Chim. Ital. 1989, 119, 435-439. [ Links ]
12. Delle Monache, F.; Di Benedetto, R.; De Moraes E Souza, M. A. Gazz. Chim. Ital. 1990, 120, 387-389. [ Links ]
13. Oliveira, F. M.; Santana, A. E. G.; Conserva, L. M.; Maia, J. G. S.; Guilhon, G. M. P.; Phytochemistry 1996, 41, 647-649. [ Links ]
14. Rios, M. Y.; Aguilar, A.B.; Charnichart, A.; Delgado, G.; Ramos C.H. Nat. Prod. Lett 2000 (enviado).
15. Gunatilaka, A. A. L.; Nanayakkara, N. P. D.; Wazeer, M. I. M. Phytochemistry 1983, 22, 991-992. [ Links ]
16. Dreyer, D. L. Fortsch. Chem. Org. Naturst. Herz, W., Ed., Springer-Verlag. New York, 1968, 26, 109-244. [ Links ]
17. Baarschers, W. H. Org. Mass Spectrom. 1972, 6, 367-372. [ Links ]
18. (a) Lehn, J. M.; Ourisson, G. Bull. Soc. Chim. France 1962, 1138. [ Links ] (b) Reynolds, W.F.; McLean, S.; Poplawski, J.; Enríquez, R.G.; Escobar, L.I.; León, I. Tetrahedron 1989, 42, 3419. [ Links ]
19. Reynolds, W. F.; Delgado, G.; Chávez, M. I.; Díaz, E. Spectroscopy Int. J. 1988, 6, 113-122. [ Links ]
20. Bohlmann, F.; Zdero, Ch. Phytochemistry 1978, 17, 1135-1153. [ Links ]
21. Coll, J. C.; Bowden, B. F. J. Nat. Prod. 1986, 49, 934-936. [ Links ]














