Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.46 no.4 Ciudad de México oct./dic. 2002
Investigación
Studies on Salts of Amine-Containing Polymers with Benzoic Acids. IV. Polymeric Salts of Some Benzoic Acids with Poly(Dimethylaminopropyl Methacrylamide) grafted onto Polyethylene Films
Emilio Bucio,1 Guillermina Burillo,1 Takeshi Ogawa2
1 Instituto de Ciencias Nucleares, Universidad Autónoma de México, Circuito Exterior, Ciudad Universitaria, Coyoacán 04510, México, D.F.
2 Instituto de Investigaciones en Materiales, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, Coyoacán 04510, Mexico D.F.
Recibido el 12 de julio del 2002.
Aceptado el 21 de noviembre del 2002.
Abstract
In order to obtain films having functional groups on the surface, polyethylene films were grafted with poly(N,N-dimethy-laminopropyl methacrylamide), and various benzoic acids were added to obtain polymeric salts. The benzoic acids having long substituents such as decynyloxy and n-octyloxy benzoic acids, showed mesophases (liquid crystalline phase), and no phase separation of the acids was observed on repeated heating and cooling. The stability is attributed to the interaction of the long substituents with polyethylene chains. Ethoxybenzoic acid having a short substituent, formed a polymeric salt but it is irreversibly separated from system on heating.
Key words: Gamma irradiation, graft polymers, polymeric salts.
Resumen
Se prepararon películas de injerto de poli(N,N-dimetil-amino propilmetacrilamida) en polietileno mediante radiación gamma de C0-60, con el objeto de tener una superficie con grupos funcionales que puedan formar sales con varios derivados del ácido benzoico. Los ácidos benzoicos con substituyentes de cadena larga tales como el deciniloxi y el n-octiloxi mostraron mesofases y no se observó separación de fases entre el ácido benzoico y la película de injerto al calentar y enfriar repetidamente. La estabilidad se puede atribuír a la interacción de las cadenas olefínicas substituyentes con las cadenas del polietileno. El etoxibenzoico, teniendo una cadena olefínica corta, forma la sal polimérica correspondiente , pero se separa del sistema en forma irreversible, al ser calentada.
Palabras clave: Irradiación gama, polímeros de injerto, sales poliméricas.
Introduction
Mixed systems of functional compounds with host polymers are of interest when the materials with the functionality are desired in the form of films or filament. When the host polymers and guest functional compounds have certain chemical interactions, such as hydrogen bonding, salt formation and dipole/dipole interactions, the concentration of the guest molecules can be increased, and the mixed system becomes more stable, and there is less possibility of phase separation and agglomeration of functional compounds. Such concept in developing novel functional materials through non-covalent bonding is often employed in obtaining polymeric liquid crystals [1-3]. The present authors have studied the systems consisting of various benzoic acids with poly(N,N-dimethy-laminoethyl methacrylate), and found that the morphology of the mixed systems varies considerably depending on the types of the acids, and the concentration of acids could be reached up to 90 % mole without phase separation [4,5]. However, the polymeric salts consisting of acids or base host polymers and base or acid guest compounds, are generally hygroscopic, and therefore water absorbed influences morphology and properties, and this is not desirable. In order to decrease hygroscopicity of the polymeric salts, and to give mechanical strength to the obtained films, the host polymer can be grafted to hydrophobic polymer films, and thus a polymer film coated with polymeric salts can be obtained. Previously, poly(N,N-dimethylaminoethyl methacrylate) was grafted onto polyethylene film [6], and several benzoic acids were added to form the corresponding salt films [7]. By the grafting to polyethylene, the phase separation between the acids and the host polymer became reversible, that is to say, the acids separate out from the amino polymer on heating, but they form salts again on cooling. However, poly(N,N-dimethylaminoethyl methacrylate) is vulnerable to the hydrolysis of the methacrylate ester which is caused by absorbed water and the dimethylaminoethyl group, and the properties change on standing for a long period. In this work, therefore, N,N-dimethylaminopropylmethacrylamide (DMPMA) was employed as a grafting polymer because it is more resistant to the hydrolysis. Thus, a commercial polyethylene films were grafted with DMPMA, and the grafted films were added with several benzoic acids to obtain novel polymer salt films. Characterization and some morphology of such products are reported in this paper.
Experimental
Materials. The benzoic acids employed include p-ethoxy, p-butoxy, p-n-octyloxy and p-n-hexylbenzoic acids, all of which were supplied by Aldrich, and used as received. p,p'-alkyl-tolancarboxyl acids and p-1-alkynylbenzoic acids were synthesized in our laboratory[4]. The polyethylene (PE) film used was a PEMEX low density PE with thickness of 0.7 mm and melting at 115 °C. N,N-Dimethylaminopropylmethacrylamide (DMPMA) was supplied by Aldrich, and it was distilled under a reduced pressure before use.
Graft polymerization. A film cut to a square of 1 cm × 5 cm was placed in an ampoule in which required amounts of DMPMA and dioxane (solvent) were added, and the ampoule was sealed off in vacuum. The ampoule was then irradiated with γ-ray using Gamma Beam 651-PT with 50,000 Curies of Nordion Corporation, Canada. After irradiation the films were poured into methanol and washed well with fresh methanol to remove the homopolymer of DMPMA, and dried in vacuum and weighed. The grafted film was then dipped in methanol containing benzoic acids, and stirred gently for 24 h. The film was then washed well with fresh methanol, dried in vacuum at room temperature, and weighed. The grafting yields were calculated from the weight increase: (Wg - W0) × 100 / W0, where Wg and W0 are the weights of the film after and before grafting, respectively. They were then converted to mole per-cent with respect to ethylene. The percentage of salt formation was calculated from the weight increase due to benzoic acids. The process is shown in Fig. 1.

Characterization. A polarizing optical microscope observation was performed using an Olympus BHC fitted with a Leitz hot stage. Differential Scanning Calorimetry (DSC) was made using a calorimeter of TA instruments, Model 910 under nitrogen. FT-IR spectra were taken using a spectrometer of Perkin Elmer Model 1600 with ATR mode. X-Ray diffraction patterns on films samples were obtained using an INEL CPS120 curved counter equipped with a quartz bent monochromator for the CuKα radiation, and a computer controlled oven, with a temperature precision of ± 0.03 °C.
Results and Discussion
Table 1 shows the results of DMPMA grafting onto PE films. The 30 % monomer concentration with respect to the solvent was chosen arbitrarily, but the homopolymer was formed with higher monomer concentrations, and this is inconvenient in separation of the homopolymer from the grafted polymer. The yield of grafting increased with increase in polymerization temperature and given dose. The greater the dose the more initiating radicals are formed in the PE films thus increasing the amount of grafted polyDMPMA. The temperature is also an important factor as can be seen from the difference between GP-1 and GP-5 (Table 1). This large difference is attributed to the increase in the rate of polymerization with temperature, and also to increase in the monomer diffusion in PE with temperature. Table 2 shows the amounts of the benzoic acids united to the films through the salt formation with the tertiary amine groups of polyDMPMA. The low percentage indicates that the benzoic acids cannot penetrate deep into the grafted films to form their salts, but they form salts with those amine groups near the surface of films. It is known that the grafting starts first on the surface of PE film as the initiating free radicals are formed on the surface, then the grafting develops toward the inside of the film [8]. This depends on swelling of the film and diffusion of monomer through the film. With respect to the benzoic acids, the small ethoxybenzoic acid is thought to penetrate more easily between the polymer chains, while the large acids will encounter with steric hindrance to penetrate in the polymer chains. This is a significant difference between the free polymer chains and the grafted polymer chains. In the case of free polymers there is no steric hindrance for the benzoic acids, and thus the salt formation reaches 90 % [4, 5]. All of the benzoic acids employed in this work are those having electron donor groups, and therefore they are week acids, and their interaction with the base tertiary amine group can be week. However, when the acids were mixed with the grafted films, the typical absorption of their carbonyl groups at 1675-1685 cm-1 decreased and shifted to around 1630 cm-1 becoming a shoulder of the peak of aromatic group at around 1600 cm-1, indicating that they form salts with the tertiary amine.
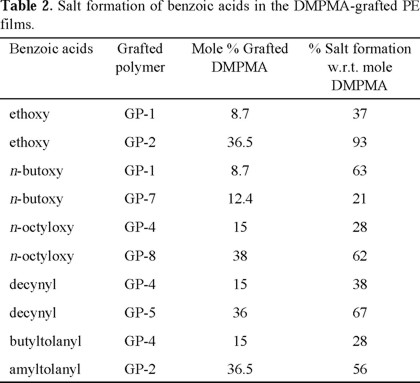
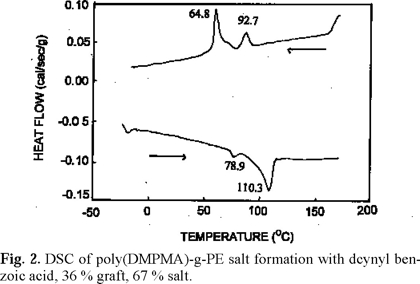
The DSC curves of these materials showed an endotherm peak due to melting of crystalline PE around 111-115 °C. The benzoic acids having p-substituents with a reasonable size which were used in this is study, exist as dimers through their hydrogen boding, and they form mesophases (nematic liquid crystals) just under their melting points [9]. However, the phase transitions of these benzoic acids dimers were not observed in the DSC curves of their mixtures with the polymer. This indicates that the benzoic acids exist as salts with the tertiary amine (polyDMPMA), and their phase transitions are different from those of benzoic acid dimers. However, mesophases were observed on DSC during cooling process for the long chain benzoic acids. For example, in the case of decynyl benzoic acid with 36 % grafting and 67 % salt formation (Fig. 2), a small endotherm peak was observed at 78.9 °C before the larger endotherm peak due to melting of crystalline PE at around 110 °C. The isotropic point of decynyl benzoic acid dimer is also 110 °C and therefore the two endotherm peaks due to the melting processes could be overlapped. However, since the acid exists as salt, this melting temperature of 110 °C is no longer valid. During the cooling process of this system, there were observed 2 exotherm peaks at 92.7 (crystallization of PE) and 64.8 °C (solidifying the salt). During the heating process, polarized optical microscope observation revealed that the mesophase lies between these two temperatures. a colorful texture appeared at 78 °C, and its brightness decreased at 110 °C, and the process was repeatable. These results suggest that the polyDMPMA-decynylbenzoic acid salt gives a mesophase between 78 °C and 110 °C. This is also supported by the X-ray diffractometry at different temperatures as shown in Fig. 3. The peak at around 3 (2θ) is the peak of decynylbenzoic acid in its mesophase, and the peak at around 20 is that of crystalline PE. The intensity of the peak due to the acid increased with temperature, and it dropped when the temperature reached to the isotropic temperature. This is commonly observed for liquid crystalline systems [10].
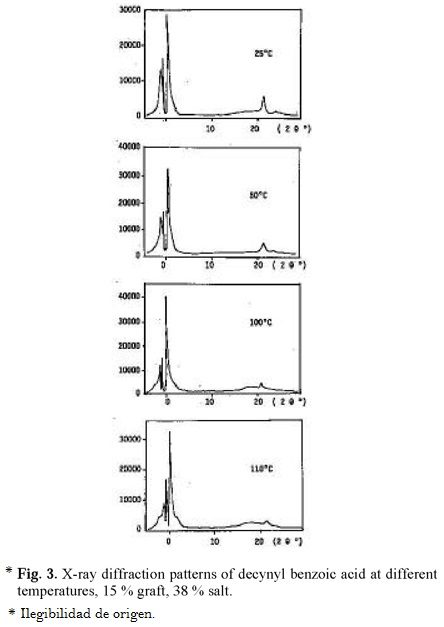
In the case of amyltolan benzoic acid with 36 % grafting and 56 % salt formation (Table 2) DSC showed 2 exotherms at 97.8 °C and 64.2 °C during cooling process. Under the microscope the texture started to lose brightness at temperature above 90 °C, and at 111 °C the texture seems to melt, and on cooling the original texture was reproduced. Table 3 summarizes the microscope observation of some of these salt systems.
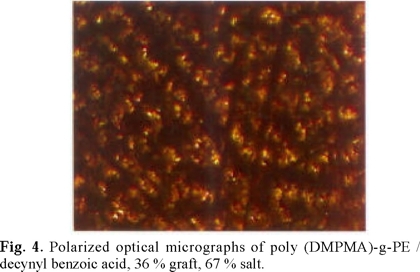
In the cases of ethoxy and butoxy benzoic acid systems, polarized microscope observation revealed that the acids separated from the polymer on heating at the melting point of PE (110 °C), giving colorful crystals of these benzoic acid, and the process was not reversible. This is probably because the ethoxy or butoxy group is too small to maintain the mutual interaction by miscibility with the polymer, probably PE, in this case. In the case of the acids with larger substitute groups, such as octyloxy, octynyl, butyltolan, pentyltolan and decynyl benzoic acids, the phase separation was not observed, and they showed the existence of mesophases below the melting point of PE. One of such textures is shown in Fig. 4. The brilliant parts in the picture are due to the mesophase, and when the temperature reaches the melting point of PE, this pase slowly fades out, but on cooling it reappears. In the previous study on the poly(dimethylaminoethyl metacrylate) systems, when the polymetahcrylate is grafted to PE [7], no phase separation was observed, while with polymethacrylate alone [5] the phase separation was observed. This is an interesting finding that PE is important to avoid the phase separation of acids. It is thought that the long substituents such as decynyl group have an attracting interaction with PE chains.
Conclusion
Stable polymeric salts can be formed between benzoic acids with long substituents and PE grafted with tertiary amine polymers. The materials showed mesophase (liquid crystals) at temperatures below the melting point of PE. It was shown that novel materials could be obtained using grafted amine-containing polymers and functional acids.
It was shown that various types of polymeric materials could be obtained by the surface treatment of common polymers by the method of this work.
Acknowledgement
The authors are grateful to DGAPA UNAM (contract No IN1004000) for their financial support, to Eng. Carmen Vázquez from IIM UNAM, MS Susana Castillo and Phys. Francisco García from ICN UNAM, and Dr. Henrich Benoit from IPCMS Strasbourg, France, for their technical assistance.
References
1. Bazuin, C.G.; Brandys, F.A.; Eve, T.M.; Plante, M. Macromol. Sym., 1994, 84, 183-196. [ Links ]
2. Bazuin,; Tork, A, Macromolecules, 1995, 28, 8877-8880. [ Links ]
3. Brandys, F.A.; Bazuin, C.G, Chem. Mater., 1996, 8, 83-92. [ Links ]
4. Calderon, M.E. Thesis for MsC., 1993, Faculty of Science, National University of Mexico.
5. Marin, M.;. Baños, L.; Moreno, A.; Burillo, G.; Ogawa, T, Polym. Bull. 1995, 34, 227-233. [ Links ]
6. Burillo G.; Oseguera, M.A.; Vazquez, C.; Castillo del L.F., Radiat. Phys. Chem. 1997, 50, 511-517. [ Links ]
7. Burillo, G.; Bucio, E., Cervera, E.; Ogawa, T, J. Appl. Polym. Sci. 2000, 78, 972-978. [ Links ]
8. Chapiro, A. 1962, Radiation Chemistry of Polymeric Systems, Interscience, New York. [ Links ]
9. Kelker, H.; Hatz, R. Handbook of Liquid Crystals, Verlag Chemie, Weinheim, 1980. Chapt. 2, pp 105. [ Links ]
10. Ruokolaainen, J.; Tenner, J.; Ikkala, O.; ten Brinke, G.; Thomas, E.L, Macromolecules 1998, 31, 3532-3536. [ Links ]














