Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias geológicas
versión On-line ISSN 2007-2902versión impresa ISSN 1026-8774
Rev. mex. cienc. geol vol.29 no.3 Ciudad de México dic. 2012
A Cenomanian aipichthyoid fish (Teleostei, Acanthomorpha) from America, Zoqueichthys carolinae gen. and sp. nov. from El Chango quarry (Cintalapa Member, Sierra Madre Formation), Chiapas, Mexico
Un pez aipichthyoideo (Teleostei, Acanthomorpha) del Cenomaniano de América, Zoqueichthys carolinae gen. y sp. nov. de la cantera El Chango (Miembro Cintalapa, Formación Sierra Madre), Chiapas, México
Jesús Alvarado-Ortega1* and Bruno Andrés Than-Marchese2
1 Instituto de Geología, Universidad Nacional Autónoma de México; Circuito de la Investigación S/N, Ciudad Universitaria, Coyoacán, D.F., 04510, México. *alvarado@geologia.unam.mx
2 Museo de Paleontología Eliseo Palacios Aguilera SEMAHN, Calzada de los Hombres Ilustres S/N, Colonia Centro, Tuxtla Gutiérrez, Chiapas, México.
Manuscript received: April 17, 2012
Corrected manuscript received: July 22, 2012
Manuscript accepted: July 24, 2012.
ABSTRACT
Zoqueichthys carolinae gen. and sp. nov. is described based on three specimens collected at the El Chango quarry, an outcrop of Cenomanian marine limestones belonging to the Sierra Madre Formation located in the Municipality of Ocozocoautla de Espinosa, near Tuxtla Gutiérrez, Chiapas State, southern Mexico. This species is identified as a new member of the superfamily Aipichthyoidea because it possesses a combination of characteristics previously recognized as diagnostic for this group. This species differs from other aipichthyoides in skeletal and meristic characteristics not previously reported in this superfamily including a completely laminar supraoccipital crest involving the participation of the supraoccipital and frontal bones, and lacking anterior thickening or spine; a pelvic fin with eight rays, and pectoralfin with twelve rays. Zoqueichthys carolinae is a basal member of the family Aipichthyoididae because it shows putative primitive characteristics including a comparatively high number of pelvicfin rays, three epurals, hypurals one and two are autogenous and in addition a complex supraoccipital crest. Zoqueichthys carolinae is the first aipichthyoid fish found in marine Cretaceous sediments deposited in the western Tethys Sea of southern North America.
Key words: Aipichthyoidea, Aipichthyoidae, Aipichthyoididae, Cenomanian, Mexico.
RESUMEN
Zoqueichthys carolinae gen. y sp. nov. es descrito a partir de tres ejemplares colectados de la cantera El Chango, un afloramiento Cenomaniano de calizas marinas pertenecientes a la Formación Sierra Madre, ubicado dentro del Municipio de Ocozocoautla de Espinosa y próximo a Tuxtla Gutiérrez, Chiapas, sureste de México. Esta especie es miembro de la superfamilia Aipichthyoidea porque presenta los rasgos diagnósticos de la misma. Esta especie es diferente de otros aipichthyoideos en rasgos merísticas y esqueléticos que anteriormente no habían sido reportadas en la superfamilia; su cresta supraoccipital es totalmente laminar, está formada por los huesos supraoccipital y frontal, y carece de un engrosamiento o espina anterior; además sus aletas pélvica y pectoral están formadas por ocho y doce radios respectivamente. Zoqueichthys carolinae es un miembro basal de la familia Aipichthyoididae ya que presenta un elevado número de radios en la aleta pélvica, tres epurales, sus dos primeros hipurales son autógenos, y su cresta supraoccipital es compleja. Esta especie es el primer pez de la superfamilia Aipichthyoidea encontrado en rocas marinas del Cretácico depositadas bajo el Dominio Occidental del Mar de Tetis.
Palabras clave: Aipichthyoidea, Aipichthyoidae, Aipichthyoididae, Cenomanian, México.
INTRODUCTION
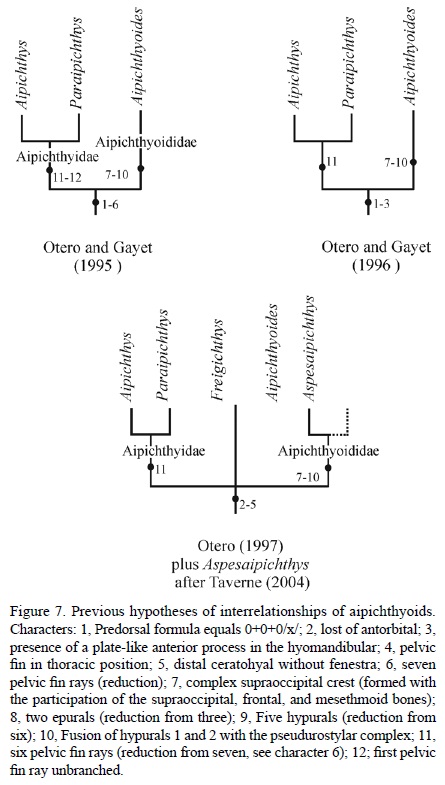
Otero and Gayet (1996) named the superfamily Aipichthyoidea. This group of basal acanthomorphs includes highly specialized small marine fishes, characterized by their rounded bodies that bear large unpaired fins armed with anterior spines and a pelvic fin in thoracic position, and by their short and high heads showing a hypertrophied supraoccipital crest ("sagittal crest" of Otero and Gayet, 1996) lacking the antorbital bone. Currently this group comprises two families, Aipichthyidae Patterson (1964) and Aipichthyiodidae and Gayet (1980a).
Until now, the superfamily Aipichthyoidea comprised primarily Cenomanian fishes discovered in eastern Tethyan outcrops of Europe and the Middle East, and included five species of Aipichthys: the type species A. pretiosus Steindachner, 1850, from Comen, Croatia (Dalmatia in Patterson, 1964: 303); A. minor Pictet, 1850, from Hakel (=Haqil) and Hadjula, Lebanon; A. velifer Woodward, 1901, and A. oblongus Gayet, 1980b, from Hakel; and A. nuchalis Dixon, 1850, from Sussex, England; as well as Paraipichthys lusitanicus Gaudant, 1978, from Laveiras, Portugal; two species of Aipichthyoides described by Gayet (1980a) under the names A. galeatus and A. for-mosus, from Ein Yabrud, Israel; Freigichthys elleipsis Otero, 1997, from marine outcrops of Hadjula (=Hgula), Lebanon; and Aspesaipichthys cavaensis Taverne, 2004, from Campanian-Maastrichtian of Nardo, Italy.
Although the first phylogenetic analysis of Aipichthyoidea performed by Otero and Gayet (1996) supports the inclusion of Paraipichthys in the family Aipichthyidae, relationships within the superfamily became unclear after the discovery of Freigichthys (Otero, 1997, fig. 5). The inclusion of Aspesaipichthys in family Aipichthyiodidae, as Taverne (2004) intended, requires further examination. Beyond these works, the phylogeny of this superfamily has not been re-examined.
The aim of this paper is to describe and discuss the relationships of Zoqueichthys carolinae gen. and sp. nov., an aipichthyoid fish known from three beautifully preserved specimens collected in Cenomanian strata of El Chango quarry, Chiapas, southern Mexico. Prior to the present paper, Aipichthyoidea were known as a marine Late Cretaceous group restricted to the eastern Tethys Sea domain of Europe and the Middle East. The discovery Zoqueichthys has important implications because it complements the taxonomic diversity of the group and improves our knowledge of the temporal and geographic distribution patterns of these primitive acanthomorphs. The presence of Zoqueichthys in Mexico suggests that the early evolution and distribution of superfamily Aipichthyoidea took place within a wider and more complex geographical setting that included both western and eastern domains of the Tethys Sea.
El Chango quarry is an outcrop of limestones near Tuxtla Gutiérrez, Chiapas State, southern Mexico (Figure 1). This site is located at 16°34'14.9"N and 093°16'12.7"W within Ocozocautla de Espinosa Municipality. Since 2006 a well-preserved and diverse fossil assemblage has been collected in the laminated and monotonous sequence exposed in this outcrop, which pertains to the Sierra Madre Formation (Ovalles-Damián et al., 2006; Alvarado-Ortega et al., 2009; among others). According Vega et al. (2007) these strata accumulated within an estuarine or salty lagoon with ephemeral freshwater influx.
The fossil assemblage thus far collected at El Chango includes fishes, plants, mollusks, crustaceans, and insects. Recently, Alvarado-Ortega et al. (2009) found that the fishes found at El Chango (and El Espinal) indicate a Cenomanian age. The fishes are the best-preserved fossils at El Chango. The specimens are tightly laterally compressed, usually complete and articulated, and often show parts of soft tissues (muscles) and stomach contents phosphatized.
Böse (1905) described the Cretaceous platform carbonate series that includes flint-bearing dolomite and limestone exposed along the Central Chiapas depression, where El Chango quarry is found, as "Cretaceous limestones with rudists". Although Wiebe (1925) named these strata the San Cristóbal Formation, Nutall (1929, in Salas, 1949) suggested the name Sierra Madre Formation that is currently in use. Salas (1949) grossly divided this formaton in two sequences, an Albian-Cenomanian unit of limestones having sugary aspect and apparently barren of fossils, and a Turonian unit of limestones bearing flint and rudists. Later, González (1963) formalized this suggestion, recognizing these units as the Cantelhá and the Jolpabuchil members respectively.
The discovery of microfossils during petroleum exploration contributed to re-ordering the units of the Sierra Madre limestones (Figure 1). Sánchez-Montes de Oca (1969, 1973), Álvarez-Mena (1975), and others, suggested that these actually comprise two formations, at the base the Sierra Madre Formation made up of the Cantelhá (Lower-middle Albian) and Cintalapa (Cenomanian) members, and at the top the Jolpabuchil Formation (Turonian-Santoniano). Steele (1986) and Waite (1986) subdivided the Sierra Madre limestones into lithologic and biostratigraphic units (herein these are simplified as 1 to 4 in Figure 1), which include Upper Albian-Santonian strata. Rosales-Domínguez et al. (1994, 1997) carried out microfossil analyses along the Suchiapa River and on the Ocozocuautla-Cintalapa road that also indicate an Albian-Santonian age for these strata. The fossiliferous strata at El Chango quarry represent a section within the 700 to 1600 meter thick sequence of the Cenomanian Cintalapa Member of the Sierra Madre Formation.
MATERIAL AND METHODS
Preparation methods. The specimens were prepared in baths of 5-10% acetic acid solution and hardened with Plexygum. Pin vises and needles were used under a binocular microscope to remove matrix from the skeletons.
Anatomical abbreviations. Most of the abbreviations used in Figures 2 through 7 follow Patterson (1964), Gaudant (1978), Otero and Gayet (1995, 1996), and Stiassny and Moore (1992).
Comparative material analyzed. The following specimens were examined for comparative purposes; all belong to the Otto Haas Collection, housed in the Bayerische Staatssammling für Paläontologie und Geologie, München, Germany, and catalogued under the following numbers. Aipichthys minor: specimens 177, 178, and 180, from Hakel, Lebanon. Aipichthy sp.: specimen Nr. 1984 I 230, from Hakel, Lebanon. Additional comparative data were obtained from available literature.
SYSTEMATIC PALEONTOLOGY
Cohort Acanthomorpha Rosen, 1973
Order incertae sedis
Superfamily Aipichthyoidea Otero and Gayet, 1996
Family Aipichthyoididae Gayet, 1980a
Emended diagnosis. Aipichthyoidea with high, long, and complex supraoccipital crest that extends over the midline of the skull from the nasal area to occiput, with the anterior and posterior edges rounded and convex; and the supraoccipital and frontal bones forming the supraoccipital crest.
Genus Zoqueichthys gen. nov.
Type species. Zoqueichthys carolinae sp. nov.
Etymology. The generic name derives from "Zoque", a native group of people in Chiapas, and "Ichthys" or fish in Greek. It means "the fish of the Zoque people".
Diagnosis. As in the type species, below.
Zoqueichthys carolinae sp. nov.
(Figures 2-6 (3, 4, 5), Table 1)


Etymology. The species name honors Mrs. Carolina Elvira Marchese y Tronkar, mother of second author of present paper.
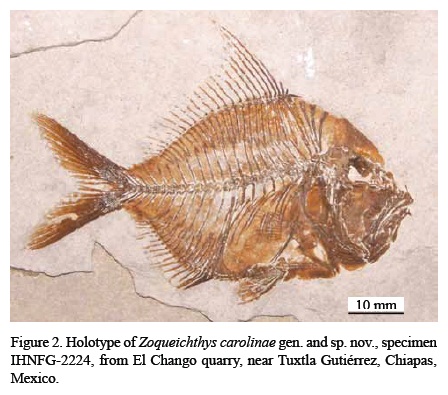
Holotype. IHNFG-2224, a complete specimen exposing the right lateral side and having total and standard length (SL) of 69 and 49 mm respectively (Figure 2).
Referred material. IHNFG-2225, a specimen in part and counterpart, incompletely preserved, and exposing its right lateral side; it lacks the whole dorsal area from the vertebral column and the caudal fin; its estimated SL is about 58 mm. IHNFG-2270, a specimen without the caudal fin and exposing its left lateral side, with an estimated SL of about 58 mm.
Diagnosis. Aipichthyoididea with an ovoid body, maximum known standard length of 49 mm and maximum body height representing 60% of SL; its supraoccipital crest is high and long, projecting over the midline of the skull from the occiput to the mesethmoid area, with its anterior and posterior edges rounded and convex, and formed by the supraoccipital and frontal bones; the vertebral column includes 11 abdominal and 16 caudal vertebrae; dorsal fin includes three anterior spines and 24 segmented and branched rays supported by 26 pterygiophores; anal fin is formed by three spines and 17 branched and segmented rays supporting 18 pterygiophores including the first pterygiophore that is transformed in the hemaxanal complex; pectoral fin includes 12 segmented and branched rays; pelvic fin in thoracic position has eight rays; all pelvic rays are segmented and branched except for the first that is unbranched; 19 principal caudal fin rays, including two unbranched and 17 segmented and branched rays plus 10 and eight dorsal and ventral procurrent rays (10-I+9—8+I-8).
Locality. El Chango quarry, Ocozocoautla de Espinosa Municipality, near Tuxtla Gutiérrez, Chiapas State, southern Mexico.
Stratigraphic horizon. Cintalapa Member (Cenomanian) Sierra Madre Formation.
Description.
General proportions. Table 1 summarizes the measurements of the specimens described herein. Zoqueichthys carolinae gen. and sp. nov is a centimetric diamond-shaped fish with a deeply forked homocercal tail, a gently rounded dorsal trunk profile continued by a large triangular supraoccipital crest, and a ventral border also rounded but having an abdominal horizontal flat profile. In this species the maximum height of the trunk is about 60% of the standard length (SL); the head is about 1.75 times higher than long, with its length being about 34.4-36% of the SL. Its trunk shows abdominal and postabdominal regions with the same length. The pectoral fin is located in the middle of the body between the vertebral column and the ventral body border. The pelvic fin position is thoracic. Anal and dorsal fins are long, the former being 50% and the latter being 26% of SL.
Skull. The skull in Zoqueichthys carolinae gen. and sp. nov. is triangular and 1.5 times longer than high, with its ocular diameter representing a third of its length (Figures 3). The mesethmoid is a well ossified and complex bone; it has a ventral arm that forms the anterior edge of the nasal capsule and joins with the parasphenoid and probably the vomer. The dorsal section of the mesethmoid has an anterior process, and joins with the ventral anterior end of the frontal. The nasal is a thick tubular bone; in IHNFG-2224 this bone is located in front of the mesethmoid but in IHNFG-2225 it is in its natural position over the nasal capsule. The lateral ethmoid is another coarse bone. This rectangular stilt forms the anterior and posterior walls of the orbit and nasal capsule respectively, and joins with the frontal bone dorsally and the parasphenoid ventrally.
The frontal is large and roofs the anterior two thirds of the skull, including all the ethmoid and ocular areas. Dorsally this bone has a triangular vertical laminar wing that forms the anterior part of the supraoccipital crest and joins with the supraoccipital section of the crest at the level of the rear of the orbital (Figure 4). The supraoccipital bone occupies the rest of the skull roof, separates the parietals and joins the rear of the frontal bone. This bone also has a vertical laminar wing that forms the posterior section of the supraoccipital crest. This crest is triangular with a pointed dorsal border forming an angle of about 90 degrees and located near the occiput, at two thirds of its length from its anterior end. The lateral surface of the central area of the supraoccipital crest is ornamented with large, shallow, and parallel ridges that run from the dorsal border of the crest to the area where the supraoccipital and frontal meet.
The parietal is an elongate bone located parallel to and below the supraoccipital (Figure 4). The posttemporal fossa is located in the anterior end of this bone but is roofed by the posterior expansion of the frontal. The lateral wall of the skull is occupied mainly by the pterotic, which forms the dorsal roof of the hyomandibular fossa. Part of the sphenotic bone is hidden below Infraorbital 5. The limits and sutures of other postocular skull bones are not discernible in the available specimens.
The parasphenoid is a large and slightly upward-curving stilt, toothless and probably triangular in cross section (Figure 4). The orbital cavity is partially occupied by the orbitosphenoid and pleurosphenoid bones that project from its internal dorsal surface, and by anterior projections of basisphenoid bones that join the ocular section of the parasphenoid. In IHNFG-2224 a smooth laminar ovoid bone of uncertain identity occupies the anterior orbital cavity (Figure 4).
The supraorbital canal runs along the whole frontal bone enclosed in tubes that open near the anterior end of this bone as well as in wide conspicuous pores located above and below the orbit. Some small pores of this canal also open in the base of the supraoccipital crest at its highest point.
Lower jaw. The lower jaw involves the dentary, angular, and dermarticular bones (Figure 4). The lateral profile of the jaw is chicken-legged in shape, having an almost straight alveolar border and the articular process for the quadrate located below the middle of the ocular orbit. The dentary bone is triangular with the posterior edge bifurcated, the anterior end narrow, and the ventral border showing an anterior hook-shaped ventral process. The dentary forms the entire alveolar border of the jaw, which is fitted with a wide patch of uniform small rounded teeth.
The angulo-articular is a coarse triangular bone strongly attached to the posterior bifurcated edge of dentary. The coronoid process area in this bone is obscured in the available specimens. The articular process rises from the posterior vertical edge of the angulo-articular. The joint between the lower jaw and the head of the quadrate is visible laterally (Figure 4). The mandibular canal runs along the ventral border of lower jaw, crossing and opening through large pores near the ventral edge of the angulo-articular bone, 5 or 6 of the pores being in the former and 2 or 3 in the latter.
Upper jaw. The premaxilla is a long complex bone that forms practically all the occlusal border of the upper jaw. The height of its triangular anterior ascendant process is a third of the length of its alveolar limb. The premaxilla has a stout and rod-like articular process, the height of which is about half of the ascending process. The alveolar surface of this bone bears a patch of numerous uniform small rounded teeth that resemble those on the lower jaw.
The maxilla is a toothless long rectangular bone with an expanded rounded posterior end and an anterior stout rod-like articular process. The maxilla articular process joins the premaxilla articular process ventrally and probably the vomer dorsally. A large long drop-shaped posterior supramaxilla lies over at least the posterior half of the premaxilla dorsal border (Figure 4). In IHNFG-2225 it is clear that Zoqueichthys carolinae has two supramaxillae, the anterior one being of a similar shape and size to the posterior one.
Infraorbtal series. The ocular orbit is incompletely surrounded by five infraorbitals, which cover it from the ethmoid area to the postocular lateral part of the skull, passing over the hymandibula and entopterygoid (Figure 4). The infraorbital 1 (also called the lacrimal bone by Gayet, 1980a: 24) is the largest in the series; it covers a large part of the nasal area. Infraorbital 1 is subrectangular in lateral view, almost as high as it is long, and bears a small dorsal process. Infraorbitals 2 through 4 are rectangular, longer than they are high, and border the orbit ventrally; whereas infraorbital 5, the smallest rectangular plate in the series, is higher than it is long and covers the rear of the orbit. The infraorbital canal runs under a thin rim of bone near the orbital border of infraorbitals 1 through 5; it opens through small pores except in infraorbital 1, where this canal branches into five to seven enclosed radiating tubes projecting downward.
Hyomandibular bones. Large parts of the suspensorium are obscured by other bones in all available specimens (Figure 4). The hymandibular is a coarse T-shaped bone with a wide stout articular head at the top, a large massive axis ventrally projecting, a short opercular process projecting backward near the top, and a wide rounded laminar anterior wing. The opercular anterior process or wing of the hyo-mandibular bone strongly attaches to the endopterygoid and metapterygoid. The quadrate is triangular bone, about two times higher than long, with a stout articular head inclined forward. The symplectic is a clove-like structure embedded near the rear of the quadrate.
The large lateral surfaces of the metapterygoid and endopterygoid are wrinkled, perhaps because these bones bear teeth internally, in contrast with the dermopalatine and ectopterygoid that show smooth lateral external surfaces, suggesting they are toothless. The articular head of the dermopalatine is hidden below the infraorbital 1.
Opercular series and brachiostegals. The opercle is a flat, ovoid bone with a wrinkled external surface that extends vertically to cover only the dorsal half of the body height, below the vertebral column and between the pectoral girdle and the rest of the head. Its anterior and ventral borders are almost straight and form an angle of about 65 degrees. The hyomandibular facet of this bone is located near the top of its anterior border (Figure 4).
The preopercle is smooth and boomerang-shaped, gently curved and two times higher than long (Figure 4). The main axis of both limbs form an angle of 130-140 degrees. A small rounded notch is present in the anterior edge of this bone, in the area where two preopercular limbs are joined. This notch is similar to the hook-like process described and illustrated in Aipichthys velifer and A. minor by Otero and Gayet (1996: 324, fig. 8A). The preopercular canal is noticeable; it runs along the middle part of both limbs and opens through a number of elongate pores, four in the horizontal limb and another two or three in the vertical limb.
The sickle-shaped subopercle is located all along the underside of the opercle. In IHNFG-2225 the height of the anterior ascending process in this bone is almost a quarter of the opercle height, and its shows a similar wrinkled external surface. The infraopercle is an inclined flat bone, in lateral view having an orange-slice shape, and barely overhanging the preopercle posterior margin. The wrinkles that ornament the infraopercle project almost in parallel from its dorsal to its ventral margin (Figure 4). In IHFG 2224 there are at least eight branchiostegal rays preserved, located below the infraopercle and behind the lower jaw. Although the first branchiostegal ray is thin, short, almost straight, and thread-like, the others tend to be longer and are flat and spatula-shaped.
Axial skeleton. The vertebral column consists of 27 centra, 11 abdominal and 16 caudal; the caudal centra include 14 preural, the pseudurostylar complex (=fusion of preural 1 and ural 1), and ural 2. All of the centra are subrectangular in lateral view, and slightly higher than long (Figure 5). The lateral surfaces of the vertebrae are ornamented with deep unordered cavities, which are conspicuous in the most posterior vertebrae.
All neural and hemal arches and spines are fused to their respective centra except for hemal arches on the last two preural centra (Figure 6) and the hemal spine in the first caudal centrum (=preural centrum 14) (Figure 5). The condition of the neural on the first abdominal vertebra is unknown. These spines are fused with their respective hemal and neural arches. The posterior five hemal spines and all neural spines on caudal vertebrae are oar-shaped; they have a central bar support of uniform thickness and a couple of bony wings on the tip (Figures 2, 5-6). The large and sharp neural spines on the first eight abdominal vertebrae show a noticeable groove that divides them in two halves. The ribs are long curved bars that enclose just the dorsal half of the abdominal cavity. Each rib has a conspicuous longitudinal hollow. The ribs join the transverse processes on the last eight abdominal vertebrae, with the height of the processes increasing with a corresponding decrease of the ribs posteriorly. The first transverse processes are very small whereas the most posterior are about three times higher (Figure 2). Epineurals are present in the abdominal and in three caudal vertebrae; these thread-like bones are about as long as three abdominal vertebrae and project upward and backward from the bases of the neural arches (Figure 4). Epipleurals are clearly associated with the four posterior abdominal and two caudal vertebrae; these fine bones project backward and downward from the transverse processes and hemal spines (Figure 5).
Three high predorsal bones are placed between the first neural spine and the occiput (Figure 4). These trumpet-shaped bones are about two times as long at the top than at their base. The first predorsal is the highest and the only one that is curved; it projects from the dorsal border to the vertebral column. The second and third predorsals are practically straight and progressively shorter; the height of the third predorsal is about two thirds that of the first. Regarding the predorsal bones, neural spines, and dorsal fin (see below), it is possible to recognize the predorsal formula (Ahlstrom et al., 1976; Johnson, 1984) in Zoqueichthys carolinae is 0+0+0/1+1/1.
Pectoral girdle and fin. The supratemporal is a narrow, short tube-like bone about as long as one abdominal vertebra; it is placed at an angle between the back of the supraoccipital crest base and the posttemporal dorsal process (Figure 4). In lateral external view the posttemporal shows a rounded central structure with two processes projecting forward forming an angle of about 60°, in which the upper process is just slightly shorter and thinner than the lower one (Figures 3 and 4).
The supracleithrum is flat and smooth, and covers the lateral surfaces of the first vertebra; in lateral external view this is an almost ovoid bone that overlaps the dorsal tip of the cleithrum and joins the posttemporal central region (Figure 4). The cleithrum is sinuous in shape and extends from the vertebral column to the ventral edge of the body. In IHNFG-2225 the cleithrum lateral surface shows the same shallow parallel ridges that ornament the opercle.
The coracoid is O-shaped with a rounded foramen in the center; this bone is located behind the cleithrum inflection area (Figure 4). There is an internal foramen formed between the ventral and dorsal joints between the coracoid and cleithrum. There are two postcleithra; the dorsal one is flat, ovoid, and two times higher than long. The ventral postcleithrum is long and tube-like, and projects from the dorsal postcleithrum to the dorsal edge of the pelvic fin base. The pectoral fin has 12 segmented rays that join the four autogenous radials, which attach this fin to the coracoid (Figure 4). This fin is triangular and covers two thirds of the abdomen length.
Pelvic girdle and fin. The pelvic fin is triangular and thoracic in position. It rises near the middle of the trunk at about 43-44% of the SL (the prepelvic length) and consists of eight segmented rays. All of these rays except the first one are branched. The first two of these rays are so long that they reach the base of the anal fin. The pelvic girdle consists of a pair of long triangular pelvic bones. The anterior tips of these bones are in contact with the pectoral girdle. The pelvic fin rays join directly on the external posterior edge of these bones. There is a long spine-like posterior process projecting backward from the internal posterior area of each pelvic bone. The pelvic bones join each other throughout their internal wings and median processes (Figure 7).
Dorsalfin. This fin is a long and acuminate structure located on the posterior half of the back between 40 and 90% of the SL (Figure 2). It exhibits 24 branched and segmented rays and three stout anterior spines that are supported by 26 pterygiophores. All spines and rays are articulated one to one with the pterygiophores, except for the last ray that is not supported. The first and second pterygiophores are located in the space between the first two neural spines. Each of the subsequent 8 pterygiophores (3 through 10 in the series) is located in the interneural spaces. Other pterygiophores (1124 in the series) are shorter and in some cases form couples that fit in a single interneural space.
The first pterygiophore is stout and hook-shaped; it is three times higher than long and its vertical and horizontal limbs form an anterior angle of 50-53 degrees. In this pterygiophore the vertical limb consists of a stout central column flanked by two laminar sections located forward and backward, whereas its horizontal limb is a stout spine projecting forward. The second pterygiophore is the highest in the series (being slightly higher than the first), and as in all the others has stout columnar and laminar sections. All subsequent pterygiophores gradually decrease in height and their laminar sections tend to be smaller; the height of the last one is about half of the first one. The laminar sections of the pterygiophores meet each other, forming interdigitating or sigmoid junctions, except for the eight most posterior ones where these sections are so small that they do not meet each other. The vertical limbs of the first and second pterygiophores are in the space between the first neural spines of vertebrae 1 and 2 (Figure 4).
The dorsal fin spines are smooth structures projecting backward. The height of the first and smallest spine is one half and one fifth the height of the second and third spines respectively. The first and second spines are stout whereas the tip of the third spine is clearly thinner. The first three anterior soft rays are higher than the posterior spine and form the anterior acuminate end of the dorsal fin; here the first ray is about two times higher than this spine. The height of the remaining dorsal rays (2 through 24 in the series) tends to decrease, and in the most posterior one this is about one fourth the height in the first ray.
Analfin. This fin is strip-like with a small acuminate anterior border. It is relatively short, opposes the posterior half of the dorsal fin, and is placed between 70 and 96% of the SL (Figure 2). This fin involves 17 branched and segmented anal rays and three stout anterior spines supported by 18 pterygiophores, the first of these represents the hemaxanal complex described by Gayet (1980a). The first two spines join the hemal complex and the last spine and all of the soft rays are articulated one to one with the remaining pterygi-ophores (Figure 5).
The first anal pterygiophore or hemaxanal complex is sickle-shaped, two times higher than long, with two limbs forming an angle of about 80 degrees and an anterior rounded concave edge. The vertical limb of this complex projects into the space between the first hemal spine (attached on preural centra 14) and the last couple of ribs (which are attached to the parapophysis of vertebra 11). It consists of a thick column running along its entire height and two laminar sections placed forward and backward and reaching only half the height. In contrast, its horizontal limb has a thick ventral edge that joins the anterior laminar section where the first two anal spines are strongly attached. The second pterygiophore is about the height of the vertical limb of the hemaxanal complex. The posterior pterygiophores gradually decrease in height; the second one slightly more than half the height of the first pterygiophore, and the last one slightly less than half. These also show a thick middle column flanked by two laminar sections of equal size that extend anteriorly and posteriorly to meet the laminar sections of adjacent pterygiophores forming almost straight joins. In the last five pterygiophores these laminar sections tend to be smaller and fail to join each another.
The spines are stout structures projecting backward with the lateral surfaces ornamented by fine longitudinal striations (Figure 5). The first and smallest spine is triangular, two times longer than it is high, and attached to the anterior pterygiophore by simple contact. The second is a better developed spine, almost as long as the first but almost three times higher; it joints the first petrygiophore through a small process developed at its base, and with the first and third spines through rounded anterior and posterior processes located near the base. The third and longest spine is almost two and a half times higher than the second one; this joins the second pterygiophore and the first soft ray through a pair of small processes present in its base and rear respectively; it also joins the second spine through a small facet formed in the anterior edge and near the base, where the posterior process of the second anal spine sits. At least the first two anal fin rays are slightly longer than the anal spines and form the acuminate anterior end of this fin. Although the height of the remaining anal rays (3 through 17 in the series) progressively decreases, they are regular in size and just slightly smaller than the third anal spine. All of the anal rays are segmented and branched from the middle to the tip.
Caudal fin. The caudal fin is homocercal, deeply forked, and has the upper and lower lobes showing the same shape and size. There are 19 principal fin rays, of which 17 are branched, with nine in the upper and eight in the lower lobes respectively (Figure 6). Ten and eight procurrent rays are present in the anterior end of the upper and lower caudal lobes respectively. A small caudal fenestra lies between the first principal and last procurent rays of the dorsal caudal lobe, similar to the fenestra that allows the insertion of the upper bundle of interradialis muscle (Winterbottom, 1974) or of the flexa dorsalis (Otero and Gayet, 1996).
Specimen IHNFG-2224 has three long epurals over the bifurcated spiny ends of the neural arch on the second preural vertebra (this is the 25th of the entire vertebral series). The first preural centrum and ural 1 are fused, forming a pseudurostylar complex. This specimen has six autogenous hypurals separated by a diastema between hypurals 2 and 3, and two long uroneurals. The first uroneural bears a wide anterior membranous outgrowth or stegural that covers the lateral dorsal surfaces the pseudurostylar complex. The par-hypural and hypurals 1 and 2 are autogenous bones. There is no evidence for the presence of urodermals (Figure 6).
Scales and scutes. Scales are cycloid and ovoid, slightly higher than long, and cover the entire body, part of the cheek and opercle. Seven or eight smooth ventral scutes of regular size are aligned and occupy the abdominal ventral border between the first anal fin spine and the pelvic fin base (Figure 5).
DISCUSSION
The unranked Aipichthyides, a monophyletic group delineated by Otero and Gayet (1995) later recognized as superfamily Aipichthyoidea, was distinguished based on the following six presumed synapomorphies: 1) predorsal formula equals 0+0+0/x/; 2) loss of antorbital; 3) presence of a plate-like anterior process in the hyomandibular; 4) pelvic fin in thoracic position; 5) distal ceratohyal without fenestra; and 6) seven pelvic fin rays (Figure 7). Subsequently, when Otero and Gayet (1996) formally named superfamily Aipichthyoidea, they did so based only on characteristics 1 through 3; and in the later phylogenetic analysis of this group, Otero (1997) resorted solely to synapomorphies 2 through 5. The inclusion of Zoqueichthys carolinae as a new member of this group is strongly supported because it shows characteristics 1 through 4 (Figures 2, 4-5).
Regarding the other two characteristics listed in the original diagnosis, 5 and 6, do not agree with the present material assigned to Zoqueichthys. However, there is scarce documentation available on the distal ceratohyal among Aipichthyoidea and this bone is obscured in Zoqueichthys carolinae, so it is impossible to provide a useful assessment of characteristic 5. On the other hand, data now available shows that pelvic fin rays are between 8 and 6 in these fishes (Table 2) and Z. carolinae is unique in having eight rays, a number comparable to that found among some Beryciformes (Fahay, 1997: 842).
Aipichthyoides and probably Aspesaipichthys (Taverne, 2004) have a long and complex supraoccipital crest involving the supraoccipital, frontal, and mesethmoid bones, and extending the entire length of the skull roof (character 7 in Figure 7). Zoqueichthys show a peculiar long crest that is comparatively simpler in its composition, involving only the supraoccipital and frontal bones (Figure 4). In contrast, all other aipichthyoid genera have a short, simple crest, which rises above the postocular and part of the ocular skull areas and involves only a dorsal laminar projection from the supraoccipital bone. In the caudal skeleton of all Aipichthyoidea, including Zoqueichthys, there are three epurals and six hypurals, except in Aipichthyoides that has only two epurals and five hypurals (characters 8 and 9 in Figure 7). Additionally, hypurals 1 and 2 are autogenous in Zoqueichthys and other Aipichthyoidea, except for Aipichthyoides and Aspesaipichthys, in which these hypurals are fused to the pseudurostylar complex (character10 in Figure 7).
Interrelationships of superfamily Aipichthyoidea
Results of the phylogenetic analysis annexed to this manuscript (see electronic complementary data) show that Aipichthys, Freigichthys, Paraipichthys, Aipichthyoides, Aspesaipichthys, and Zoqueichthys form the monophyletic superfamily Aipichthyoidea (node 2, Figure 8), which is supported by two synapomorphies: the plate-like anterior process of the hyomandibular [15(1)] and the occurrence of three predorsal bones in front of the first neural spine [17(1)]. Additionally, this clade, Amblyopsidae, and Aphredoderidae share a homoplasic characteristic, absence of the antorbital bone [14(1)].

Results of the phylogenetic analysis show two mono-phyletic groups outlined within superfamily Aipichthyoidea, these are the families Aipichthyoidae and Aipichthyioididae. The composition and supporting characteristics of these groups depict some differences with previous hypotheses (compare Figures 7 and 8). Remarkable in this new cla-distic array are the inclusion of Paraipichthys in family Aipichthyidae together with Freigichthys and Aipichthys, and the position of Zoqueichthys within family Aipichthyoididae together with Aipichthyoides and Aspesaipichthys.
Monophyly of Aipichthyoididae is supported by two characters (node 3, Figure 8). Although in Aspesaipichthys the supraoccipital crest is unknown, in this family the crest is partially formed by the frontal [18(1)], and it is a long laminar projection from the nasal area to the occiput [21(1)]. Among aipichthyoidids, Aspesaipichthys and Aipichthyoides share one synapomorphy, inclusion of the mesethmoid bone in the supraoccipital crest [19(1)], but the crest is unknown in the first taxon. Additionally these latter genera also share two homoplasic characteristics, they have only five hypurals [24(1)] as in Omosomopsidae, and hypurals 1 and 2 are fused with the pseudurostylar complex [25(1)] as in Amblyopsidae and Apheredoderidae. In this analysis Zoqueichthys is unique among Aipichthyoidea in having eight branchistegal rays [4(1)] and eight pelvic fin rays [10(0)].
The natural status of family Aipichthyidae is weakly supported on the combination of two homoplasic characteristics (node 5, Figure 8), the occurrence of six pelvic fin rays [10(2)] as in Omosomopsidae, and the occurrence of one supramaxilla [28(1)] as in Omosomopsidae and Sphenocephalidae. In this family the monophyletic group Aipichthys + Freigichthys is supported by two syn-apomorphies (node 6, Figures 8), the anterior edge of the supraoccipital is thickened [22(1)] and the interfrontal is flat [27(1)] as previously noted by Otero and Gayet (1996). These species also share one homoplasic condition, the half boomerang shape of the supraoccipital crest [20(1)] also found in crown acanthomorph clades (i.e. Amblyopsidae, Percopsidae, among others). In this phylogenetic hypothesis Aipichthys is presented as a paraphyletic group with A. velifer apart from other species of Aipichthys. In contrast, Freigichthys, A. minor, and A. oblongus constitute a monophyletic group weakly supported on a single homoplasic characteristic, opening of the frontal branch of the sensory canal through pores [3(0)]. Finally, in this group Freigichthys is unique in having seven pelvic fin rays [10(1)], which represents a homoplasy.
Although Taverne (2004) pointed out that the single specimen thus far known of Aspesaipichthys does not preserve the diagnostic features (or synapomorphies) of Aipichthyoidea (Otero and Gayet, 1996), he supported its inclusion in this superfamily because it shows a caudal fin with 19 principal rays, true epipleural bones, and three predorsal bones located in front of the first neural spine. Nevertheless the first two plesiomorphic features are also present in other primitive acanthomorphs (i.e., Johnson and Patterson, 1993; Patterson, 1993); whereas the predorsal arrangement is doubtful according to the illustrations of Aspesaipichthys published by Taverne (2004, figs. 3, 4), in which these bones appear disarticulated and are located behind the first two neural spines. Tavern (2004) also justified inclusion of Aspesaipichthys in family Aipichthyoididae because it shares with Aipichthyoides some common characteristics, which include a reduced neural arch on preural 2, the lack of fenestra in the caudal fin, and first two hypurals fused to the pseudurostylar complex (ural 1 + preural 1). These arguments appear weak because the first condition is a plesiomorphic one present in other primitive acanthomorphs, and Aipichthyoides has a real caudal fin fenestra (Gayet 1980a, figs. 4 and 15). Fusion of the first two hypurals to the pseurodtylar complex is a feature also present in derived acanthomorphs, i.e., Amblyopsidae (Otero and Gayet, 1996).
CONCLUSIONS
The phylogenetic hypothesis presented in this paper attempts to depict the relationships of Zoqueichthys carolinae within Aipichthyioidea. Recognition of the phylogenetic position of this superfamily within Acanthomorpha requires a comprehensive study, which in the future should include all possible forms of basal recent and fossil acanthomorphs. The mixture of primitive and derived characteristics of Zoqueichthys allows its recognition as a new genus and species in the family Aipichthyoididae, and validates this Mexican fish as the first Aipichthyoidea found in America.
Diagnostic characteristics of the superfamily Aipichthyoidea include some of those described by other authors (Otero and Gayet, 1995, 1996; Otero, 1997). Both families Aipichthyidae and Aipichthyodidae are shown here to be organized differently than in previous hypotheses (compare Figures 7 and 8). Today the former includes Paraipichthys, Aipichthys, and Freigichthys whereas the latter includes Zoqueichthys, Aipichthyoides, and Aspesaipichthys. In this new classification the family Aipichthyidae is well characterized by a supraoccipital crest that is half-boomerang shaped, relatively short, projects over the posterior half of the orbit and the postocular skull areas, and has a thickened anterior edge. Otero and Gayet (1995, 1996) discussed generic characteristics that distinguish Aipichthys, and later Otero (1997) did the same for Freigichthys. It is noticeable that the six pelvic fin rays shared by Aipichthys and Paraipichthys, and once recognized as a synapomorphy, is here considered a homoplasic condition.
On the other hand, family Aipichthyoididae is diagnosed by a high and extremely long supraoccipital crest. The crest includes participation of the supraoccipital and frontal bones, and extends over the whole skull roof, from occiput to the nasal area. Zoqueichthys represents the sister group of other genera in this family.
The discovery of Zoqueichthys complements our understanding of the taxonomic diversity of basal acantho-morphs. Efforts to describe the processes involved in their evolution, origin, and early diversification must now include an expanded biogeographical framework, and involve new fossil evidence from the Cretaceous deposits of America.
ACKNOWLEDGEMENTS
We thank M.A. Coutiño and G.F. Carbot for the facilities to perform the present study. M. Gayet, O. Otero, B. Khalloufi, and D. Mayrinck provided us with a valuable bibliography. L.E. Gómez, F. Riquelme, and A. Alaniz helped us during the fieldwork. C. Ross kindly helped us improve the English. A. López-Arbarello an O. Rauhut gave us the facilities to review fossils of the Bayerische Staatssammling für Paläontologie und Geologie. L.P.C. Machado took the photographs shown in this manuscript.
We are indebted to S. Cevallos and his students, who collected the fossils described in this manuscript. Our friend L. Espinosa-Arrubarrena provided critical technical support for the development of this research. Valuable critical suggestions by O.Otero, L.Taverne, and T. Lehman significantly improved the content of this article. Universidad Nacional Autónoma supports J.A.O. through the project PAPIIT IN106011. B.A.T.M. acknowledges the support of the project: Prospección y Resguardo del Patrimonio Paleontologico de Chiapas.
REFERENCES
Ahlstrom, E.H., Butler, J.L., Sumida, B.Y., 1976, Pelagic stromateoid fishes (Pisces, Perciformes) of the eastern Pacific: kind, distributions, and early life histories and observations on five of these from the northwest Atlantic: Bulletin of Marine Sciences 26(3), 285-402. [ Links ]
Alvarado-Ortega, J., Ovalles-Damián, E., Blanco-Piñón, A., 2009, The fossil fishes from the Sierra Madre Formation, Ocozocoautla, Chiapas, Southern Mexico: Palaeontologia Electronica 12(2, 4A), 1-22. [ Links ]
Álvarez-Mena, A., 1975, Estratigrafía del Cretácico de la región central de Chiapas: México, Instituto Politécnico Nacional, Escuela Superior de Ingeniería y Arquitectura, Bachelors thesis, 50 pp.+ 9 maps. [ Links ]
Böse, E., 1905, Reseña acerca de la geología de Chiapas y Tabasco: Boletín del Instituto Geológico de México 20, 1-116. [ Links ]
Dixon, F., 1850, The geology and fossils of the Tertiary and Cretaceous formations of Sussex. London: Longman, Brown, Green and Longmans, 422 pp. + xvi [ Links ]
Fahay, M.P., 1997, Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras), Volume one, Acipenseriformes through Sygnathiformes: Northwest Atlantic Fisheries Organization, 931 pp. [ Links ]
Gaudant, M., 1978, Contribution à l'étude anatomique et systématique de la faune ichthyologique cénomanienne du Portugal. Les "Acanthoptérygiens.": Comunicações dos Serviços Geológicos de Portugal, 63, 105-149. [ Links ]
Gayet, M., 1980a, Recherches sur l'ichthyofauna cénomanienne des Monts de Judée: les Acanthopterygii: Muséum National d'Histoire Naturelle, Annales de Paléontologie (Vertebrés), 66(2), 75-128. [ Links ]
Gayet, M., 1980b, Contribution a l'etude anatomique et systématique des Poissons cénomaniens du Liban anciennement placés dans les Acanthopterygii: Memoires du Muséum National d'Histoire Naturelle, Paris, Série C, Science de la Terre, 44, 1-149. [ Links ]
González, A.J., 1963, Levantamiento del Área San Cristóbal-Bachajón: Petróleos Mexicanos, Zona Sur, Informe Geológico 498 (unpublished report), 59 pp. [ Links ]
Johnson, G.D., 1984, Percoidei: Development and Relationships, in Moser, H.G., Richards, W.J., Cohen, D.M., Fahay, M.P., Kendall, Jr. A.W., Richardson, S.L. (eds.), Ontogeny and Systematics of Fishes: American Society of Ichthyologists and Herpetologists, Special Publication, 1, 464-498. [ Links ]
Johnson, G.D., Patterson, C., 1993, Percomorph phylogeny: a survey of acanthomorphs and a new proposal: Bulletin of Marine Sciences 52(1), 554-625. [ Links ]
Otero, O., 1997. A new genus of Aipichthyoidea (Teleostei, Acanthomorpha) from the Lower Cenomanian of Hgula (Lebanon): description and phylogenetic relationships: Comptes Rendus de l'Académie des Sciences, Paris, Earth & Planetary Sciences, 325, 453-458. [ Links ]
Otero, O., Gayet, M., 1995, Étude phylogénétique des Aipichthyides poissons Téléostéens de la Téthys Cénomanienne: Geobios 19, 221-224. [ Links ]
Otero, O., Gayet, M., 1996, Anatomy and phylogeny of the Aipichthyoidea nov. of the Cenomanian Tethys and their place in the Acanthomorpha (Teleostei): Neues Jarbuch für Geologie und Paläontologie, Abhandlungen, 202, 314-344. [ Links ]
Ovalles-Damián, E., Alvarado-Ortega, J., Blanco-Piñón, A. 2006, Los peces fósiles del Cretácico inferior de Ocozocoautla, Chiapas, in Memoria X Congreso Nacional de Paleontología y libreto guía de la excursión a Tepexi de Rodríguez, Puebla: Instituto de Geología, publicación especial 5, p. 61. [ Links ]
Patterson, C., 1964, A review of Mesozoic acanthopterygian fishes, with special reference to those of the English Chalk: Philosophical Transactions of the Royal Society of London, Series B, 247, 213-482. [ Links ]
Patterson, C., 1993, An overview of the early fossil record of acanthomorphs: Bulletin of Marine Science, 52(1), 29-59. [ Links ]
Pictet, F.J., 1850, Description de quelques poissons fossiles du Mont Liban: Genève, J.-G. Fick, 59 pp. [ Links ]
Rosales-Domínguez, M.C., Caus-Gracia, E., Bermúdez-Santana, J., Aguilar-Piña, M., 1994, Evidencias de exposición subaérea en el Cretácico de Chiapas, Primer reporte de Microcodium en la Caliza Sierra Madre: Boletín de la Asociación Mexicana de Geólogos Petroleros, 44(1), 1-15. [ Links ]
Rosales-Domínguez, M.C., Bermúdez-Santana, J.C., Aguilar-Piña, M., 1997, Mid and Upper Cretaceous foraminiferal assemblages from the Sierra de Chiapas, southeastern Mexico: Cretaceous Research, 18, 697-712. [ Links ]
Rosen, D.E., 1973, Interrelationships of higher euteleosteans fishes, in Greenwood, P.H., Miles, R.S., Patterson, C. (eds.), Interrelation of fishes: London, Zoological Journal of the Linnean Society, 53, Supplement 1, 397-513. [ Links ]
Salas, G.P., 1949, El Cretácico de la Cuenca de Macuspana y su correlación: Boletín de la Sociedad Geológica Mexicana, 14, 47-65. [ Links ]
Sánchez-Montes de Oca, R., 1969, Estratigrafía y paleogeografía del Mesozoico de la Sierra del Sur: Instituto Mexicano del Petróleo, Seminario sobre exploración petrolera, Mesa 4, Capítulo 5, 31 pp. [ Links ]
Sánchez-Montes de Oca, R., 1973, Proyecto Mesozoico Arrecifal, Sierra de Chiapas: Mexico, Petróleos Mexicanos, Zona Sur, Informe Geológico 581 (unpublished report), 59 pp. [ Links ]
Steele, D.R., 1986, Contributions to the Stratigraphy of Sierra Madre Limestone (Cretaceous) of Chiapas, Part 1. Physical stratigraphy and petrology of the Cretaceous Sierra Madre Limestone, west-central Chiapas: Universidad Nacional Autónoma de México, Instituto de Geología, Boletín 102, 1-101. [ Links ]
Steindachner, F., 1850, Beiträge zur Kentniss der Fische Oesterreichs. II Folge, I. Uber einen neuen Vomer-ähnlichen Fish von Comen am Karst: Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften, Mathematisch-naturwissenchaftliche Classe, 38, 763-777 + 1pl. [ Links ]
Stiassny, M.L.J., Moore, J.A. 1992, A review of the pelvic girdle of acanthomorph fishes, with comments on Hypotheses of acanthomorph interrelationships: Zoological Journal of the Linnean Society, 104, 209-242. [ Links ]
Taverne, L., 2004, Les poissons crétacés de Nardò. 17°. Aspesaipichthys cavaensis gen. et sp. nov. (Teleostei, Acanthomorpha, Aipichthyoidea): Bollettino del Museo Civico di Storia Naturale di Verona, Geologia Paleontologia Preistoria, 28, 3-15. [ Links ]
Vega, F.J., Álvarez, F., Carbot-Chanona, G., 2007, Albian penaeoidea (Decapoda: Dendrobranchiata) from Chiapas, Southern Mexico, 3rd Symposium on Mesozoic and Cenozoic Decapoda Crustaceans, Museo di Storia Naturale di Milano: Memorie della Societá Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano, 35(2), 6-8. [ Links ]
Waite, L.E., 1986, Contributions to the Stratigraphy of Sierra Madre Limestone (Cretaceous) of Chiapas, Part 2, Biostratigraphy and paleoenvironmental analysis of the Sierra Madre Limestone (Cretaceous), Chiapas: Universidad Nacional Autónoma de México, Instituto de Geología, Boletín 102, 103-245. [ Links ]
Wiebe, W.A., 1925, Geology of southern Mexico oil fields: Pan American Geology, 94, 121-138. [ Links ]
Winterbottom, R., 1974, A descriptive synonymy of the striated muscles of the Teleostei: Proceedings of the Academy of Natural Sciences of Philadelphia, 125, 225-317. [ Links ]
Woodward, A.S., 1901, Catalogue of the fossil fishes in the British Museum (Natural History), Part IV: British Museum (Natural History), London, 636 pp. + viii pls. [ Links ]














