Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias geológicas
versión On-line ISSN 2007-2902versión impresa ISSN 1026-8774
Rev. mex. cienc. geol vol.31 no.1 Ciudad de México abr. 2014
CaK-clinoptilolite, KNa-chabazite, KNa-heulandite, KNa-erionite and Na-phillipsite from tuffaceous rocks, Province of the Mesa Central, Mexico
CaK-clinoptilolita, KNa-chabazita, KNa-heulandita, KNa-erionita y Na-phillipsita de rocas tobáceas, Provincia de la Mesa Central, México
Liberto de Pablo1*, Mercedes Doval†2, Angel La Iglesia3, Jesús Soriano4
1 Instituto de Geología, Universidad Nacional Autónoma de México, Ciudad Universitaria, 04510 México D.F., Mexico. *liberto@unam.mx.
2 Departamento de Cristalografía y Mineralogía, Facultad de Ciencias Geológicas, Universidad Complutense de Madrid, 28040 Madrid, Spain.
3 Instituto de Geociencias CSIC-UCM, Facultad de Ciencias Geológicas, Avda. Antonio Novais 2, 28040 Madrid, Spain.
4 Laboratorio Central de Estructuras y Materiales CEDEX, Ministerio de Fomento, Madrid, Spain.
Manuscript received: August 14, 2013
Corrected Manuscript received: January 21, 2014
Manuscript accepted: January 26, 2014
ABSTRACT
This paper presents the occurrence, mineralogy and process of formation of CaK-clinoptilolite, KNa-chabazite, KNa-heulandite, KNa-erionite and Na-phillipsite in pyroclastic rocks from the Calvillo-Jalpa-Nochixtlán area in the Province of the Mesa Central, Mexico. CaK-clinoptilolite, (Si30.600-30.150Al5760-5.220)(Ca1890-1.800K1080-0.810Mg0.540-0450)O72, of 5.04-5.86 Si/Al, 0.83-0.86 Si/(Si+Al) and 0.26-0.31 (Na+K)/ (Na+K+Ca+Mg) ratios, free of Na, crystallized from siliceous alkaline pore fluids and glasses of composition (Si0.890-0825Al0.202-0.102)(K0.072-020Ca0.020-0.010Fe0.050-0.010)O2 in tuffaceous rocks northeast of Jalpa. KNa-heulandite, (Si28.080Al8.010)(K4.680Na2.250Ca0.270Fe0.180)O72, KNa-chabazite, (Si9.210-9.000Al3.090-2.940)(K0.750-1.180Na0.360-1.260Ca0.150-0.030Fe0.210-0.090Mg0.380-0.210) O24, NaK-erionite, (Si27.990Al7.830)(K2.000)(K4.210Na1.620Ca0.090Fe0.090Mg0.180)O72, and Na-phillipsite, (Si12.080Al3.920(Na3.160K0.240Ca0.160Fe0.040) O32, of 2.91-3.57 Si/Al, 0.74-0.78 Si/(Si+Al) and 0.72-0.97 (Na+K)/ (Na+K+Ca+Mg) ratios, dominant exchange cations K and Na, formed from siliceous alkaline high-pH fluids and glasses of composition (Si0.910-0.833Al0.170-0.092) (K0.101-0.050Na0-0.033-0.027)02 in tuffs northwest of Nochixtlán. The zeolites were formed in the Jalpa area by diagenetic alteration of the tuff; in the Nochixtlán area, enrichment of Na and formation of alkali-rich zeolites was attributed to alkaline saline brines in an open hydrologic system. They occur in Oligocene - early Miocene tuffaceous rocks associated to the volcanism of the Sierra Madre Occidental, exposed in the Calvillo-Jalpa-Nochixtlán area in the states of Aguascalientes and Zacatecas, in the Province of the Mesa Central, Mexico.
Keywords: CaK-zeolites, KNa-zeolites, zeolites, Mexico.
RESUMEN
Este trabajo presenta la ocurrencia, mineralogía y proceso de formación de CaK-clinoptilolita, KNa-chabazita, KNa-heulandita, KNa-erionita y Na-phillipsita en rocas piroclasticas del área de Calvillo-Jalpa-Nochixtlán, en la Provincia de la Mesa Central, México. CaK-clinoptilolita, (Si30.600-30.150Al5760-5.220)(Ca1890-1.800K1080-0.810Mg0.540-0450)O72, de relaciones 5.04-5.86 Si/Al, 0.83-0.86 Si/(Si+Al) y 0.26-0.31 (Na+K)/(Na+K+Ca+Mg), libre de Na, cristalizó de fluidos de poro silícicos alcalinos y vidrios de composición (Si0.890-0825Al0.202-0.102)(K0.072-020Ca0.020-0.010Fe0.050-0.010)O2 en tobas al noreste de Jalpa. KNa-heulandita, (Si28.080Al8.010)(K4.680Na2.250Ca0.270Fe0.180)O72KNa-chabazita, (Si9.210-9.000Al3.090-2.940)(K0.750-1.180Na0.360-1.260Ca0.150-0.030Fe0.210-0.090Mg0.380-0.210) O24,NaK-erionita, (Si27.990Al7.830)(K2.000)(K4.210Na1.620Ca0.090Fe0.090Mg0.180)O72, y Na-phillipsita, (Si12.080Al3.920(Na3.160K0.240Ca0.160Fe0.040) O32, de relaciones 2.91-3.57 Si/Al, 0.74-0.78 Si/ (Si+Al)y 0.72-0.97 (Na+K)/(Na+K+Ca+Mg), cationes intercambiables dominantes K y Na, cristalizaron de fluidos de poro silicicos alcalinos de alto pH y de vidrios de composición (Si0.910-0.833Al0.170-0.092) (K0.101-0.050Na0-0.033-0.027)02 en tobas al noroeste de Nochixtlán. Las zeolitas se formaron, en Jalpa, por diagenesis de la toba;en Nochixtlán, el enriquecimiento de Na y la formación de zeolitas alcalinas se atribuye a la acción de salmueras alcalinas salinas, en un sistema hidrológico abierto. Las zeolitas ocurren en tobas del Oligoceno - Mioceno temprano asociadas al volcanismo de la Sierra Madre Occidental y expuestas en el área de Calvillo-Jalpa-Nochixtlán, estados de Aguascalientes y Zacatecas, en la Provincia de la Mesa Central, México.
Palabras clave: CaK-zeolitas, KNa-zeolitas, zeolitas, México.
INTRODUCTION
This paper presents the occurrence, mineralogy and process of formation of alkali-rich zeolites in volcanoclastic deposits in the states of Zacatecas and Aguascalientes in the Province of the Mesa Central, México. Alkali-rich zeolites are a not so common rarity, opposed to the widely distributed sedimentary zeolites clinoptilolite-heulandite and mordenite of CaKNaMg-exchange components. Alkali-rich zeolites have not been reported previously in Mexico. Known deposits of sedimentary zeolites in Mexico are mostly of clinoptilolite-heulandite, in the state of Oaxaca in the Province of the Sierra Madre del Sur (Wilson and Clabaugh, 1970; Mumpton, 1973; de Pablo, 1986), in the vicinity of Guanajuato and San Miguel de Allende in the Province of the Mesa Central (de Pablo et al., 1996), in the Mexican Volcanic Belt (de Pablo and Chavez, 1996) and in the state of Sonora in the Basin and Range Province (Münch and Cochemé, 1993; Münch et al., 1996). Extensive deposits of sedimentary zeolites are known in western United States, in the states of Arizona, California, Nevada and Oregon (Passaglia and Sheppard, 2001), in Italy (Passaglia and Vezzalini, 1985) and elsewhere. Alkali-rich zeolites are known from western United States (Passaglia and Sheppard, 2001) and which generally have, relative to their equivalent Italian zeolites, less K and larger Si/Al ratios (Passaglia, 1970; De'Genaro and Franco, 1976; Passaglia and Vezzalini, 1985; Passaglia and Sheppard, 2001).
The formation of zeolites in volcanoclastic deposits is associated to the peculiarities of the precursor glass, the percolating fluids and the local environment, thus a close relationship prevails between the newly formed authigenic minerals and the chemistry of the glasses and pore fluids, which may change. The transformation to zeolites is possible in: (1) hydrologic closed alkaline saline systems, (2) hydrologic open systems, (3) marine environments, (4) hydrothermal systems, (5) geoautoclave systems and (6) by burial diagenesis (Hay and Sheppard, 2001). In this paper are presented the mineralogy and origin of CaK-, KNa- and Na-zeolites from the Province of the Mesa Central, Mexico. The area studied is known for a high incidence of pulmonary diseases (Reyes-Veyna, 2007). The interest is on the origin of the alkali-rich zeolites, contribute to the knowledge on the minerals, their occurrence in Mexico, possible harmful health effects and eventual technological interest. Mineral zeolites have economic interest as low-cost adsorbers in chemical and environmental applications, in agricultural practice and in animal feedstocks and husbandry (Mumpton, 1977); their commercial use in Mexico has been mostly limited to application as dimension stone, in agriculture and in animal feedstocks.
MATERIALS AND METHODS
Materials
The tuffs discussed herein are part of the Cenozoic volcanic that extends over the Mesa Central, in the states of Zacatecas and Aguascalientes in Mexico. The area of study extends approximately 25,000 km2, between the 103°40' - 102°00'W longitude and the 22°00' - 21°15'N latitude; it is located to the west of the city of Aguascalientes and south of Calvillo, Zacatecas, and includes the towns of Jalpa and Nochixtlán. The geology setting of the area was introduced in a previous publication (de Pablo et al., 2013). The dominant lithology are the Oligocene-Miocene rhyolitic tuffs and rhyolite-ignimbrites associated with the volcanism of the Sierra Madre Occidental; exposed in the area are Miocene andesites and basalts, Pliocene calcareous conglomerates, conglomerates and shales. The tuffs were sampled at different locations and which together with mineralogical and compositional data have been presented (de Pablo et al., 2013).
Methods
The mineralogy and petrography of the tuffs were determined by the usual procedures of optical microscopy, X-ray powder diffraction (XRD) and X-ray fluorescence (XRF). X-ray powder diffraction (XRD) analyses were performed using a Siemens diffractometer with filtered CuKa radiation on powdered bulk material and clay-sized fractions scanned at 1o 2θ min-1. Whole-rock major and trace elements analyses were performed by X-ray fluorescence (XRF) on bulk powders fused as glass beads. Total Fe was analyzed by XRF and calculated as FeO; methods to differentiate Fe3+ from Fe2+ were not considered. Loss on ignition was determined by wet chemistry (de Pablo et al., 2013). XRD characterization of some zeolites was limited due to the difficulty of obtaining adequate amounts for their detection. Heulandite and clinoptilolite were differentiated by their framework composition: minerals with the framework ratio Si/Al<4 were labeled as heulandite and those with Si/Al>4 were labeled as clinoptilolite, after the recommended nomenclature of the Commission on New Minerals and Mineral Names of the International Mineralogical Association (Coombs et al., 1997, 1998).
The morphology, microtexture and composition of minerals were initially determined by scanning electron microscopy (SEM) at 25 kV operating voltage coupled with energy dispersive X-ray (EDX) analysis, on unpolished fragments that were carbon-coated when analyzed for composition and gold-coated when studied morphologically. More precise studies were performed by high resolution scanning electron microscopy (HRSEM) using a JEOL 2000FX scanning electron microscope operated at an acceleration voltage of 200 kV and equipped with a double inclined sample holder with a resolution of 3.1 A and an EDX Oxford ISIS spectrometer with a resolution of 136 eV at 5.39 keV. The chemical compositions determined by EDX were obtained at short time periods to avoid alkali volatilization, from selected areas of uniform morphology and mineralogy presumably larger than the cross section of the incident electron beam (de Pablo et al., 2013).
RESULTS
The dominant lithology in the area is an assemblage of ignimbrite and rhyolitic tuffs of Oligocene-Miocene age (de Pablo et al., 2013). Variations in the chemical composition (Table 1), mineralogy (Table 2) and stratigraphic position of the tuffs allowed recognition of four distinct groups (de Pablo et al., 2013). Group 1, located 10 km northeast of Jalpa, elevation 1334 m, comprises tuffs of comparatively low contents of SiO2 and MgO and high contents of Al2O3, Fe2O3 and K2O (sample 2 in Tables 1 and 2). Group 2, 20 km northeast of Jalpa, elevation 1350 m, where the tuffs are noted by the unusual association of cristobalite, tridymite and minor alunogen-alunite to feldspar, pla-gioclase, heulandite-clinoptilolite and smectite and by comparatively high contents of SiO2 and low of Al2O3, Fe2O3, CaO, Na2O, Rb and Ba (sample 3 in Tables 1 and 2). Group 3, 90 km northwest of Nochixtlán at elevations of 1370 m and 1440-1478 m and east of Calvillo at elevations of 1521-1570 m, includes tuffs of high and low SiO2/Al2O3 ratio and high contents of MgO, Sr, Ba and Y and predominant authigenic heulandite-clinoptiolite and smectite (samples 14, 12, 13, 1 and 15 in Tables 1 and 2). Group 4, northwest of Nochixtlán, elevation 17801880 m, includes tuffs noted by the presence of primary quartz and authigenic smectite, high contents of Na2O, K2O, Rb, Y and Nb and low of MgO, CaO, Sr and Ba (samples 10 and 11 in Tables 1 and 2).
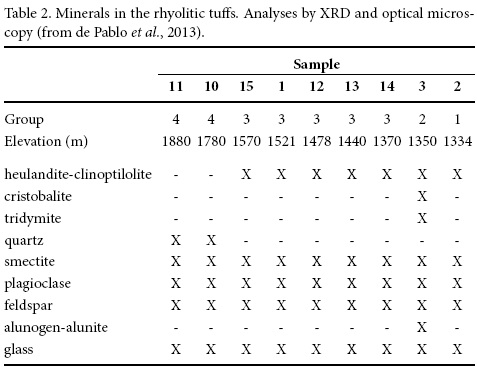
The contents of major and trace elements in the tuffs relative to Zr are presented in Figure 1. Zirconium was selected as an indicator of differentiation because of its incompatibility. From low to high concentrations of Zr slight descending compositional trends are shown by SiO2 and Na2O and upward trends are exposed by Al2O3 and MgO that depict continuous compositional changes in the tuffs. High concentrations of SiO2 and low of Al2O3, Na2O and K2O occur in tuffs from the 1350 m elevation site northeast of Jalpa, opposed to the more abundant K2O and Na2O noted in tuffs from the 1880 m elevation site northwest of Nochixtlán. Differences in the ignition loss imply hydration and reaction with H2O, H+ and H3O+.
Changes in the mineralogy of the tuffs among the four groups are significant (Table 2, Figure 2). X-ray diffraction data showed the presence of predominant heulandite-clinoptilolite and minor smectite at intermediate elevations in Group 3. Northeast of Jalpa, in Group 2 at an elevation of 1350 m, primary cristobalite and tridymite are significant and northwest of Nochixtlán, in Group 4 at an elevation of 1880 m, quartz crops. Altogether, XRD data depict an extensive area of tuffs zeolitized to predominant heulandite-clinoptilolite. The tuffs from groups 2 and 4 showed the most relevant changes and were selected for presentation in further detail, represented respectively by sample 3, location 102°50'W longitude, 21°42'N latitude, elevation 1350 m, northeast of Jalpa and by sample 11, location 102°47'W longitude, 21°26'N latitude, elevation 1880 m, northwest of Nochixtlán.

High-resolution scanning electron microscopy of the rhyolitic tuff cropping 20 km northeast of Jalpa (sample 3) shows rhyolitic glasses of 78.22-80.01 wt% SiO2, free of Na and Mg, K>Ca, of 4.07-5.65 Si/Al, 0.80-0.88 R [R=Si/(Si+Al)] and 0.50-0.80 alkali [(Na+K)/ (Na+K+Ca+Mg)] ratios, occurring as irregular non-vesicular fragments over 30 urn in size (Figure 3a, composition 3.4.1 in Table 3), slabs ~150 nm thick and 800 nm long (Figure 3b, compositions 3.9.1 and 3.9.2 in Table 3) and thinner irregular slabs of round edges (Figure 3b). These thin slabs occasionally are oriented around orthogonal empty spaces and are crowned by vesicular glass (Figure 3c, compositions 3.14.1 and 3.14.2 in Table 3). Glass spheres of 50-100 nm in diameter formed (Figure 3d, composition 3.11.1 in Table 3). A more siliceous glass of84.67 wt% SiO2, no Na and Mg, 8.68 Si/Al, 0.89 R and 0.94 alkali ratios fills voids between crystals (Figure 3e, composition 3.37.1 in Table 3).
The authigenic mineralogy includes blocky, tabular and pseudo hexagonal thick crystals 2-10 um thick and 10-20 um long of clinoptilolite of5.86-5.04 Si/Al, 0.85-0.83 R and 0.31-0.26 alkali ratios, Ca>K, dominant exchange cations (DEC) Ca, K, subordinate exchange cation (SEC) Mg, free of Na, in vesicles and fissures (Figure 3f, 3g, 3h, compositions 3.32.2, 3.34.1, 3.35.1 in Table 3) (Coombs et al., 1997). This clinoptilolite differs from the clinoptilolite from the Suchilquitongo Formation, Etla, Oaxaca, which is sodic, K+Na>Ca+Mg and 5.05-4.31 Si/Al, 0.83-0.81 R and 0.59-0.53 alkali ratios, DEC K, Ca, Na, SEC Mg and from clinoptilolite from the Chichíndaro Formation, San Miguel Allende, Guanajuato, which is sodic, 4.49 Si/Al, 0.81 R and 0.69 alkali ratios, DEC K, Ca, Na, SEC Mg (de Pablo, 1986; de Pablo et al., 1996; de Pablo and Chavez, 1996). Differences in the Si, Al and alkalis contents depict distinct degrees of framework ordering and channel occupancy between the clinoptilolite from Jalpa and the clinoptilolite from the Suchilquitongo and the Chichíndaro Formations (Armbruster and Gunter, 2001). Clinoptilolite of high R ratio occurs in rhyolitic tuffs at Cañadón Hondo, Patagonia, Argentina (Mason and Sand, 1960); of low alkali ratio is known from the Báucarit Formation, Mexico (Münch and Cochemé, 1993) and Na-free clinoptilolite crops in the Malpais Hill, Arizona (Wise and Tschernich, 1976). The composition and morphology of the clinoptilolite from Jalpa has some resemblance to tabular dachiardite. Dachiardite of 0.86 R ratio has been described from Altoona, Washington (Wise and Tschernich, 1978) and of Ca>Na from Elba Island, Italy (Gottardi, 1960; Vezzalini, 1984), Yellowstone National Park, Wyoming (Bargar et al., 1987) and from the Kagoshina and Ogasawara Islands, Japan (Nishido and Otsuka, 1981; Passaglia and Sheppard, 2001).
Irregular thick wavy glassy slabs of a composition that resembles heulandite, of2.64-2.30 Si/Al, 0.72-0.70 R and 0.60-0.51 alkali ratios, DEC Na, Ca and minor K, enclose empty orthogonal cavities (Figure 3i, compositions 3.38.1 and 3.38.2 in Table 3). Heulandite of high alkali ratio was found in burial diagenetic clay-rich horizons in Denmark (Nornberg, 1990) and high alkali ratio almost K-free crystals are known from Yellowstone National Park, Wyoming (Bargar and Beeson, 1981), from the Shimane Prefecture, Japan (Minato and Aoki, 1978), Caravaca, Spain (Vannucci et al., 1992) and the Barstow Formation in California (Sheppard and Gude, 1969; Passaglia and Sheppard, 2001).
The tuff that crops 11 km northwest of Nochixtlán at an elevation of 1880 m (sample 11), contains ~16 urn quartz crystals of predominant hexagonal bipyramidal faces and diminished prismatic faces that depict crystallization from the melt (Figure 4a, composition 11.35.1 in Table 4). Smaller quartz crystals are enclosed by 67.63 wt% SiO2 glass (Figure 4f, composition 11.34.1 in Table 4). Albite crystals are noted (Figure 4b, composition 11.21.1 in Table 4).
The glasses in sample 11 show a wider range of composition than the glasses from sample 3. In sample 11 there are glasses of 91.8681.06 wt% SiO2, low 9.54-5.52 wt% Al2O3, free of Na, 13.89-7.27 Si/ Al, 0.93-0.88 R and 0.92-0.50 alkali ratios, non-vesicular (Figures 4c, 4d, compositions 11.25.1, 11.25.2, 11.24.1 in Table 4). They associate to less siliceous glasses of 86.98-76.74 wt% SiO2, 10.97-7.52 wt% Al2O3, I. 55-1.28 wt% Na2O, 9.84-4.90 Si/Al, 0.91-0.83 R and 1.00-0.92 alkali ratios which form small lens-shaped shards (Figure 4c, compositions II. 25.3 to 11.25.6 in Table 4) and to lower-temperature glasses of dacitic and trachydacitic composition of67.63-65.01 wt% SiO2, 7.09-3.17 wt% K2O, 3.13-2.51 wt% MgO, free of Na, low 3.20-2.43 Si/Al, 0.76-0.71 R and 0.60-0.43 alkali ratios, often occurring around irregular and roughly orthogonal cavities (Figures 4e, 4f, 4g, compositions 11.37.1, 11.34.1 and 11.39.1 in Table 4) (Le Bas et al., 1986).
Intimately associated with the glasses are crystallites of rhombohedral pseudocubic morphology and 'herschelitic' habit of chabazite, ~0.25 um in size, 3.13-2.91 Si/Al, 0.76-0.74 R and 0.89-0.72 alkali ratios, DEC K, Na, SEC Mg and essentially free of Ca (Figure 4d, compositions 11.24.2 and 11.24.3 in Table 5). Chabazite crystallites of 0.89 alkali ratio, DEC Na, K, SEC Mg and very minor Ca are on phillipsite in vesicles (Figure 4i, composition 11.20.2 in Table 5). These chabazites have an R ratio greater than the 0.73-0.68 R ratio of the Italian hydrothermal chabazites (Passaglia, 1970; De'Genaro and Franco, 1976; Passaglia and Vezzalini, 1985) but similar to the sedimentary calcic chabazite from the John Day Formation, Oregon (Sheppard and Gude, 1970; Passaglia and Sheppard, 2001) and to chabazite from the Big Sandy Formation, Arizona (Sheppard and Gude, 1973). Chabazite of 0.78 R ratio has been observed in rhyolitic tuffs and a ratio R of 0.77 is known for chabazite from 'closed systems' (Sheppard et al., 1978).
Erionite of 3.57 Si/Al, 0.78 R and 0.97 alkali ratios, DEC K, Na, SEC Mg, Ca, occurs in bundles of prismatic crystals in vesicles (Figure 4h, composition 11.23.1 in Table 5). The R ratio of this erionite is within the range of 0.79-0.68 common to sedimentary erionite but, whereas its exchange cations are K and Na, the general rule is to have K and Ca and rarely Na as exchangeable cations (Passaglia et al., 1998; Passaglia and Sheppard, 2001). This erionite has the composition K621NaL62Ca009Mg018(Al7 83Si2799)O72, more potassic than the erionite-K, K4Na2Ca(Al8Si28)O72, from Rome, Oregon (Ballinaro et al., 2009) and distinct from the erionite-Ca and erionite-Na from the erionite series (Dogan and Dogan, 2008).
Phillipsite amygdaloidal of 3.08 Si/Al, 0.76 R and 0.95 alkali ratios, DEC Na, K, SEC Ca, an unusually high content of 0.79 Na+ per 8O2- and almost no K+, (Ca+Mg)/(Na+K) 0.047, Na/(Na+K) 0.93 (Figure 4i, compositions 11.20.1 Table 5) occurs in vugs; its composition approaches that of analcime (Passaglia and Sheppard, 2001). Heulandite thick slabs and blocky crystals of high cationic and low Si contents have 3.51 Si/Al, 0.78 R and 0.96 alkali ratios, 0.32 Na/Na+K, Na+K>Ca+Mg, DEC K, Na, SEC Ca (Figure 4d, composition 11.24.4 in Table 5). The R ratio is normal for heulandite in sediments; a ratio of 0.85 is known for mineral from rhyolitic tuffs of Canadon Hondo, Argentine (Mason and Sand, 1960); the alkali ratio ranges from 0.07 in sedimentary crystals from silicic volcanic sandstone of the Baucarit Formation, Mexico (Münch and Cochemé, 1993) to 1.0 in crystals from a burial diagenetic clay-rich horizon in a chalk soil from Sangstrup Klint, Denmark (Nornberg, 1990). The 0.32 Na/ Na+K ratio ranges from 0 in Na-free crystals to 0.94 in almost K-free crystals from Yellowstone National Park, Wyoming (Bargar and Beeson, 1981). Heulandite of Na+K>Ca+Mg ratios is known in samples from deep-sea sediments and burial diagenic sediments (Boles and Wise, 1978; Vannucci et al., 1992; Ogihara, 1994; Passaglia and Sheppard, 2001).
Anorthoclase occurs as few rare thick crystals of defective cation content (Figure 4j, composition 11.17.1 in Table 5). Crystals show a pitted surface on which mineralized strings 1600 nm long and 350-400 nm diameter of possible cemented glass bubbles that resemble microbes grew (Figure 4k, composition 11.18.1 in Table 5). Thin hexagonal crystallites of fayalite occur in Fe-rich glass (Figure 4l, composition 11.15.1 in Table 5).
The empirical formulas and the compositions of minerals are shown in Table 6 and Figure 5. Glasses and zeolites define compositional trends (Figure 5). In the Jalpa district occur glasses of 84.6778.22 wt% SiO2, 8.68-4.07 Si/Al and 0.89-0.80 R ratios, framework modifiers K and Ca, free of Na and Mg. Clinoptilolite free of Na crystallized from these glasses and pore fluids when the ratios were 5.86 Si/Al and 0.85 R (Figure 5a). Glasses coexist with lower-temperature glasses of 66.04-63.16 wt% SiO2, 2.64-2.30 Si/Al and 0.70-0.72 R ratios, higher contents of Na and Ca and lower of K (Figure 5a). In the Nochixtlán area, glasses of 91.86-81.06 wt% SiO2, free of Na, 13.89-7.27 Si/Al, 0.93-0.88 R and 0.92-0.50 alkali ratios coexist with glasses of 86.98-76.74 wt% SiO2, 1.55-1.28 wt% Na2O, 9.84-4.90 Si/ Al, 0.91-0.83 R and 1.00-0.92 alkali ratios and with dacitic glasses of 67.63-65.01 wt% SiO2, 7.09-3.17 wt% K2O, free of Na, 3.20-2.43 Si/Al, 0.76-0.71 R and 0.60-0.43 alkali ratios. When the ratios were Si/Al<3.57 and R<0.78 KNa-chabazite, KNa-heulandite, NaK-erionite and KNa-phillipsite crystallized (Figure 5b). Glasses and zeolites show continuous compositional correlations: diminishing contents of SiO2 and of the Si/Al and R ratios correspond to increasing concentrations of Al2O3, K2O, Na2O and CaO (Figure 5c). The correlations extend to the SiO2-glass spherules filled with tridymite and cristobalite-tridymite nanocrystals and the high-temperature AlFe-rich minerals kyanite, Fe-cordierite, Fe-amphibole and fayalite- and lepysheres of cristobalite-tridymite in vesicles in the glasses from Jalpa (Figure 5d) (de Pablo et al., 2013).

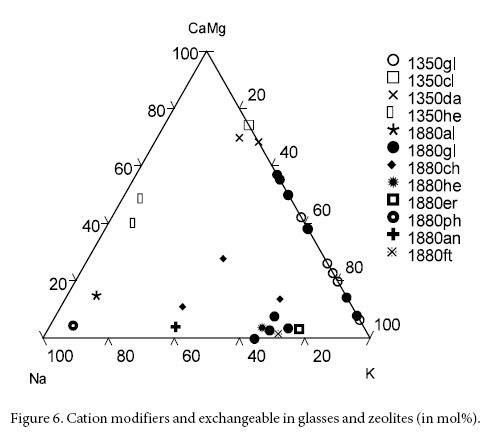
The distribution of the framework cation modifiers in glasses and zeolites is shown in Figure 6. In the Na-CaMg-K system the glasses from Jalpa fall on the CaMg-K axis, not far from clinoptilolite which is more calcic and displaced towards the Ca corner of the system (Figure 6). Residual sodic glasses from Jalpa fall towards the central part of the system. The Na-free glasses from Nochixtlán are on the CaMg-K axis and those containing Na are closer to the Na-K axis; chabazite, heulandite, phillipsite and erionite move towards the central part of the system (Figure 6). When the zeolite framework is incorporated in the system (CaMg)0.25Al0.50Si0.50O2-SiO2-(NaK)0.50Al0.50Si0.50O2 glasses and zeolites remain in the upper half of the system; those from Jalpa stay closer to the SiO2 corner and those from Nochixtlán are displaced towards the central part of the system and above the 50-50 line (Figure 7).

DISCUSSION
In the Jalpa district, Provincia de la Mesa Central, occurs clinoptilo-lite, DEC Ca, K, free of Na, 5.86 Si/Al, 0.85 R and 0.31-0.26 alkali ratio, distinct from clinoptilolite of DEC K, Na, Ca from the Sulchiquitongo Formation, Oaxaca and from the Chichíndaro Formation, Guanajuato, and of possible different degree of framework ordering and channel occupancy. In the Nochixtlán district crop heulandite, DEC Na, K and significant 0.96 alkali ratio known only from few worldwide locations and deep-sea sediments; chabazite, DEC K, Na, 0.76 R ratio, comparable to chabazite from the John Day Formation; erionite, DEC K, Na, free of Ca, a rarity compare to erionite that normally contains Ca and K and seldom Na; phillipsite, DEC Na and only minor K.
Minerals and glasses resulted from the fractionation of a precursor explosive magma into a high-temperature Fe-rich magma from which high-temperature AlFe-minerals crystallized and into an immiscible siliceous melt. The siliceous melt cooled from a high-temperature SiO2-rich liquid, predominant in the Jalpa district, to a lower temperature more aluminous liquid common to the Nochixtlán district; upon cooling, the framework cation modifiers Na, K and Ca and the framework former Al progressively increased their concentration. (Roedder 1951; Muan 1957; Muan and Osborn 1965; Thompson et al., 2007; de Pablo et al. 2013). In Jalpa, CaK-clinoptilolite free of Na, of 80.69-78.98 wt% SiO2, 5.86-5.04 Si/Al, 0.85-0.83 R and 0.31-0.26 alkali ratios was formed in veins and amygdules from altered glass of similar composition and from siliceous pore fluids of low alkalinity. The transformation of the Na-free glass to smectite may have raised the activity of Si and Ca in the pore fluid, eventually leading to the crystallization of clinoptilolite in a relatively high-temperature environment free of Na. Associated residual AlCaNa-glasses of low Si content did not intervene in the formation of the zeolite. In Nochixtlán, chabazite, heulandite, erionite and phillipsite, of70.11-60.70 wt% SiO2, 3.57-2.91 Si/Al, 0.78-0.74 R and 0.97-0.72 alkali ratios crystallized from siliceous alkaline high-pH pore fluids in an environment of comparatively lower temperature. Pore fluids removed Na, K, Al and Si from glasses of SiO2 content higher than the zeolites and which in the present case were over 76 % SiO2. Zeolitization implied desilication of glass and displacement of Na, K and minor Ca to the pore fluid. Zeolites crystallized from glasses altered through hydration, H2O, H+ and H3O+ interdifusion and from enriched pore fluids. Once the structure of the glass was opened and the glass saturated with water, formation of gel and crystallization of zeolites was simple, with the Si free to diffuse outwards to attain equilibrium with the surrounding aqueous fluid. In Jalpa, the diagenetic alteration of the tuff was significant. In Nochixtlán, the notable enrichment of Na and crystallization of NaK-rich zeolites is attributed to reaction with alkaline saline brines in an open hydrologic system, without formation of saline minerals.
The zeolitized tuffs from Jalpa and Nochixtlán have low content of zeolites, insufficient to be of economic interest. The tuffs from Jalpa contain the health-hazardous minerals cristobalite-tridymite, tridymite, SiO2-glass, amorphous and cryptocrystalline silica, and siliceous glasses and clinoptilolite of not well-defined health effects. The tuffs from Nochixtlán enclose erionite of known health-damaging effects and potentially damaging heulandite, chabazite and phillipsite. The high alkali content of the tuffs could preclude their application in agricultural practice and the association of quartz should result inconvenient for their use in animal feedstocks.
CONCLUSIONS
Sedimentary CaK-clinoptilolite occurs in the vicinity of Jalpa, state of Zacatecas; it crystallized from Na-free glasses and siliceous pore fluids of low alkalinity. Alkali-rich KNa-chabazite, KNa-heulandite, KNa-erionite and Na-phillipsite occur northwest of Nochixtlán, Aguascalientes; they formed from Ca-low glasses and siliceous highly alkaline pore fluids. Zeolites in Jalpa may have formed by diagenetic alteration of the tuffs while zeolitization and alkali enrichment in Nochixtlán is attributed to alkaline saline brines, in an open hydro-logic system.
ACKNOWLEDGEMENTS
This work was supported by Consejo Nacional de Tecnología, CONACYT, Project D47075F. The principal author is indebted to DGAPA, Universidad Nacional Autónoma de México, for financial support while on a sabbatical leave. The electron microscopy studies were conducted at the Electron Microscopy Laboratory of the Universidad Complutense and the Laboratorio Central de Estructuras y Materiales CEDEX, Madrid. P. Girón assisted with the analytical work.
REFERENCES
Armbruster, T. and Gunter, M.E., 2001, Crystal structure of natural zeolites, in Bish, D.L., Ming, D.W. (eds.), Natural Zeolites: Occurrence, Properties, Applications: Reviews in Mineralogy and Geochemistry, Chantilly, Virginia, Mineralogical Society of America, 45, 1-67. [ Links ]
Ballinaro, P., Andreozzi, G.B., Dogan, M., Dogan, A.U., 2009, Crystal structure and iron topochemistry of erionite-K from Rome, Oregon, USA: American Mineralogist, 94, 1262-1270. [ Links ]
Bargar, K.E. and Beeson, M.H., 1981, Hydrothermal alteration in research drill hole Y-2, Lowe Geyser Basin, Yellowstone National Park, Wyoming: American Mineralogist, 66, 473-490. [ Links ]
Bargar, K.E., Erd, R.C., Keith, T.E.C., Beeson, M.H., 1987, Dachiardite from Yellowstone National Park, Wyoming: Canadian Mineralogist, 25, 475-483. [ Links ]
Boles, J.R. and Wise, W.S., 1978, Nature and origin of deep-sea clinoptilolite, in Sand, L.B., Mumpton, F.A., (eds.), Natural Zeolites. Occurrences, Properties, Uses: Reviews in Mineralogy, Oxford, Pergamon Press, 4, 235-243. [ Links ]
Coombs, D.S., Alberti, A., Arrmbruster, T., Artioli, G., Colella, C., Galli, E., Grice, J.D., Liebau, F., Mandarino, J.A., Minato, H., Nickel, E.H., Passaglia, E., Peacor, D.R., Quarteri, S., Rinaldi, R., Ross, M., Sheppard, R.A., Tillman, E., Vezzalini, G., 1997, Recommended nomenclature for zeolite minerals: Report of the subcommittee on zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names: Canadian Mineralogist, 35, 1571-1606. [ Links ]
Coombs, D.S., Alberti. A., Armbruster, T., Artioli, G., Colella, C., Galli, E., Grice, J.D., Liebau, F., Mandarino, J.A., Minato, H., Nickel, E.H., Passaglia, E., Peacor, D.R., Quarteri, S., Rinaldi, R., Ross, M., Sheppard, R.A., Tillmanns, E., Vezzalini, G., 1998, Recommended nomenclature for zeolite minerals: report of the subcommittee on zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names, American Mineralogist Special Feature, 28 pp. [ Links ]
De'Genaro, M., Franco, F., 1976, La K-chabazite di alcuni "tufi del Vesubio": Rendues Academia Nazionale Linceir, Serial 8 60, 490-497. [ Links ]
De Pablo, L., 1986, Geochemical trends in the alteration of Miocene vitric tuffs to economic zeolite deposits, Oaxaca, México: Applied Geochemistry, 1, 273-285. [ Links ]
De Pablo, L., Chávez, M. L., 1996. Diagenesis of Miocene vitric tuffs to zeolites, Mexican Volcanic Belt: Clays and Clay Minerals, 44, 324-338. [ Links ]
De Pablo, L., Chávez, M. L., Cruz-Sánchez, M., 1996, Sedimentary zeolites in the Sierra Madre del Sur and Sierra Madre Occidental, México: Revista Mexicana de Ciencias Geológicas, 13, 188-200. [ Links ]
De Pablo, L., Doval, M., La Iglesia, A., Soriano, J., Chavez, L., 2013, Occurrence of silica polymorphs nanocrystals in tuffaceous rocks, Province of the Mesa Central, México, and their formation from subcritical Si-rich fluids: American Mineralogist, 98, 977-985. [ Links ]
Dogan, A.U., Dogan, M., 2008. Re-evaluation and reclassification of erionite series minerals: Environmental Geochemistry and Health, 30, 355-366. [ Links ]
Gosen, B.S. van, Blitz, T.Q., Plumbe, G.S., Meeker, G.P., Pierson, M.P., 2013, Geological occurrence of erionite in the United States: an emerging national public health concern for respiratory disease: Environmental Geochemistry Health, 35, 431-438. DOI 10.10017/s 10653-012-9504-9. [ Links ]
Gottardi, G., 1960, Sul dimorfismo mordenite-dachiardite: Periodice Mineralogie, 29, 183-191. [ Links ]
Hay, R.L., Sheppard, R.A., 2001, Occurrence of zeolites in sedimentary rocks: An overview, in Bish, D.L., Ming, D.W. (eds.), Natural Zeolites: Occurrence, Properties, Applications: Reviews in Mineralogy and Geochemistry, Chantilly, Virginia, Mineralogical Society of America, 45, 217-234. [ Links ]
Le Bas, M.J., Le Maitre, R.W., Streckeisen, A., Zanettin, B., 1986, A chemical classification of volcanic rocks based on the total alkali-silica diagram: Journal of Petrology, 27, 745-750. [ Links ]
Mason, B., Sand, L.B., 1960, Clinoptilolite from Patagonia: the relationship between clinoptilolite and heulandite: American Mineralogist, 45, 341-350. [ Links ]
Minato, H., Aoki, M., 1978, The mode of formation of clinoptilolite from volcanic glass-In the case of Tmatsukuri, Shimane Prefecture, Japan: Scientific Papers Collection General Education, University of Tokyo, 28, 205-214. [ Links ]
Muan, A., 1957, Phase equilibrium relationships at liquidus temperature in the system FeO-Fe2O3-Al2O3-SiO2 system: Journal of the American Ceramic Society, 40, 420-431. [ Links ]
Muan, A., Osborn, E.F., 1965, Phase equilibria among oxides in steelmaking: Addison-Wesley, Massachusetts, p. 115-116. [ Links ]
Mumpton, F. A., 1973, First reported occurrence of zeolites in sedimentary rocks of México: American Mineralogist, 58, 287-290. [ Links ]
Mumpton, F.A., 1977. Utilization of natural zeolites, in Mumpton, F.A. (ed.) Mineralogy and Geology of Natural Zeolites: Reviews in Mineralogy, Mineralogical Society of America, 4, 177-204. [ Links ]
Münch, P., Cochemé, J.J., 1993, Heulandite-group zeolites in volcanoclastic deposits of the southern Basin and Range Province, México: European Journal of Mineralogy, 5, 171-180. [ Links ]
Münch, P., Duplay, J., Cochemé, J.J., 1996, Alteration of silicic vitric tuffs interbedded in volcanoclastic deposits of the Southern Basin and Range Province, México: Evidences for hydrothermal reactions: Clays and Clay Minerals, 44, 49-67. [ Links ]
Nishido, H., Otsuka, R., 1981, Chemical composition and physical properties of dachiardite group zeolites: Mineralogy Journal, 10, 371-384. [ Links ]
Nornberg, P., 1990, A potassium-rich zeolite in soil development on Danian chalk: Mineralogical Magazine, 54, 91-94. [ Links ]
Ogihara, S., 1994, Ba-bearing clinoptilolite from ODP Leg 127, Site 795, Japan Sea: Clays and Clay Minerals, 42, 482-484. [ Links ]
Passaglia, E., 1970, The crystal chemistry of chabazites: American Mineralogist, 55, 1278-1301. [ Links ]
Passaglia, E., Vezzalini, G., 1985, Crystal chemistry of diagenetic zeolites in volcanoclastic deposits of Italy: Contributions to Mineralogy and Petrology, 90, 190-198. [ Links ]
Passaglia, E., Artioli, G., Gualtieri, A., 1998, The crystal chemistry of the zeolites erionite and offretite: American Mineralogist, 83, 577-589. [ Links ]
Passaglia, E., Sheppard, R.A., 2001, The crystal chemistry of zeolites, in Bish, D.L., Ming, D.W. (eds.), Natural Zeolites: Occurrence, Properties, Applications: Reviews in Mineralogy and Geochemistry, Chantilly, Virginia, Mineralogical Society of America, 45, 70-116. [ Links ]
Reyes-Veyna, L. del R., 2007, Registro de neoplasias malignas México-Zacatecas 1999-2003, Servicios de Salud de Zacatecas, 3 pp. (E. B. Ilgreen, personal communication). [ Links ]
Roedder, E., 1951, Low-temperature liquid immiscibility in the system K2O-FeO-Al2O3-SiO2: American Mineralogist, 36, 282-286. [ Links ]
Sheppard, R.A., Gude, A.J., 1969, Diagenesis of tuffs in the Barstow Formation, Mud Hills, San Bernardino County, California: U.S. Geological Survey Professional Paper 634, 35 pp. [ Links ]
Sheppard, R.A., Gude, A.J., 1970, Calcic siliceous chabazites from the John Day Formation, Grant County, Oregon: U. S. Geological Survey Professional Paper 700D, p. 176-180. [ Links ]
Sheppard, R.A., Gude, A.J., 1973, Zeolites and associated authigenic silicate minerals in tuffaceous rocks of the Big Sandy formation, Mohave County, Arizona. U.S. Geological Survey Professional Paper 830, 36 pp. [ Links ]
Sheppard, R.A., Gude, A.J., Edson, G.M., 1978, Bowie zeolite deposit, Cochise and Graham Counties, Arizona, in Sand, L.B., Mumpton, F.A., (eds.), Natural Zeolites. Occurrences, Properties, Uses: Reviews in Mineralogy, Oxford, Pergamon Press, 4, 319-328. [ Links ]
Thompson, A.B., Aerts, M., Hack, A.C., 2007, Liquid immiscibility in silicate melts and related systems, in Liebscher, A., Heinrich, C.A. (eds.), Natural Zeolites: Occurrences, Properties, Applications: Reviews in Mineralogy and Geochemistry, Mineralogical Society of America, Chantilly, Virginia, 65, 99-127. [ Links ]
Vannucci, S., Pancani, M. G., Vasselli, O., Corandossi, N., 1992, Presence of clinoptilolite in the Maastrichtian pelagic sediments of the Barranco del Gredero Section (Caravaca, SE Spain): Chemistry Erde, 52, 165-177. [ Links ]
Vezzalini, G., 1984, A refinement of Elba dachiardite: opposite acentric domains simulating a centric structure: Zeitskrift fur Kristallographie, 166, 63-71. [ Links ]
Wilson, J. A, Clabaugh, S. E., 1970, New Miocene formation and a description of volcanic rocks, northern valley of Oaxaca, in Segura, L.R., Rodriguez, R., (eds.), Libro Guía de la Excursión México Oaxaca: México, Sociedad Geológica Mexicana, 120-128. [ Links ]
Wise, W.S., Tschernich, R.W., 1976, The chemical composition and origin of the zeolites offretite, erionite and levyne: American Mineralogist, 61, 853-863. [ Links ]
Wise, W.S., Tschermich, R.W., 1978, Dachiardite-bearing zeolites assemblages in the Pacific Northwest. in Sand, L.B., Mumpton, F.A., (eds.), Natural Zeolites, Occurrences, Properties, Uses: Reviews in Mineralogy, Oxford, Pergamon Press, 4, 105-111. [ Links ]














