Introduction
In modern vegetable production systems, transplanting has largely replaced direct seeding. The use of containerized transplants allows for an increase in yields, crop uniformity and a more predictable timing of production, in comparison with direct seeding. However, the need to reduce transplant costs has prompted the use of trays with small cells. This imposes a physical constraint on the development of the root system (Di Benedetto, 2011). The consequences of early root growth restrictions may span over the whole plant life cycle, negatively affecting production.
Lettuce is one of the most important vegetables worldwide (Stagnari, Galieni, & Pisante, 2015; Shehata, Schmidhalter, Valšíková, & Junge, 2016; Kim, Moon, Tou, Mou, & Waterland, 2016); it is often raised in small cell trays, and overcoming root restriction is thus needed to minimize yield losses. Root restriction effects on shoot growth are mainly caused by a low cytokinin supply from roots, and may be partially overcome by exogenous supply of this hormone (Richards & Rowe, 1977), but its application on vegetables has only recently gained full attention (Di Benedetto, Giardina, de Lojo, Gandolfo, & Hakim, 2020a). Cytokinins are synthesized in root apices and transported via the xylem to the shoot, where they promote cell division, leaf expansion, and photosynthetic apparatus development, and delay senescence, thus resulting in an enhanced plant development and size (Hönig, Plíhalová, Husičková, Nisler, & Doležal, 2018; Glanz-Idan, Tarkowski, Turečková, & Wolf, 2020).
Exogenous supply of cytokinin to the foliage may counteract the effects of root restriction in several horticultural and ornamental species (Di Benedetto, Tognetti, & Galmarini, 2010; Di Benedetto et al., 2020b). Early sprays with the synthetic cytokinin 6-benzylaminopurine (BAP) often promote the relative growth rate in association with an enhanced net assimilation rate and photosynthetic rate, rather than with an increased carbon partitioning to the shoot (Di Benedetto, Tognetti, & Galmarini, 2015a). Cytokinin may enhance carbon fixation through the development and maintenance of functional chloroplasts and chlorophyll synthesis (Boonman et al., 2007; Cortleven & Schmülling 2015; Liu, Li, & Zhong, 2017).
Cytokinin supplied to in vitro plants has been found to increase chlorophyll contents (Muniz-de Oliveira et al., 2008a; Dobránszki & Drienyovszki, 2014), while in plants at an advanced stage it helps delay senescence (Hönig et al., 2018). However, virtually no changes in chlorophyll content were found after exogenous supply of BAP on root-restricted ornamental Epipremnum aureum (Di Benedetto et al., 2015a) and Ficus benjamina (Di Benedetto, Galmarini, & Tognetti, 2020c), despite the promoting effect of the hormone on the net assimilation rate. Elucidating whethercytokinin may promote chlorophyll concentration in lettuce under root restrictions is important because of the association between chlorophyll and lettuce quality.
Cytokinins are also positively associated with nitrogen and photosynthetic protein concentration in leaves, by regulating the expression of genes involved in N uptake and carbon assimilation as well (Ruffel et al., 2011). Cytokinins also enhance N partitioning to leaves (Ookawa, Naruoka, Sayama, & Hirasawa, 2004) and within them (Gu et al., 2018) from older, shaded leaves to younger, sunlit ones, where they stimulate Rubisco expression (Ookawa et al., 2004; Boonman et al., 2007). Cytokinin delays senescence-associated declines in leaf nitrogen, protein and Rubisco levels, and in photosynthesis rates (Jordi et al., 2000).
Previous work on root-restricted Epipremnum aureum and Ficus benjamina showed an increased leaf N concentration as a consequence of BAP sprays (Di Benedetto et al., 2010; Di Benedetto, Galmarini, & Tognetti, 2018; 2020c), which was in turn associated with an increase in the net assimilation rate (NAR) over untreated controls. A possible increase in leaf nitrogen content of lettuce plants supplied with cytokinin could also be a desirable attribute from a nutritional quality point of view.
Changes in carbon assimilation prompted by cytokinin may also be the consequence of an increased CO2 diffusion due to anatomical changes, although somewhat conflicting results has been reported. Bosselaers (1983) found a decreased stomatal density in Phaseolus vulgaris leaves treated with BAP, but not with another cytokinin (kinetin). Conversely, Gandolfo et al. (2014) found a BAP-induced increase in the stomatal density of Impatiens wallerana, which was more pronounced in seedlings subjected to more severe root restriction. This effect depended on BAP concentration, being lower doses (5 mg·L-1) the most effective.
The proportion of intercellular spaces in leaf mesophyll, which impacts CO2 diffusion within the leaf, is also modified by cytokinin, although reports are again conflicting. Bosselaers (1983) found a decrease in the proportion of intercellular air spaces in Phaseolus vulgaris by both kinetin and BAP, and a similar response was reported by Muniz-de Oliveira et al. (2008b) in Annona glabra. However, Gandolfo et al. (2014) found that in leaves of Impatiens wallerana the proportion of intercellular spaces increased when treated with BAP at a low concentration (5 mg·L-1) but instead higher BAP concentrations reversed the effect.
Several reports indicate that cytokinin may promote an increase in leaf thickness (Bosselaers, 1983; Di Benedetto, Tognetti, & Galmarini, 2015b, 2018) which may also play a role on CO2 diffusion. A possible increase in leaf thickness as a consequence of cytokinin application would be an interesting response in lettuce due to its association with shelf life (Lee, Nath, Goswami, & Nou, 2017).
Preliminary observations on BAP-sprayed summer butter-head lettuce raised in small pots suggest that this species also responds to exogenous cytokinin by increasing net assimilation rate, although the biological processes involved remain unknown (Campolongo et al., 2020). Understanding lettuce response to cytokinin under different degrees of root restrictions is needed not only to obtain higher commercial yields but also because of the potential impact on quality attributes. In order to optimize the commercial use of this treatment, information about the best timing (either pre- or post-transplant application) is also needed (de Lojo, Gandolfo, Boschi, Giardina, & Di Benedetto, 2019; Hakim, Gandolfo, Giardina, & Di Benedetto, 2017).
The aim of this work was to test the hypotheses that, first, BAP sprays may improve lettuce biomass accumulation through higher carbon assimilation, depending on plug cell size, hormone concentration, and moment of application, and second, that BAP-driven changes in chlorophyll and nitrogen content, together with changes in leaf anatomy, may explain the promotion in carbon assimilation by this hormone.
Materials and methods
The experiment was carried out in the campus of the Faculty of Agronomy, University of Buenos Aires, Argentina (34° 35’ 59’’ LS and 58° 22’ 23’’ LW) from March 6th to May 15th 2017 and repeated once from March 5th to May 21th 2018.
Lettuce (Lactuca sativa L.) Crimor INTA seeds were sown and grown in 128-, 200- and 288-cell trays (17.37, 13.90 and 6.18 cm3 per cell respectively) in a Klasmann411® substrate (GmbH, Germany) containing white peat 0-5 mm, pH: 6, structure extra fine with Hydro S as wetting agent for 35 days. Lettuce seedlings were sprayed with BAP (6-benzylaminopurine) (EC 214-927-5, Sigma-Aldrich®, USA) solutions (0, 5, 50 or 100 mg·L-1) when the first true leaf pair were developed (pre-transplant treatments for BAP). Additionally, seedlings without pre-transplant treatment were sprayed with BAP seven days after transplant (post-transplant treatments). BAP was previously diluted in ethyl alcohol 80 %.
Lettuce seedlings were transplanted into 3 L pots filled with a Sphagnum maguellanicum-river waste-perlite (40:40:20, v/v) medium where they were grown for about 60 days. River waste or ‘temperate peat’ is the result of the accumulation of plant residues under an anaerobic environment, which is dredged from river or lake banks placed under a subtropical climate with high rainfalls over 1,000 mm. At the beginning of the experiments, total porosity (%), air-filled porosity (%), container capacity (%) and bulk density (g·cm-3) were 63.50, 17.06, 10.06 and 0.35, respectively. Weeds were manually removed.
Plants were irrigated with high quality tap water (pH: 6.64; electrical conductivity: 0.486 dS·m-1) using intermittent overhead mist and one weekly fertigation (1N: 0.5P: 1K: 0.5Ca v/v). Nutrient concentration was modified according to seedling development. From radicle protrusion to first true leaves expansion: 50 mgN·L-1; from this stage to transplant: 100 mgN·L-1 and during pot experiments: 150 mg·L-1. Fertilization solution volume per pot varied according to pot volume.
Half hourly averages of the air temperature were measured using a data logger (H08-001-02, HOBO®, USA) protected from direct radiation by aluminum foil shades. Global solar radiation was recorded with a PAR HOBO data logger. The mean air temperatures ranged between 14.4 to 16.1 °C and mean photosynthetic active radiation ranged between 13.46 to 17.33 mol photons·m-2·day-1 during the experiments. Pots were arranged at a density of 6 plants·m-2 avoiding mutual shading.
Plants for destructive measurements were harvested (ten per treatment) at the transplant stage and afterwards at 20-days intervals. Roots were washed and root, stem, leaf and petioles fresh weights (FW) were recorded as well as root length. Dry weights (DW) were obtained after drying roots, stems and leaves to constant weight at 80 °C for 96 h. The number of leaves was recorded and each leaf area was determined using the ImageJ® software.
The rate of leaf appearance (RLA), the relative leaf area expansion rate (RLAER), the relative growth rate (RGR), the net assimilation rate (NAR), the leaf area ratio (LAR) and were calculated according to Di Benedetto and Tognetti (2016).
RLA was calculated as the slope of the number of visible leaves (including unrolled ones > 1 cm) vs. time (in weeks). RLAER was calculated as the slope of the regression of the natural logarithm of total leaf areas versus time (in days). Whole plant RGR was calculated as the slope of the linear regression of the natural logarithm of DW versus time (in days). Mean LAR was calculated as RGR/NAR. Mean NAR was calculated as:
where W 0 is the extrapolated value of total DW (g) at time zero, k w is the RGR (days-1), A 0 is the extrapolated value of leaf area (cm2) at time zero, k a is the RLAER (days-1), t is the time (days) at the midpoint of the experimental period and e is the base of natural logarithm.
The net photosynthetic rate was measured at ambient O2 and CO2 concentrations at a saturating photon flux density (> 1,700 µmol photons·m-2·s-1) between 11:30 - 13:00 h of a sunny day just before final harvest. The youngest fully expanded leaf on three plants from each hormone treatment was selected for measurements, which were performed using a portable LICOR LI-6200 photosynthetic system (LI-COR®, USA).
For chlorophyll analysis, leaf disks were cut from the central area near the mid-vein of each leaf and placed in vials containing 3 cm3 of N, N-dimethyl formamide. Leaf disks were vacuum infiltrated and stored for three days in complete darkness. At this time, chlorophyll had completely eluted to the solvent. Absorbance at 647 and 664 nm was measured using a (Metrolab 1600, Metrolab UV-Vis, Argentina) spectrophotometer. Chlorophyll content was calculated as indicated by Inskeep and Bloom (1985).
Leaf nitrogen concentration at the final sampling was analysed using the Kjeldahl method. Leaf samples were placed in a tube containing sulfuric acid and heating for about 1.5 h raises the temperature to 310 to 320 °C. After a 1-h digestion at this temperature, the tubes were removed and cooling to 20 °C. The acid digest was diluted to 10 mL with water, and an aliquot of 3 mL was transferred to another tube where the Nessler reagent was added. Absorbance at 500 nm was measured using a spectrophotometer.
Leaf non-reducing sugars at the final sampling were analysed using the Anthrone method. Leaves were exhaustively extracted with 70 % (v/v) ethanol and the extracts evaporated to dryness in vacuum. They were then taken up in warm water and cleared with aluminium hydroxide. The Anthrone reagent (5 mL) was pipetted into Pyrex tubes and chilled in ice water. The solution to be tested (1 mL) was layered on the acid, cooling for a further 5 min and then thoroughly mixed while still immersed in ice water. The tubes were loosely fitted with corks, heated (80 °C) in a vigorously boiling, constant level water bath and then cooled in water for 5 min.
Samples of young fully expanded leaves were collected to examine leaf anatomy i.e. overall leaf thickness and volume of intercellular spaces, on the final harvest. Tissue from the middle region of the lamina was fixed in a mixture of 70 % ethanol, 5 % formalin, 5 % glacial acetic acid, and 20 % distilled water prior to dehydration in an ethanol and tert-butyl alcohol series. Samples were sectioned at 10 - 20 µm thick with a rotary microtome and stained with safranin-crystal violet-fast green. Data obtained were the means of three leaves per treatment using ten leaf cross-sections per leaf. Quantitative anatomical data were obtained using Image Pro Express version 6.0 (Media Cybernetics, USA).
The experimental design was a randomized factorial with three blocks of five single-pot replications of each treatment combination (plug cell volume × BAP concentration × experiment). Data were subjected to three-way analysis of variance using STATISTICA 8 (StatSoft) software. Since there were no significant differences between the two yearly experiments, they were considered together for analysis (Table 1). Means were separated by Tukey’s test (P ≤ 0.05). Slopes from straight-line regressions of RLAER, RGR and NAR were tested using the SMATR package (Warton, Duursma, Falster, & Taskinen, 2012).
Table 1 Results of a three-way analysis of variance with cell size (CS), BAP treatment and experiment (df = 2, 6 and 1, respectively) as factors.
| Variables | CS | BAP | Experiment | CS x BAP | CS x E | BAP x E | CS x BAP x E |
|---|---|---|---|---|---|---|---|
| Rate of leaf appearance | *** | *** | ns | *** | ns | ns | ns |
| Relative leaf area expansion rate | *** | *** | ns | *** | ns | ns | ns |
| Total leaf area | *** | *** | ns | *** | ns | ns | ns |
| Individual leaf area | *** | *** | ns | *** | ns | ns | ns |
| Leaf thickness | *** | *** | ns | ** | ns | ns | ns |
| Intercellular spaces | *** | *** | ns | ** | ns | ns | ns |
| Chlorophyll | * | * | ns | * | ns | ns | ns |
| Leaf nitrogen | *** | *** | ns | *** | ns | ns | ns |
| Non-reducing sugars | *** | *** | ns | *** | ns | ns | ns |
| Relative growth rate | *** | *** | ns | *** | ns | ns | ns |
| Net assimilation rate | *** | *** | ns | *** | ns | ns | ns |
| Leaf area ratio | *** | *** | ns | *** | ns | ns | ns |
| Photosynthetic rate | *** | *** | ns | *** | ns | ns | ns |
*** = P ≤ 0.001; ** = P ≤ 0.01; * = P ≤ 0.05; ns = no significant.
Results
Leaf area development
During the experiment, both the rate of leaf appearance (RLA) and the relative leaf area expansion rate (RLAER) were directly related to plug cell volume in control plants. A BAP spray at both pre- and post-transplant significantly increased RLA and RLAER (Table 2).
Table 2 Rate of leaf appearance (RLA) and relative leaf area expansion rate (RLAER) of lettuce plants grown in 128-, 200- or 288-cell trays-1 and sprayed with pre- or post-transplant cytokinin 6-benzylaminopurine (BAP) (5, 50 or 100 mg·L-1).
| Treatments | RLA (leaves·week-1) | RLAER (cm2·cm-2·day-1) | |||||
|---|---|---|---|---|---|---|---|
| 128-cell | 200-cell | 288-cell | 128-cell | 200-cell | 288-cell | ||
| Control | 0.241 bAz | 0.229 cB | 0.188 dC | 0.0616 eA | 0.0552 dB | 0.0520 dC | |
| BAP pre-5 | 0.248 abA | 0.242 aA | 0.196 cB | 0.0645 cA | 0.0656 bA | 0.0615 dB | |
| BAP pre-50 | 0.256 abA | 0.241 aB | 0.228 bC | 0.0633 dA | 0.0631 cA | 0.0622 dB | |
| BAP pre-100 | 0.255 abA | 0.241 aB | 0.252 aA | 0.0652 cA | 0.0644 bA | 0.0655 bA | |
| BAP post-5 | 0.264 aA | 0.238 aB | 0.256 aA | 0.0668 cA | 0.0635 cB | 0.0672 aA | |
| BAP post-50 | 0.267 aA | 0.242 aB | 0.253 aB | 0.0680 bA | 0.0650 abB | 0.0650b B | |
| BAP post-100 | 0.271 aA | 0.243 aA | 0.232 bB | 0.0728 aA | 0.0678 aB | 0.0636 cC | |
zDifferent lower case letters indicate significant differences (Tukey, P ≤ 0.05) between control and BAP-sprayed treatments. Different capital letters indicate significant differences (Tukey, P ≤ 0.05) between plants from different cell number per tray. RLA and RLAER slopes were in all cases statistically different from zero (Tukey, P ≤ 0.001).
Leaf area development variables (including RLA, RLAER, total leaf area and individual leaf area) were highly significantly affected by BAP treatment, cell size, and their interaction (Table 1). At the last harvest, lettuce plants from the lower plug cell volume (288-cell trays) had significantly lower both totals and individuals leaf areas than those grown in larger plug cells. Pre-transplant BAP spray at any dose significantly increased leaf area, irrespective of plug cell size. However, maximum leaf area promotion in 200-cell plants was achieved at higher BAP concentrations than in 128-cell and 288-cell plants. Post-transplant BAP spray also promoted leaf area accumulation in comparison with controls (Table 3).
Table 3 Total leaf area and mean individual leaf area at final harvest in lettuce plants raised in 128-, 200- and 288-cell trays, and sprayed at either pre- or post-transplant stage with cytokinin 6-benzylaminopurine (BAP) (5, 50 or 100 mg·L-1) (n = 6).
| Treatments | Total leaf area (cm2·plant-1) | Individual leaf area (cm2·leaf-1) | |||||
|---|---|---|---|---|---|---|---|
| 128-cell | 200-cell | 288-cell | 128-cell | 200-cell | 288-cell | ||
| Control | 506.2 bAz | 503.5 cA | 328.0 eB | 21.62 cA | 21.11 cA | 16.77 dB | |
| BAP pre-5 | 662.0 aA | 611.8 aB | 415.2 dC | 25.14 bA | 26.86 aA | 17.97 dB | |
| BAP pre-50 | 603.1 aA | 570.3 bB | 568.6 aB | 25.00 bA | 23.76 bB | 23.58 aB | |
| BAP pre-100 | 561.3 aA | 572.7 bA | 509.2 bB | 23.49 bcA | 23.54 bA | 20.74 cB | |
| BAP post-5 | 623.3 aA | 580.5 bB | 575.7 aB | 24.71 bA | 23.53 bB | 23.44 aB | |
| BAP post-50 | 693.3 aA | 490.2 cC | 530.7 bB | 27.73 aA | 24.88 bB | 21.13 bC | |
| BAP post-100 | 605.1 aB | 636.4 aA | 478.2 cC | 24.75 bB | 26.92 aA | 22.07 abC | |
zDifferent lower case letters indicate significant differences (Tukey, P ≤ 0.05) between control and BAP-sprayed treatments. Different capital letters indicate significant differences (Tukey, P ≤ 0.05) between plants from different cell number per tray.
Biomass accumulation
A highly significant effect of cell size, BAP treatment, and their interaction was observed for RGR, NAR and LAR (Table 1). The effect of exogenous BAP sprays on lettuce DW accumulation is shown in Figure 1. Strong RGR increases, relative to controls, were found in 288-grown plants although, in a lesser extent, most BAP concentrations increased RGR also in 128- and 200-cell grown plants (Figure 1A ). Regarding RGR components, a relative increase in NAR (Figure 1B) and a relative decrease in LAR were found as a consequence of BAP application. In both cases, the higher responses were found in 288-cell grown plants.
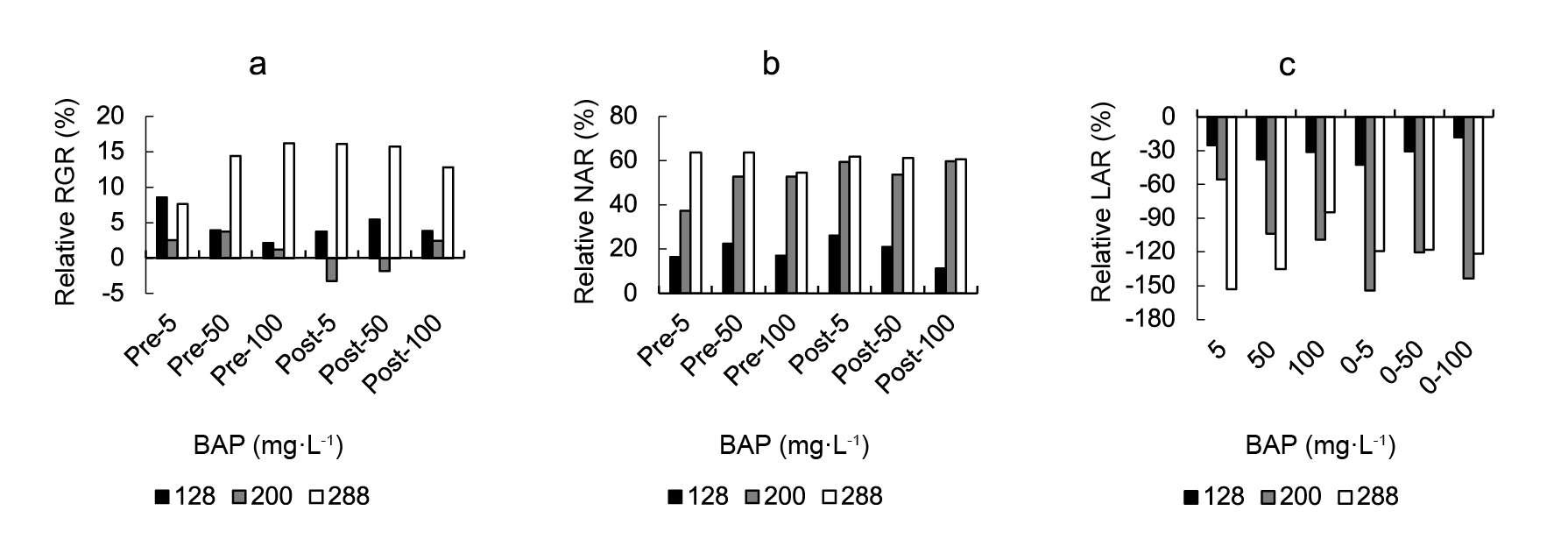
Figure 1 The effect of a 5, 50, or 100 mg·L-1 cytokinin 6-benzylaminopurine (BAP) spray at the pre- or post-transplant stage on lettuce mean: a) relative growth rate (RGR), b) net assimilation rate (NAR) and c) leaf area ratio (LAR). Data are expressed as percentages of change in control values. Control data from 128-, 200- and 288-cell trays were RGR: 0.0823, 0.0808, 0.0788 (g·g-1·day-1); NAR: 21.28, 16.73, 13.21 (g·cm-2·day-1 x 10-5) and LAR: 287.09, 537.97, 611.49 (cm2·g-1), respectively.
To analyze the contribution of changes in NAR and in LAR to BAP-driven variation in RGR, a regression analysis was performed, which showed that NAR was strongly and directly associated with RGR, while an inverse, moderate association between RGR and LAR was found (Figure 2).
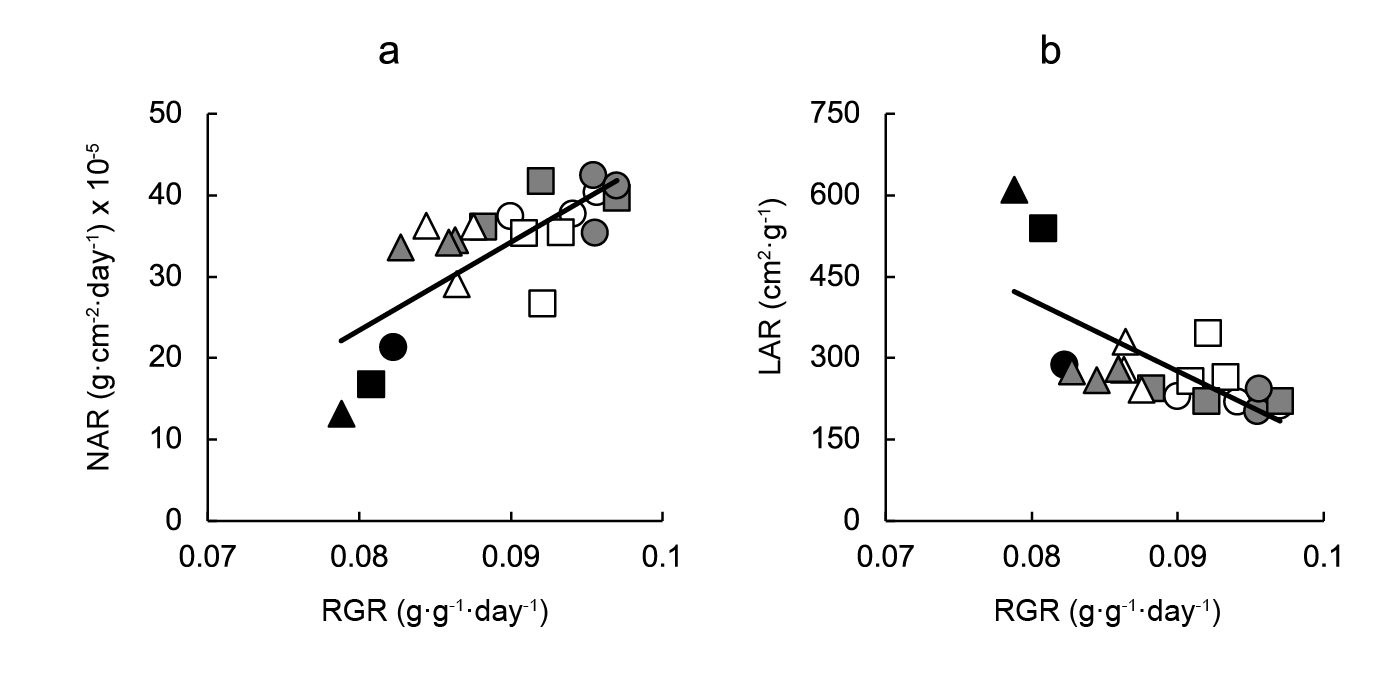
Figure 2 Contribution of changes: a) net assimilation rate (NAR) and b) leaf area ratio (LAR) to variation in relative growth rate (RGR) of lettuce plants grown in 128-, 200- or 288-cell trays and sprayed with pre- or post-transplant cytokinin 6-benzylaminopurine (BAP) sprays at 0, 5, 50 or 100 mg·L-1. Linear regression equations are NAR = 1081.60RGR - 63.11 (r2 = 0.581; P < 0.001); LAR = -13106.00RGR + 1455.30 (r2 = 0.495; P < 0.05). Symbols indicate controls (black), pre-transplant BAP (white) and post-transplant BAP (grey), corresponding to 128- (circles); 200- (squares) and 288- (triangles) cell trays.
Carbon fixation
The photosynthetic rate at the end of the experiment was highly significantly affected by cell size, BAP treatment, and their interaction (Table 1). The photosyntehtic rate showed a similar response pattern than NAR, this is, a general significant increase with BAP application in relation to control plants, with the highest responses being observed in 288-cell grown plants (Figure 3A). In agreement with these responses, the photosynthetic rate was linearly correlated with NAR (Figure 3B).
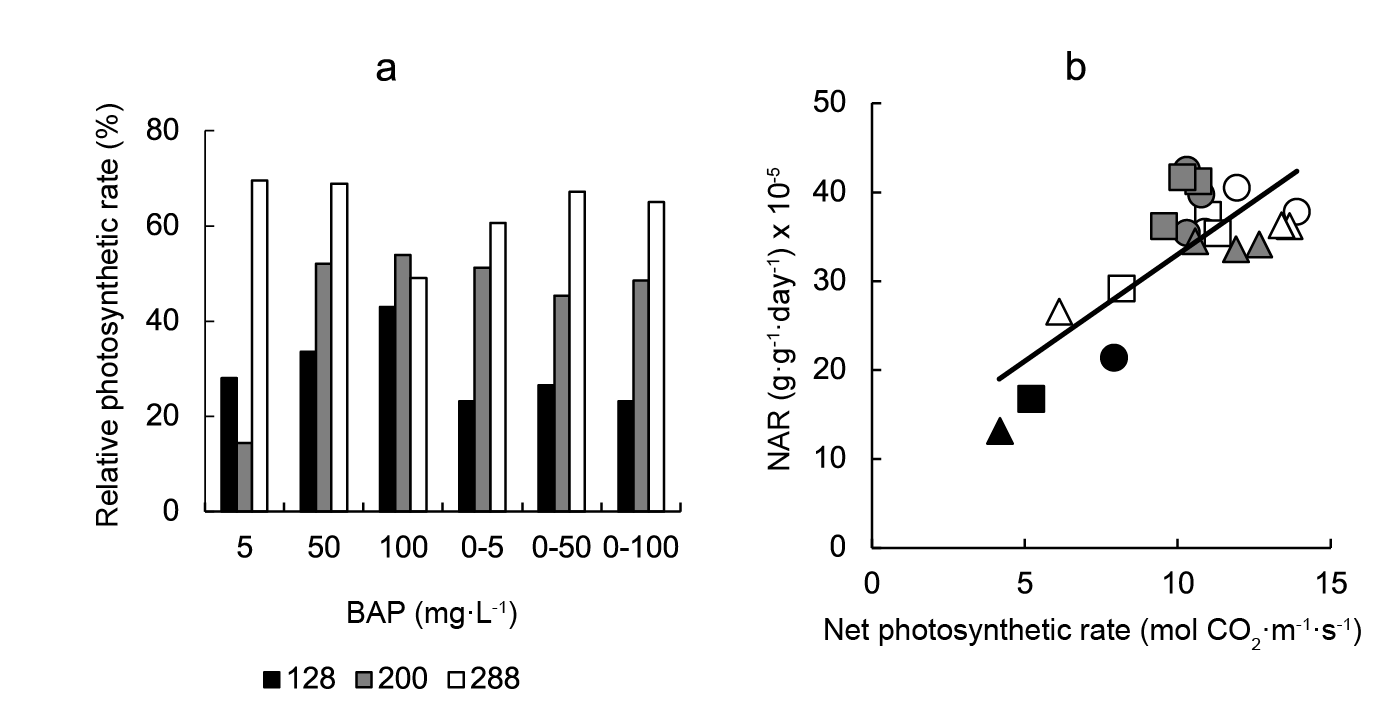
Figure 3 The effect of a 5, 50, or 100 mg·L-1 cytokinin 6-benzylaminopurine (BAP) spray application on the relative photosynthetic rate (a). Data are expressed as percentage changes observed following BAP application at 5, 50, or 100 mg·L-1 relative to plants sprayed with 0 mg·L-1 BAP. Panel b showed the inter-relationship between the net assimilation rate (NAR) and the net photosynthetic rate (μmol CO2·m-2·s-1). Linear regression equation was NAR = 2.40 photosynthetic rate + 9.00 (r2 = 0.631; P ≤ 0.001). Symbols indicate controls (black), pre-transplant BAP (white) and post-transplant BAP (grey), corresponding to 128- (circles); 200- (squares) and 288- (triangles) cell trays. Control data from 128-, 200- and 288- cell trays were 7.93, 5.22 and 4.16 mol CO2·m2·s-1, respectively.
Leaf anatomy
Both leaf anatomical variables considered, i.e. leaf thickness and percentage of intercellular spaces at the end of the experiments, were highly significantly (P ≤ 0.001) affected by cell size, BAP treatment, and their interaction (Table 1). The lowest values of leaf thickness and proportion of intercellular spaces were observed in plants grown in the smallest cells. BAP sprays at either pre- or post-transplant stage increased values of leaf thickness irrespective of cell size. On the other hand, pre- transplant BAP applications were more effective than post-transplant ones regarding the promotion of intercellular spaces percentage (Table 4).
Table 4 Leaf thickness and percentage leaf intercellular spaces in the youngest fully expanded leaf at final harvest of lettuce plants grown in 128-, 200- or 288-cell trays and sprayed with cytokinin 6-benzylaminopurine (BAP) at 0, 5, 50 or 100 mg·L-1 at the pre- or post-transplant stage (n = 6).
| Treatments | Leaf thickness (µmm) | Intercellular spaces (%) | |||||
|---|---|---|---|---|---|---|---|
| 128-cell | 200-cell | 288-cell | 128-cell | 200-cell | 288-cell | ||
| Control | 178.03 cAz | 164.77 cA | 157.20 bB | 60.91 cA | 38.06 bB | 23.84 cC | |
| BAP pre-5 | 227.27 bA | 213.64 bB | 202.65 aC | 71.90 aB | 47.32 aB | 47.77 aB | |
| BAP pre-50 | 242.42 aB | 263.64 aA | 206.44 aC | 66.93 aA | 29.64 cC | 43.77 aB | |
| BAP pre-100 | 261.36 aB | 280.30 aA | 212.12 aC | 72.99 aA | 36.60 bB | 42.94 aB | |
| BAP post-5 | 196.97 bB | 217.80 bA | 177.27 bC | 59.92 cA | 29.72 cB | 24.33 cB | |
| BAP post-50 | 200.76 bA | 215.91 bA | 155.30 bB | 68.89 aA | 35.61 bB | 29.47 bB | |
| BAP post-100 | 210.23 bA | 202.65 bA | 160.88 bB | 63.00 bA | 32.81 bB | 34.08 bB | |
zDifferent lower case letters indicate significant differences (Tukey, P ≤ 0.05) between control and BAP-sprayed treatments. Different capital letters indicate significant differences (Tukey, P ≤ 0.05) between plants from different cell numbers per tray.
Leaf metabolite concentration
The effects of cell size, BAP treatment, and their interaction were significant at the P < 0.05 level for chlorophyll, and at the P < 0.001 level for nitrogen and sugar contents (Table 1). In control plants, chlorophyll concentration was unaffected by cell size, and BAP sprays has relatively little effect, although in some cases differences between treatments or cell size were statistically significant (Table 5). Leaf nitrogen content per unit leaf area of control plants was the highest in 128-cell plants, and values decreased in parallel with decreasing cell size (Table 5). Significant increases due to BAP application were observed depending on BAP concentration and timing of application, especially in 128- and 200-cell plants.
Table 5 Leaf chlorophyll content and leaf nitrogen content at final harvest of lettuce plants grown in 128-, 200- or 288-cell trays and sprayed with cytokinin 6-benzylaminopurine (BAP) at 0, 5, 50 or 100 mg·L-1 at the pre- or post-transplant stage (n = 6).
| Treatments | Chlorophyll (mg·m-2) | Leaf nitrogen (mg·cm-2) | |||||
|---|---|---|---|---|---|---|---|
| 128-cell | 200-cell | 288-cell | 128-cell | 200-cell | 288-cell | ||
| Control | 213.50 aAz | 211.69 bA | 205.22 cA | 0.971 dA | 0.652 cdB | 0.567 bC | |
| BAP pre-5 | 195.92 bB | 192.04 cB | 228.49 aA | 1.439 aA | 0.489 dB | 0.425 cB | |
| BAP pre-50 | 211.94 aB | 224.87 aA | 209.88 cB | 1.160 bA | 0.723 cB | 0.645 aC | |
| BAP pre-100 | 208.59 aB | 214.79 bB | 233.10 aA | 1.141 bA | 0.648 cdB | 0.436 cC | |
| BAP post-5 | 175.23 cC | 186.35 cB | 202.90 cA | 0.965 dA | 0.458 dB | 0.371 dC | |
| BAP post-50 | 211.95 aA | 191.52 cB | 219.70 bA | 1.033 cA | 1.085 bA | 0.560 bB | |
| BAP post-100 | 213.76 aB | 229.01 aA | 184.02 dC | 1.422 aB | 1.563 aA | 0.532 bC | |
zDifferent lower case letters indicate significant differences (Tukey, P ≤ 0.05) between control and BAP-sprayed treatments. Different capital letters indicate significant differences (Tukey, P ≤ 0.05) between plants from different cell number per tray.
Non-reducing sugar concentration was substantially lower in plants grown in the smallest cells, in comparison with 128- and 200-cell plants, irrespective of BAP treatment. In general, BAP application resulted in higher concentrations, independently of cell size (Table 6).
Table 6 Shoot non-reducing sugar concentration at final harvest of lettuce plants grown in 128-, 200- or 288-cell trays and sprayed with cytokinin 6-benzylaminopurine (BAP) at 0, 5, 50 or 100 mg·L-1 at the pre- or post-transplant stage (n = 6).
| Treatments | Non-reducing sugars (mg·g-1 fresh weight) | ||
|---|---|---|---|
| 128-cell | 200-cell | 288-cell | |
| Control | 3.11 dAz | 2.97 dB | 1.23 dC |
| BAP pre-5 | 3.60 cA | 3.59 cA | 1.70 bB |
| BAP pre-50 | 5.95 bA | 5.60 bA | 1.57 bA |
| BAP pre-100 | 6.62 aB | 7.75 aA | 1.13 bcC |
| BAP post-5 | 3.91 cA | 3.97 cA | 2.51 aB |
| BAP post-50 | 3.62 cA | 3.58 cA | 1.53 bB |
| BAP post-100 | 6.63 aA | 1.83 eB | 1.39 cdB |
zDifferent lower case letters indicate significant differences (Tukey, P ≤ 0.05) between control and BAP-sprayed treatments. Different capital letters indicate significant differences (Tukey, P ≤ 0.05) between plants from different cell number per tray.
Relationships between carbon fixation and leaf anatomical variables
Both the net assimilation rate (Figure 4a) and the photosynthetic rate (Figure 4b) were directly correlated with leaf thickness, and the best fits were obtained with curvilinear equations (Figure 4a and b, respectively). Both variables related with carbon fixation were also directly correlated with the proportion of intercellular spaces (Figure 4c and d).
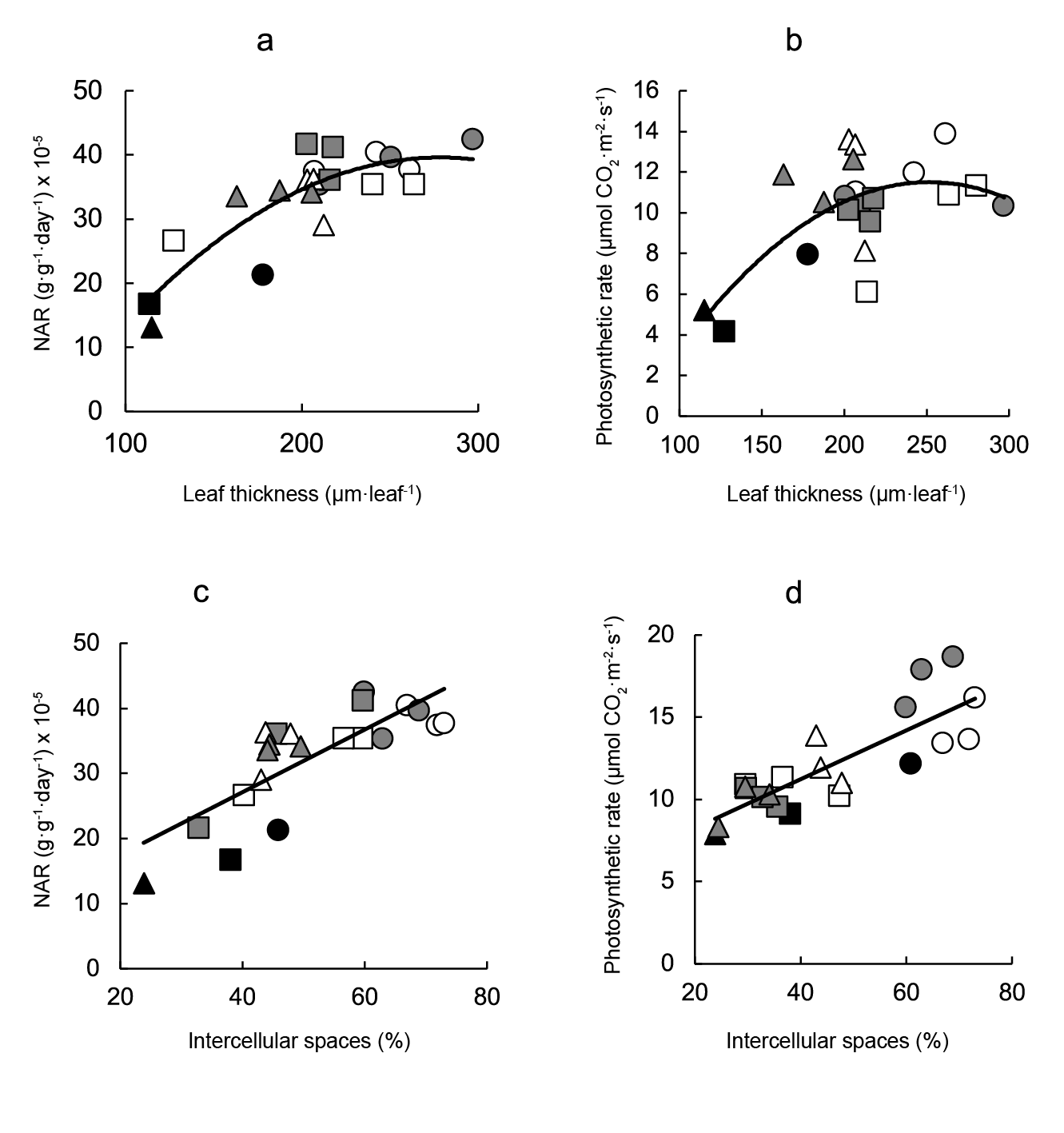
Figure 4 Relationship between carbon assimilation and leaf anatomical variables. Linear regression equations are: a) NAR = -0.0008leaf thickness2 + 0.455leaf thickness - 23.63 (r2 = 0.726; P ≤ 0.001), b) photosynthetic rate = -0.0004leaf thickness2 + 0.181leaf thickness - 11.25 (r2 = 0.442; P ≤ 0.001), c) NAR = 0.48intercellular spaces + 7.87 (r2 = 0.618; P ≤ 0.001) and d) photosynthetic rate = 0.15intercellular spaces + 5.28 (r2 = 0.679; P ≤ 0.001). Symbols indicate controls (black), pre-transplant BAP (white) and post-transplant BAP (grey), corresponding to 128- (circles); 200- (squares) and 288- (triangles) cell trays.
Relationships between carbon fixation and leaf nitrogen content
A direct association between both NAR, or photosynthetic rate, and leaf nitrogen content per unit area was observed. The best fits were obtained with a curvilinear relationship, plant response reaching an optimum above (Figure 5a and b), although correlations were somewhat weaker than those observed between carbon fixation and leaf anatomical variables.
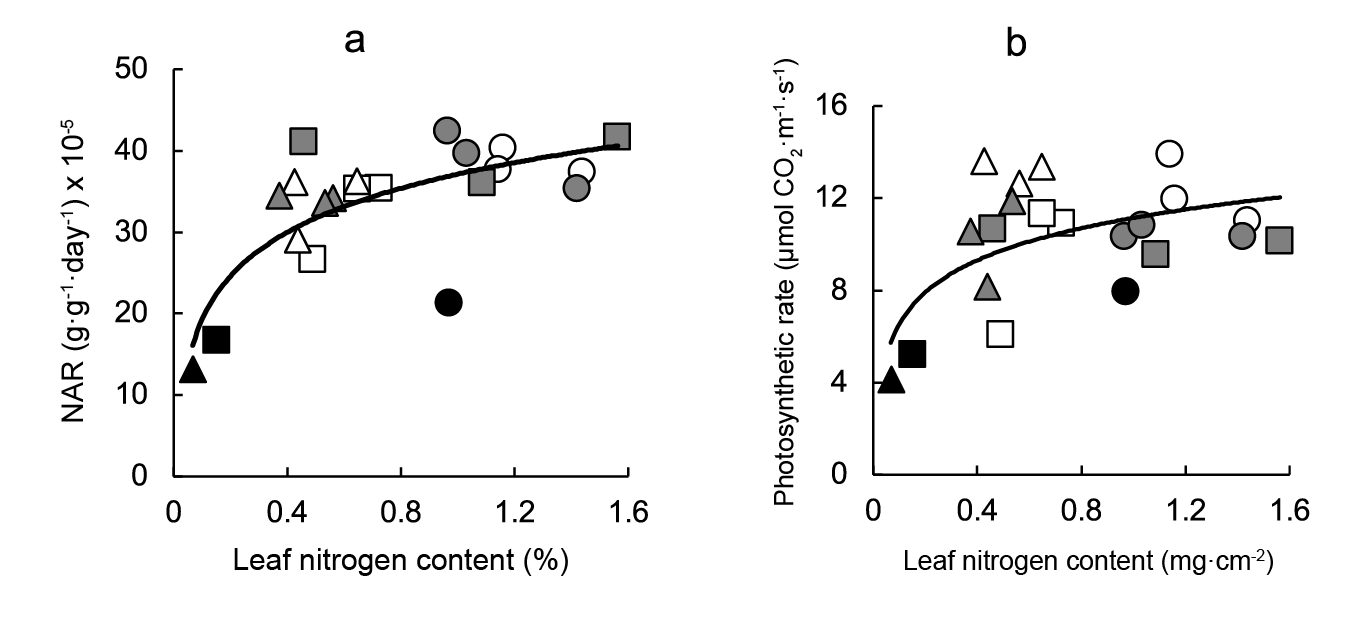
Figure 5 Relationship between carbon assimilation and leaf metabolic variables. Linear regression equations are: a) NAR = 7.78leaf nitrogen content + 37.12 (r2 = 0.558; P ≤ 0.001) y b) photosynthetic rate = 2.00leaf nitrogen content2 + 11.14 (r2 = 0.338; P ≤ 0.01). Symbols indicate controls (black), pre-transplant BAP (white) and post-transplant BAP (grey), corresponding to 128- (circles); 200- (squares) and 288- (triangles) cell trays.
Relationship between leaf thickness and anatomical or metabolic variables of leaves
The possibility of autocorrelation between leaf thickness and other variables analyzed (percentage of intercellular spaces, nitrogen per unit area, chlorophyll per unit area, non-reducing sugars concentration) was studied through regression analyses. While non-significant relationships between the intercellular spaces proportion (Figure 6a), leaf nitrogen content (Figure 6b), chlorophyll content (Figure 6c) and leaf thickness were observed, a significant correlation between non-reducing sugars content and leaf thickness (Figure 6d) (r2 = 0.606) was found.
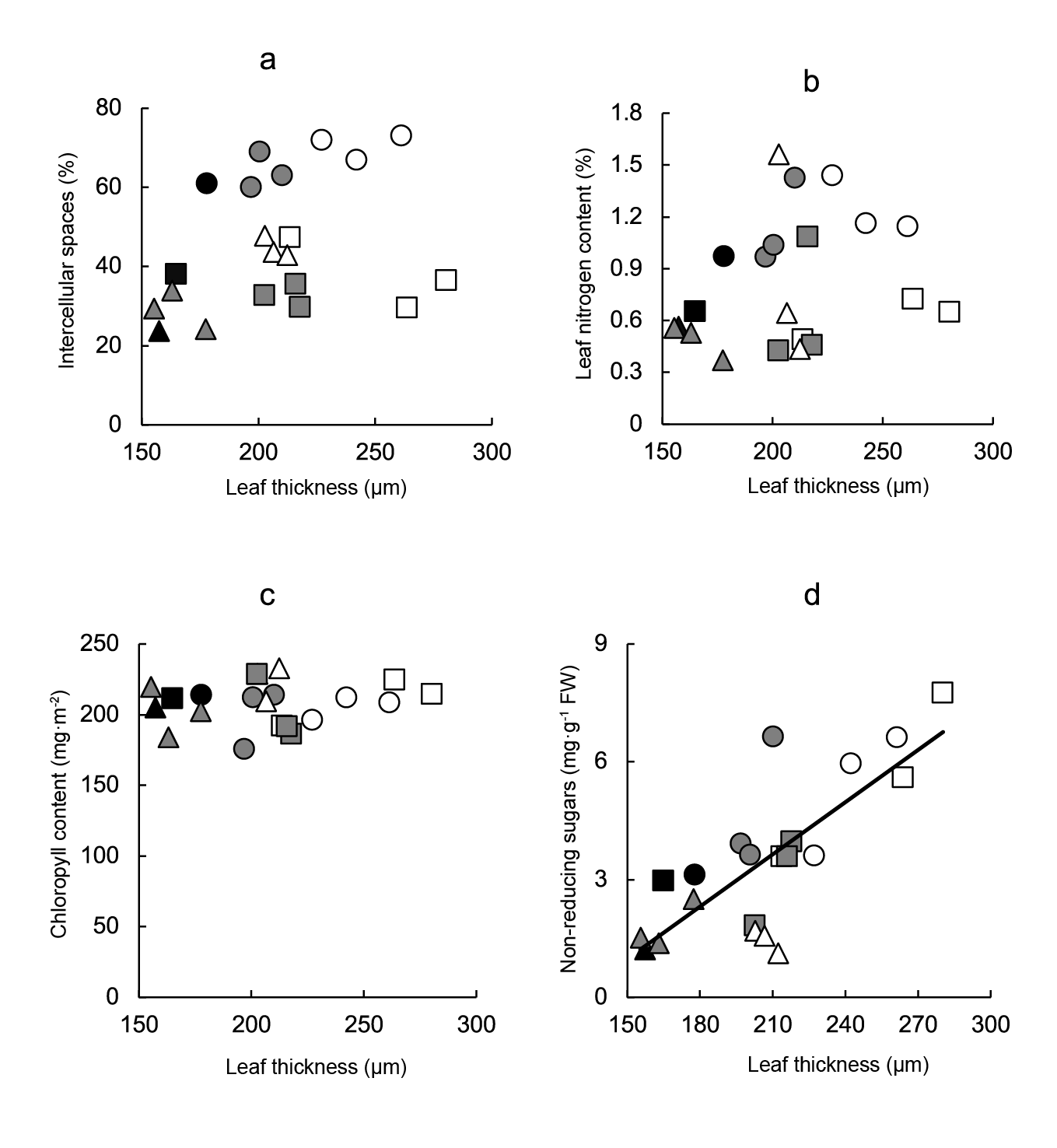
Figure 6 Relationship between leaf thickness and anatomical and metabolic variables of leaves. Linear regression equations are: a) Intercellular spaces = 0.15leaf thickness + 14.17 (r2 = 0.103), b) leaf nitrogen content = 0.001leaf thickness + 0.22 (r2 = 0.076), c) chlorophyll content = 0.07leaf thickness + 194.25 (r2 = 0.021) y d) non-reducing sugars = 0.04leaf thickness - 5.67 (r2 = 0.606; P ≤ 0.001). Symbols indicate controls (black), pre-transplant BAP (white) and post-transplant BAP (grey), corresponding to 128- (circles); 200- (squares) and 288- (triangles) cell trays.
When all photosynthetic rate data were plotted against leaf mesophyll volume (Figure 7a) a significant direct relationship (r2 = 0.612) was observed. However, closer correlations were found by analyzing pre- and post-transplant data by separate (r2 = 0.757 and r2 = 0.866, respectively) (Figure 7).
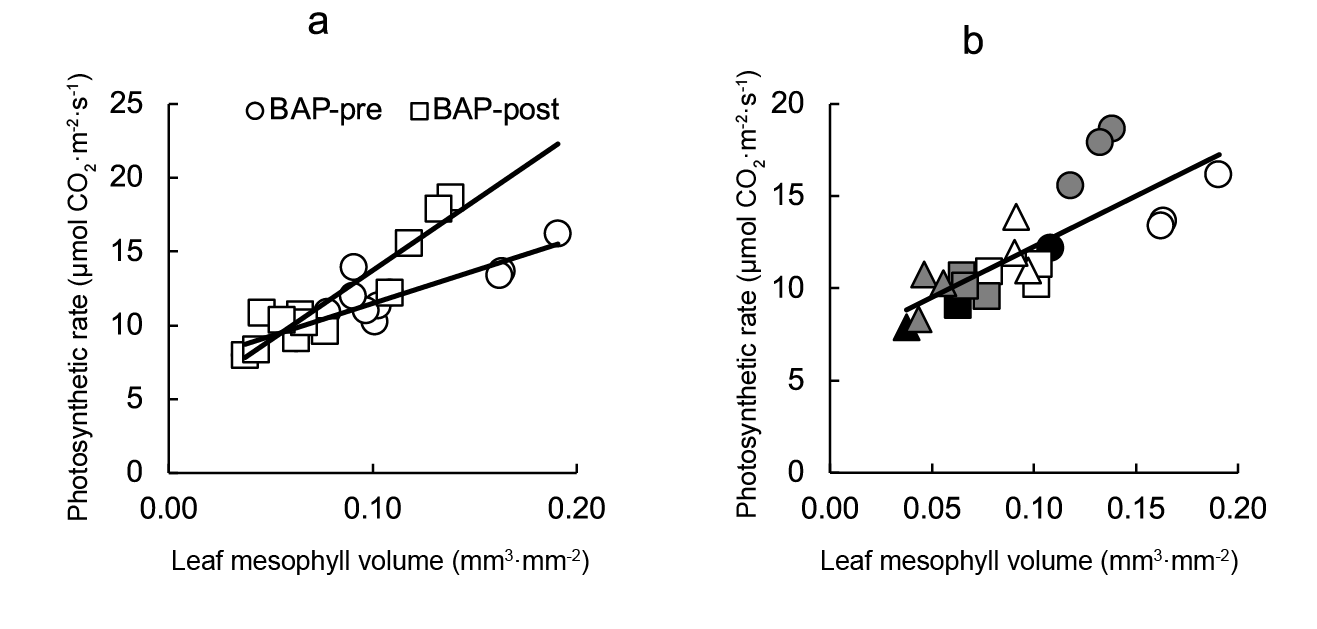
Figure 7 Relationship between photosynthetic rate and leaf mesophyll volume for lettuce plants BAP-sprayed at pre- or post-transplant (a). Plate B showed data differences for each treatment (plug cell number and BAP sprays). Linear regression equation is: photosynthetic rateBAP pre-transplant = 0.0004air space volume + 7.06 (r2 = 0.757), photosynthetic rateBAPpost-transplant = 0.0009air space volume + 4.29 (r2 = 0.866), and photosynthetic rateCombined data = 54.92air space volume + 6.77 (r2 = 0.612). Symbols indicate controls (black), pre-transplant BAP (white) and post-transplant BAP (grey), corresponding to 128- (circles); 200- (squares) and 288- (triangles) cell trays.
Discussion
A large amount of evidence, gathered from studies on a wide range of horticultural and ornamental species, indicates that root restriction effects due to small transplant pots may be at least partially overcome by the exogenous supply of cytokinin (Di Benedetto et al., 2020b). Applications of this hormone either in pre- or early post-transplant stages often reverts root restriction by promoting carbon assimilation, ultimately resulting in recovery of plant development and biomass accumulation in a manner that may depend on severity of root restriction, hormone concentration and timing of cytokinin application (Di Benedetto et al., 2020a). Our present data on BAP-sprayed lettuce seedlings subjected to root-restriction are largely in agreement with previous reports on other species, since an increased plant biomass accumulation, and a promotion of photosynthetic area development (Table 2) associated with faster leaf production and higher individual leaf area, were observed.
Decreasing plug cell volume from 128 to 288 cells per tray resulted in a steep decrease in NAR (Figure 1b) and net photosynthesis values (Figure 3a). However, the impact on RGR was in general smaller (Figure 1a), due to the progressive increase in LAR observed in plants grown in smaller cells (Figure 1c). Then, the change in resource allocation, prioritizing leaf area development, can be considered as an adaptive response tending to compensate for the strong negative impact of root restriction on carbon fixation. Impairment of carbon assimilation due to root restriction has been reported before for horticultural and ornamental crops such as tomato (Shi, Ding, Dong, Zhou, & Yu, 2008), pothos (Di Benedetto et al., 2015b) and ficus (Di Benedetto et al., 2020c).
Exogenous cytokinin application, as expected, generally counteracted the negative impact of root restriction on plant growth, resulting in higher leaf area development and whole plant growth than untreated controls (Table 3), but especially, in increased mean NAR (Figure 1c) and net photosynthetic rate (Figure 3a). Furthermore, BAP sprays were relatively more effective in promoting NAR on seedlings grown in the smallest plugs (i.e. those cultivated in 288-cell trays). This is consistent with expectations, since plants under severe root restriction are supposed to produce the least amount of cytokinin, which are known to be synthesized in root apices (Zhang et al., 2014).
Previous work on other species has shown that plant response to increasing BAP concentration of sprays often reaches a plateau, suggesting the existence of an optimum hormone concentration in tissues (Araki, Rattin, Di Benedetto, & Miravé, 2007). In agreement with this, our results show that doubling BAP concentration from 50 to 100 mg·L-1 had no significant effect on growth promotion, similar responses being found when BAP was applied either at the pre- or post-transplant stages.
Despite promoting leaf area development, BAP treatment resulted in a decrease in LAR, and this was the most remarkable in plants grown in smaller cells. This decrease in LAR could be attributed to an enhanced root growth together with lower specific leaf area, which was in turn associated with increased leaf thickness (Table 5) and higher sugar concentration in sprayed plants, in comparison with controls (Table 6). Thus, BAP-treated plants showed morpho-physiological changes that tended to compensate for those responses elicited by root restriction.
The physiological processes involved in cytokinin-mediated growth promotion in root-restricted plants have received relatively little attention. Cytokinin are well known to promote the development of the photosynthetic apparatus, including chloroplast ultrastructure and chlorophyll synthesis, especially during etioplast-to-chloroplast transition (Cortleven & Schmülling, 2015).
Among the most important processes related to carbon assimilation, cytokinin upregulates protein abundance to exert its role on chloroplast development and function. Cytokinin enhances the expression of plastid-related nuclear genes such as CAB, encoding chlorophyll a binding proteins of photosystem II, and also genes encoding both the small and large Rubisco subunits (Cortleven & Schmülling, 2015; Liu et al., 2017). Furthermore, cytokinin accelerates chlorophyll biosynthesis by promoting synthesis of the precursor 5-aminolevulinic acid, and by enhancing the activity of NADPH:protochlorophyllide oxidoreductase that converts protochlorophyllide into chlorophyllide (Cortleven & Schmülling, 2015).
It has been hypothesized that growth-promoting effects of exogenous cytokinin in root restricted seedlings could be explained either by increased chlorophyll and/or nitrogen content, ultimately resulting in the promotion of the photosynthetic rate (Di Benedetto et al., 2020c).
Our results show that nitrogen content was mostly affected by plug cell size (Table 5), being quite variable the impact of BAP sprays, and that variation in nitrogen content could only explain changes in carbon fixation at very low N values (i.e., lower than about 0.5 mg·cm-2, observed in plants raised in the smallest plugs) (Figure 5). In our experiments, because of weekly-applied fertigation, N availability in substrate may be considered as non-limiting, and under these conditions C3 plants such as lettuce are expected to synthesize large amounts of photosynthetic proteins, which makes photosynthesis limited by Rubisco activity mostly unlikely (Sage & Coleman, 2001).
Our work also shows that chlorophyll concentration in leaves was not modified by BAP, in either pre- or post-transplant application, irrespective of plug cell size (Table 5). It was also observed that any variation in chlorophyll concentration among treatments was not associated with carbon fixation variables, which contrasts with Croft et al. (2017) suggestion that leaf chlorophyll content may be considered as a proxy for leaf photosynthetic capacity. Although the reason for this apparent discrepancy is not clear, it should be noted that chlorophyll synthesis promotion by cytokinin has been mostly observed in dark grown seedlings (Cortleven & Schmülling, 2015), and that previous studies from our laboratory on greenhouse grown plants of several species have also shown a lack of responsiveness of chlorophyll to BAP sprays (Di Benedetto et al., 2015a). This may be interpreted from an eco-physiological point of view: since maximum daily light intensity values in the present experiments were close to those reported as saturating intensities for lettuce (Wang, Lu, Tong, & Yang, 2016), any further increase in light capture would not be expected to significantly promote photosynthesis.
A further possibility to explain the cytokinin-induced promotion of carbon fixation in root-restricted plants is that morpho-anatomical changes prompted by the hormone may affect carbon diffusion to chloroplasts. In our study, the proportion of intercellular spaces was severely diminished by decreasing plug cell volume, and conversely, BAP induced a remarkable recovery of its values in plants raised in 288-cell trays, particularly in pre-transplant applications (Table 4). This response, together with an increase in leaf thickness often observed in BAP-treated plants (Table 4), determined a large increase in free space volume per unit leaf surface area, which was strongly correlated with photosynthetic rate (Figure 4b) which may help CO2 diffusion to chloroplasts.
Bosselaers (1983) early addressed the possible association between photosynthesis promotion by cytokinin and increased gas diffusion in his study on Phaseolus leaf architectural changes as a consequence of kinetin or BAP application. However, in that study it was concluded that changes due to cytokinin application seemed of little impact on leaf diffusion resistance. Nevertheless, it must be considered that in Booselaers' study plants were not root-restricted, and that high hormone doses were applied for five consecutive days, which may have increased endogenous cytokinin concentration above optimum levels.
In any case, there is a renewed interest in studies of the leaf anatomy impact on CO2 diffusion (Evans, Kaldenhoff, Genty, & Terashima, 2009; Kaldenhoff, 2012; Han et al., 2018; Ren, Weraduwage, & Sharkey, 2019; Roig-Oliver et al., 2020) and recent work on the aroid vine Rhodospatha oblongata subjected to contrasting light environments emphasizes the impact of thicker leaves with larger spongy parenchyma and increased intercellular spaces on maximum photosynthetic rate (Mantuano, Ornellas, Aidar, & Mantovani, 2021). Thus, the role of cytokinin on changes in leaf anatomy as associated with photosynthetic capacity deserves further studies.
Under non-limiting irradiance conditions, the net photosynthetic rate of C3 plants is usually limited by CO2 (Sage & Coleman, 2001), a condition that has likely driven plant evolution (Beerling, 2012). Decades of studies focused on the effect of free air CO2 enrichment in plant biology have made it evident that C3 plants, unlike C4 ones, increase their photosynthetic rate and biomass production (Long, Ainsworth, Leakey, Nösberger, & Ort, 2006; Donohue, Roderick, McVicar, & Farquhar, 2013). In lettuce, CO2 enrichment (from 355 to 800-900 ppm; from 400 to 800 ppm and from 200 to 1000 ppm in Mortensen [1994] , Kitaya, Niu, Kozai, & Ohashi [1998] and Becker & Kläring [2016] , respectively) has shown a promotion of dry mass accumulation in the aerial part ranging from 18 % to more than 100 %, according to experiments.
The possibility that root restriction reversion by cytokinin is mediated by changes in the CO2 diffusion pathway to chloroplasts is consistent with the fact that several plant responses observed in the present work are similar to those observed in lettuce plants grown under CO2 enrichment, such as increased leaf thickness (Giri, Armstrong, & Rajashekar, 2016), increased sugar concentration (Becker & Kläring, 2016) and decreased specific leaf area (Kitaya et al., 1998), being the latter a variable that depends on both leaf thickness and metabolite concentration. In our work, a significant correlation between sugar concentration and leaf thickness was found (Figure 6d ), which might be associated with osmotic-driven mesophyll cell enlargement.
Besides, increases in sugar concentration and leaf thickness are desirable quality traits in lettuce, especially because they contribute to an extended shelf life (Zhang et al., 2007; Lin et al., 2013).
Lettuce is a very important horticultural crop worldwide and maximizing both production and quality is a central goal to be achieved. Modern production systems may impose stressful conditions that need to be overcome in order to accomplish this goal (Rao, Laxman, & Shivashankara, 2016; Wien & Stützel, 2020). The use of different bio-stimulants have been suggested as alleviators to different abiotic stresses, including root restrictions during nursery, and many of these compounds contain plant hormones like auxin and cytokinin (Bulgari, Franzoni, & Ferrante, 2019; Di Benedetto et al., 2020a, 2020b).
The present work shows, first, that both production and certain quality attributes of lettuce can be enhanced in root-restricted plants when subjected to a single exogenous application of cytokinin, and secondly, that these responses may be associated with changes in certain leaf anatomical characters that may improve CO2 diffusion and therefore enhance the photosynthetic capacity of plants.
Conclusions
Carbon assimilation of lettuce seedlings grown in small cells was enhanced by exogenous BAP application and this effect was associated with changes in leaf anatomy such as an increase in the proportion of free air spaces, and an increased leaf thickness, which together may help CO2 diffusion to chloroplasts. Instead, despite previous assumptions, no changes in chlorophyll concentration were observed while increases in leaf N concentration per unit leaf area appeared to play a relatively minor role. A significant correlation between BAP-driven increases in sugar concentration and leaf thickness was found, which may represent desirable quality traits in this species.
In sum, the present work shows that both production and certain quality attributes of lettuce can be promoted in root-restricted plants when subjected to a single exogenous cytokinin application, in association with leaf anatomical changes that could enhance plant photosynthetic capacity.











 texto en
texto en 


