Abstract
Seed germination is influenced by imbibition capacity, viability and seed vigor; these characteristics could help to explain the few successful germination rate of the genus Opuntia. The aim of this study was to evaluate the imbibition, viability, and vigor of the seeds of nine variants of O. streptacantha, O. hyptiacantha, O. megacantha and O. ficus-indica and its relation to seed biomass and time of collect. The hypothesis was that the imbibition, viability, and vigor of the Opuntia seeds are low and independent of the species and time of harvest. Imbibition was quantified as moisture gain over the original seeds weight. Viability and vigor of the embryos were determined by the tetrazolium test. The experimental design was completely random, with nine treatments (or accessions) and a variable number of repetitions. The results were analyzed with ANOVA, means comparison with the Tukey (p≤0.05) and Pearson correlation tests. The nine variants showed accelerated seed imbibition during the first 8 h. The maximum mean imbibition remained constant at 29%, for 50-68 h, except for one O. megacantha variant and one O. ficus-indica, both from the Estado de México, which imbibed 60%. The maximum imbibition was not directly related (p>0.05) with seed biomass of the wild species O. streptacantha; in contrast, in the nearly wild O. hyptiacantha, the fairly domesticated O. megacantha, and the highly domesticated O. ficus-indica, there was a positive and significant correlation (r=0.731 to 0.947). On average, the four species showed 97.6% seed viability and 94% vigor, with no significant (p>0.05) differences. The seeds of the four species reach maximum imbibition within hours, its viability and vigor are significantly high and independent of the species and harvest time. The imbibition ability of the Opuntia seeds is modified by the domestication process.
Keywords::
Sexual reproduction, seed biomass, imbibition, vigor, prickly pear, Opuntia
Introduction
The genus Opuntia (Cactaceae) is native to the American continent, it includes 188 species and 78 of them are native to México (Anderson, 2001). The plants of these species are known as “nopales” and are a valuable genetic resource due to the wide range of uses, of both, their tender and mature cladodes, and fruits as food for humans, livestock and wild animals, in herbal medicine and as host of the hemipteran parasite Dactylopius coccus, from which a natural carmine pigment is extracted (Bravo-Hollis and Sánchez-Mejorada, 1991).
-
Anderson, 2001The Cactus Family, 2001
-
Bravo-Hollis and Sánchez-Mejorada, 1991Las Cactáceas de México, 1991
According to Callen (1966), Opuntia and Homo sapiens have a close relationship for over 9000 years. Domesticated species of Opuntia cacti evolved from the wild nopales, from a continuous and systematic harvest, to the management as formal cultivation on commercial plantations. This included an intermediate stage of plants establishment in close to home orchards. The abundance of wild variants and the plethora of cultivars with exceptional features, such as producing large sweet and succulent fruit, turgid cladodes, or promoting Dactylopius coccus infection, allowed to establish a domestication gradient among the species of this genus, by morphological hole plant characters, nopales and fruits (Reyes-Agüero et al., 2005). This gradient was supported by biophysical, biochemical and physiological studies of tender cladodes (López-Palacios et al., 2012; García-Nava et al., 2015) and fruit and seed morphology (López-Palacios et al., 2015).
-
Callen (1966Analysis of the Tehuacan coprolitesThe Prehistory in Tehuacan Valley. I. Environment and Subsistence, 1966
-
Reyes-Agüero et al., 2005Variación morfológica de Opuntia (Cactaceae) en relación con su domesticación en la Altiplanicie Meridional de MéxicoInterciencia, 2005
-
López-Palacios et al., 2012Effects of domestication on structural polysaccharides and dietary fiber in nopalitos (Opuntia spp.)Genet. Resour. Crop Ev, 2012
-
García-Nava et al., 2015Biophysical and physiological characteristics of nopalitos (Opuntia spp. Cactaceae) as influenced by domesticationGenet. Resour. Crop Ev, 2015
-
López-Palacios et al., 2015Inter- and intra-specific variation in fruit biomass, number of seeds, and physical characteristics of seeds in Opuntia spp., CactaceaeGenet. Resour. Crop Ev, 2015
Vegetative propagation in Opuntia is common and is the most successful method (Colunga-García et al., 1986; Reyes-Agüero et al., 2005; Mandujano et al., 2007) in wild populations, home gardens and commercial plantations (Hamilton, 1970; Sáenz et al., 2006; Lenzi and Orth, 2012). Establishment of Opuntia seedlings under natural and laboratory conditions is low; in the first case, out of 600 000 seeds only one plant will appear (Mandujano et al., 1996). But,Colunga-García et al., (1986) state that plants in home gardens could grow from seed.
-
Colunga-García et al., 1986Variación morfológica, manejo agrícola tradicional y grado de domesticación de Opuntia spp. en el Bajío GuanajuatenseAgrociencia, 1986
-
Reyes-Agüero et al., 2005Variación morfológica de Opuntia (Cactaceae) en relación con su domesticación en la Altiplanicie Meridional de MéxicoInterciencia, 2005
-
Mandujano et al., 2007Effect of reproductive modes and environmental heterogeneity in the population dynamics of a geographically widespread clonal desert cactusPopul. Ecol, 2007
-
Hamilton, 1970Seedling development of Opuntia bradtiana (Cactaceae)Am. J. Bot, 1970
-
Sáenz et al., 2006Utilización agroindustrial del nopalBoletín de Servicios Agrícolas de la FAO, 2006
-
Lenzi and Orth, 2012Mixed reproduction systems in Opuntia monacantha (Cactaceae) in Southern BrazilBraz. J. Bot, 2012
-
Mandujano et al., 1996Reproductive ecology and inbreeding depression in Opuntia rastrera (Cactaceae) in the Chihuahuan Desert: Why are sexually derived recruitments so rare?Am. J. Bot, 1996
-
Colunga-García et al., (1986Variación morfológica, manejo agrícola tradicional y grado de domesticación de Opuntia spp. en el Bajío GuanajuatenseAgrociencia, 1986
Seeds of Opuntia spp. have physiological dormancy because they need an after-ripening period to germinate. Also, seeds have a lignified hard seed coat that hinders germination. Its seed coat needs between 0.2 to 4.6 kN to fracture, according to López-Palacios et al. (2015), or 1.59 to 1.68 kN, according to Aguilar-Estrada et al. (2003) and Reyes-Agüero et al. (2005). Furthermore, embryos have low growth potential (Orozco-Segovia et al., 2007). Methods of mechanical scarification, washing with water, immersion in hot water or acid (HCl, H2SO4 or gibberellic), and exposure to cold or dry heat are applied to seeds of some Opuntia species. Nevertheless, they do not have a similar effect in all species, partly increasing the germination of some or having a negative effect on others (Mandujano et al., 2005; Rojas-Aréchiga et al., 2011). At the same time, some fungi of the genus Phoma sp., Trichoderma koningii and Penicillium chrysogenum can erode their seed coat and facilitate the radicle to emergence (Delgado-Sánchez et al., 2013). In connection with the above, a dormancy classification is 1) exogenous or pericarp or seed coat dormancy, 2) endogenous or embryonic dormancy and 3) combined or simultaneous seed coat dormancy, and the embryo (FAO, 1991). According to this classification, the seeds of most species of the Opuntia genus show combined dormancy.
-
López-Palacios et al. (2015Inter- and intra-specific variation in fruit biomass, number of seeds, and physical characteristics of seeds in Opuntia spp., CactaceaeGenet. Resour. Crop Ev, 2015
-
Aguilar-Estrada et al. (2003Caracterización de la semilla de 403 variantes de nopal (Opuntia spp.)Memoria del IX Congreso Nacional y VII Internacional sobre Conocimiento y Aprovechamiento del Nopal, 2003
-
Reyes-Agüero et al. (2005Variación morfológica de Opuntia (Cactaceae) en relación con su domesticación en la Altiplanicie Meridional de MéxicoInterciencia, 2005
-
Orozco-Segovia et al., 2007Seed anatomy and water uptake in relation to seed dormancy in Opuntia tomentosa (Cactaceae, Opuntioideae)Ann. Bot, 2007
-
Mandujano et al., 2005Breaking seed dormancy in Opuntia rastrera from the Chihuahuan desertJ. Arid Environ, 2005
-
Rojas-Aréchiga et al., 2011Effect of gibberellic acid on germination of seeds of five species of cacti from the Chihuahuan Desert, Northern MexicoSouthwest. Nat, 2011
-
Delgado-Sánchez et al., 2013Effect of fungi and light on seed germination of three Opuntia species from semiarid lands of central MexicoJ. Plant Res, 2013
For a seed to generate a seedling, seed germination must happen, this involves metabolic and morphogenetic mechanisms, summarized as follows, phase: 1) imbibition or water absorption by seed, 2) activation of metabolism and respiration process, protein synthesis and mobilization of embryo reserve substances, and 3) elongation of the embryo and seed coat rupture through which the output of the radicle is observed (Suárez and Melgarejo, 2010). However, imbibition may still occur in non-viable seeds, meaning that it does not guarantee germination by itself (Azcón-Bieto and Talón, 2000). Thus, the determination of seed viability, among other characteristics such as the amount and reserves types, and the absence of germination inhibitors, allows knowing its potential for germination.
-
Suárez and Melgarejo, 2010Biología y germinación de semillasExperimentos en Fisiología Vegetal, 2010
-
Azcón-Bieto and Talón, 2000Fundamentos de Fisiología Vegetal, 2000
Among the variables that define the seeds to originate new plants are the adequate imbibition capacity, and high viability and vigor (Baskin and Baskin, 1977). These characteristics are necessary for seeds germination and seedlings establishment. Seeds of the Opuntia genus have barely been evaluated, although those characteristics could help to explain their unsuccessful germination.
-
Baskin and Baskin, 1977Seed and seedling ecology of Opuntia compressa in Tennessee cedar gladesJ. Tennessee Acad. Sci, 1977
The aim of this study was to evaluate the maximum seed imbibition, viability and vigor in nine variants of O. streptacantha, O. hyptiacantha, O. megacantha and O. ficus-indica and its relationship to seed biomass. Our hypothesis was that seed imbibition, viability and vigor of the embryos are remarkably low and independent of species and time of harvest.
Materials and Methods
Plant material
In this study, seeds of ripe fruits of nine variants were evaluated. These correspond to four species of Opuntia, with a different domestication degree (Reyes-Agüero et al., 2005) and harvest time of 3 to 5 years. The biological material was obtained from 1) the Germplasm Bank at the Centro Regional Universitario Centro-Norte, Universidad Autónoma Chapingo (CRUCEN-UACh) in Zacatecas, México (22° 44’ 49.6” N, 102° 46’ 28.2” O), with BS1kw(w) climate, 382 mm of annual rainfall, rainy season in summer and cold winters (García, 2004); 2) an orchard backyard in the town of San Salvador Atenco, Estado de México (19° 33’ 30’’ N, 98° 54’ 45’’ O), with BSkw (w)(i) semidry climate, 604 mm annual rainfall, summer rainy season and cold winters (García, 2004); 3) a commercial orchard of the municipality of Teotihuacán de Arista, Estado de Mexico (19° 41’ N, 98° 51’ W), with BS1kw (w)(i’)g semiarid climate, less than 5% winter rains and low thermal oscillations (García, 2004); 4) a wild population from San Luis de la Paz, Guanajuato, México (21° 41’ N, 100° 45’ W), with a BS0kw semidry climate, with 400 mm per year (García, 2004).
-
Reyes-Agüero et al., 2005Variación morfológica de Opuntia (Cactaceae) en relación con su domesticación en la Altiplanicie Meridional de MéxicoInterciencia, 2005
-
García, 2004Modificaciones al Sistema de Clasificación Climática de Köppen, 2004
The fruits were harvested between July and August 2011, 2012 and 2013 (Table 1). To obtain the seeds, fruit pulp was placed with water in a domestic blender, slightly sharp blades were driven 30 s at low speed and the mixture was sieved. The seeds were then rinsed with copious water, placed in plastic trays and dried 5 d at room temperature in the shade (26±1 °C). The dried seeds were placed in paper envelopes at the same temperature and stored until used.
Thumbnail
Table 1
Opuntia species and variants evaluates in the study.
Opuntia species and variants evaluates in the study.
Seed selection
A visual classification of the seeds of the nine variants allowed to identify groups with small, intermediate and large size, abortive, malformed, light brown color and those that float when placed in water (Cerezal and Duarte, 2004). For this study, normal seeds, those appearing fully developed, without or apparent damage or defects, which would not float in water, and of the relatively uniform size, of each species were selected (López-Palacios et al., 2015).
-
Cerezal and Duarte, 2004Influencia sensorial de aditivos químicos en tunas (Opuntia ficus-indica (L.) Miller) peladas en almíbar conservada por métodos combinadosJ. Prof. Assoc. Cactus Dev, 2004
-
López-Palacios et al., 2015Inter- and intra-specific variation in fruit biomass, number of seeds, and physical characteristics of seeds in Opuntia spp., CactaceaeGenet. Resour. Crop Ev, 2015
Seed biomass and moisture content
The average biomass of the seeds of the nine accessions was determined on 100 seeds and each seed was weighed on an analytical balance (Scientech SA120, USA, 0.0001 g precision).
Seed moisture was individually determined in 25 seeds of each accession. The seeds were dried 4 d in an oven (Riossa digital E-71, México) at constant temperature (70±1 °C), in the dark (Roberts et al., 1988). Initial and final weights were recorded on an analytical balance (Scientech SA120, USA; with an accurately of ±0.0001 g) and seed moisture was expressed as a percentage of weight loss relative to the initial weight (International Seed Testing Association, 2010).
-
Roberts et al., 1988Medición de la biomasa vegetal y de la producción primaria netaTécnicas en Fotosíntesis y Bioproductividad, 1988
-
International Seed Testing Association, 2010International Rules for Seed Testing, 2010
Imbibition
Seeds of the nine variants from the four species of Opuntia were placed in Petri dishes, on filter papers moistened with distilled water with a cotton ball soaked as support, and then kept in a controlled environment chamber (Seedburo ATTGPT-B, USA) at constant temperature (30±1 °C) and in the dark. Three repetitions of each variant were evaluated, with 10 seeds each (n=30). Initial weight was recorded every 4 h for 48 h, a final record was made after 72 h of the onset. Imbibition was expressed as the increase percentage in seed weight, by water absorption, relative to the initial seed weight.
Seed viability and vigor
The seeds viability and vigor were quantified with the tetrazolium test described by the International Seed Testing Association (2010) and Maldonado-Peralta et al. (2016), in five randomized variants of the nine included in the study which comprised at least one of each species (G, Z1, EM, EM1 and EM2; Table 1). Five repetitions with 10 seeds per variant were kept in Petri dishes with a soaked cotton ball as a humidity support and a completely moistened filter paper, for 18 d, at 30±1 °C in a dark controlled environment chamber (Seedburo ATTGPT-B, USA). Then the seed coat was removed (with pliers), and the embryos were extracted. These were placed in Petri dishes, water was then replaced by a 1% tetrazolium (2, 3, 5 triphenyltetrazolium chloride) water solution (w:v) (Altare et al., 2006) and maintained for 24 h in the same conditions within the chamber. The embryos were then rinsed with distilled water and observed using a stereo microscope (Leica Microsystems EZ4, Switzerland).
-
International Seed Testing Association (2010International Rules for Seed Testing, 2010
-
Maldonado-Peralta et al. (2016Seed viability and vigour of two nanche species (Malpighia mexicana and Byrsonima crassifolia)Seed Sci. Technol, 2016
-
Altare et al., 2006Stimulation and promotion of germination in Opuntia ficus-indica seedsJ. Prof. Assoc. Cactus Dev, 2006
The embryos were classified according to their coloring (International Seed Testing Association, 2010; Maldonado-Peralta et al., 2016), in 1) live with high vigor, when they were totally and fully intense red stained, 2) live with low vigor, when their coloration was pale red or with discolored sections and 3) nonviable, when remained colorless (the printed version shows gray shades and the electronic version shows the original embryos color). Viability was expressed as a percentage of live embryos of all evaluations for each accession. The sum of class 1 and 2 represented the proportion of viable embryos, class 1 and 2 represented the embryos with high and low vigor. These values were expressed as a percentage.
-
International Seed Testing Association, 2010International Rules for Seed Testing, 2010
-
Maldonado-Peralta et al., 2016Seed viability and vigour of two nanche species (Malpighia mexicana and Byrsonima crassifolia)Seed Sci. Technol, 2016
Experimental design and statistical analysis
The experimental design to assess seed imbibition dynamics was completely randomized, with four treatments or species, nine samples or variants, and 30 repetitions per variant, each represented by a single seed. The experimental design to quantify seed viability and vigor was completely randomized with five treatments (five variants) and five replicates of 10 embryos each.
The results were analyzed with ANOVA, Tukey multiple means comparisons test (p≤0.05) and Pearson correlation.
Results and Discussion
Biomass and seed moisture
Seed biomass showed significant differences (p≤0.05) among the nine variants. Among nine variants the seeds of O. ficus-indica cv. Atlixco (EM1) were the least heavy and on average accounted for 39.37% of the heaviest, which were Amarilla Olorosa of O. hyptiacantha (Z2). The seeds of the other variants showed intermediate values between these extremes. Differences in seminal biomass were also significant (p≤0.05) within and between species. Wild species O. streptacantha had the smallest difference (9.4%) among its variants and O. ficus-indica, the species with the highest domestication degree, the contrast was higher (p≤0.05); in the latter the seed biomass of the Atlixco variant (EM1) represented 48% of the correspondent Copena V1 (EM2) (Figure 1).
Thumbnail
Figure 1
Seed biomass (±e.e.) of nine variants of four species of Opuntia collected at Estado de México (EM), Guanajuato (G) and Zacatecas (Z), México (n=50).
Seed biomass (±e.e.) of nine variants of four species of Opuntia collected at Estado de México (EM), Guanajuato (G) and Zacatecas (Z), México (n=50).
Lopez-Palacios et al. (2015) quantified and physically characterized the seeds of 89 variants of five Opuntia species. They observed a seed biomass gradient with the degree of domestication: seeds of O. streptacantha were small and its mean biomass was the lowest (13.11 mg) on the gradient. Among the heaviest were those of O. ficus-indica (15.91 mg). Our results are different because they showed no direct relationship of seeds biomass with the domestication degree of the species, but the mean seed biomass was in the range documented by those authors. It is likely that the biomass variability or size of Opuntia seeds is a characteristic related to the species level of domestication or variant (López-Palacios et al., 2015), also depends on consistent vegetative multiplication, which is common and successful in the plants of this genus, the selection processes for the multiplication of vegetative (cladodes) or reproductive (fruit) structures relevant to humans, and the interaction of these factors. But with the current information, it is not possible to identify which factors have major effects on the seeds of the variants in the domestication gradient because both, sexual reproduction and asexual propagation seem to have contributed to the ecological and evolutionary success of the genus. The vegetative propagation seems to be more efficient than the sexual reproduction in plantations and in wild populations. The reproduction, mainly vegetative, of the species of the Opuntia genus is well documented; and O. ficus-indica, the species with the highest degree of domestication, reproduces sexually but propagates vegetatively, for which multiplication by cladodes is the most commonly used cropping technique (Barbera et al., 1994; Reyes-Agüero et al., 2005).
-
Lopez-Palacios et al. (2015Inter- and intra-specific variation in fruit biomass, number of seeds, and physical characteristics of seeds in Opuntia spp., CactaceaeGenet. Resour. Crop Ev, 2015
-
López-Palacios et al., 2015Inter- and intra-specific variation in fruit biomass, number of seeds, and physical characteristics of seeds in Opuntia spp., CactaceaeGenet. Resour. Crop Ev, 2015
-
Barbera et al., 1994Seed content and fruit characteristics in cactus pear (Opuntia ficus-indica Mill.)Sci. Hortic, 1994
-
Reyes-Agüero et al., 2005Variación morfológica de Opuntia (Cactaceae) en relación con su domesticación en la Altiplanicie Meridional de MéxicoInterciencia, 2005
Multiplication for the production of vegetative structures prevents the fruit and seed production, as young cladodes or “nopales” are harvested before reaching their reproductive stages. Vegetative propagation replaces sexual reproduction, at least partially, and seems essential because of the Opuntia seeds dormancy, independent of the species (Barbera et al., 1994; Reyes-Agüero et al., 2005; López-Palacios et al., 2015). In addition, a species variants may have normal seeds with uniform width and thickness, as in O. megacantha and O. ficus-indica, or heterogeneous as in O. streptacantha and O. hyptiacantha, and these trends influence the individual seed weight, regardless of their proportion in the fruit (López-Palacios et al., 2015). These variations in seed biomass between species and variants, besides those already discussed, may also be due to different climatic conditions at the growth sites of the plants from which the seeds were obtained, as well as the maturation time of the seed after their harvest.
-
Barbera et al., 1994Seed content and fruit characteristics in cactus pear (Opuntia ficus-indica Mill.)Sci. Hortic, 1994
-
Reyes-Agüero et al., 2005Variación morfológica de Opuntia (Cactaceae) en relación con su domesticación en la Altiplanicie Meridional de MéxicoInterciencia, 2005
-
López-Palacios et al., 2015Inter- and intra-specific variation in fruit biomass, number of seeds, and physical characteristics of seeds in Opuntia spp., CactaceaeGenet. Resour. Crop Ev, 2015
-
López-Palacios et al., 2015Inter- and intra-specific variation in fruit biomass, number of seeds, and physical characteristics of seeds in Opuntia spp., CactaceaeGenet. Resour. Crop Ev, 2015
The moisture content of the seeds showed significant differences (p≤0.05) between the variants. In six of the nine variants, including O. streptacantha, O. hyptiacantha, O. megacantha EM and O. ficus-indica Red Vigor (Z), seeds averaged 4.97% moisture and two variants of O. ficus-indica averaged the highest humidity (6.94%) of the group (Figure 2). According to the Pearson correlation analysis, the moisture content variants was not significantly associated with the seminal biomass (r: 0.064-0.285; p>0.224). The slightly higher humidity of the O. ficus-indica variants may result from less storage time because at least one of them was the last to be harvested (Table 1).
Thumbnail
Figure 2
Seed humidity (±e.e.) of nine variants of four species of Opuntia collected at Estado de México (EM), Guanajuato (G) and Zacatecas (Z), México (n=50).
Seed humidity (±e.e.) of nine variants of four species of Opuntia collected at Estado de México (EM), Guanajuato (G) and Zacatecas (Z), México (n=50).
Imbibition
The time-dependent seed imbibition showed differences and similarities between the variants and within and between species. The main similarity was the accelerated water imbibition by the seeds of the nine variants in the first 8 h, although O. megacantha EM and O. ficus-indica EM2 (Copena V1) extended that for another 4 or 8 h. Furthermore, after this accelerated phase the average imbibition remained constant over the next 50-68 h in eight of the nine variants. The exception was O. ficus-indica EM1 that continued to gain moisture, although less accelerated than in the first 4 h. The mean maximum seed imbibition of seven of the variants (both O. streptacantha and O. hyptiacantha, O. megacantha Z and O. ficus-indica EM2 and Z) was 29% (p>0.05) of moisture its initial biomass (Figure 3 A-D). In contrast, the seeds of the other two variants (O. megacantha EM and O. ficus-indica EM1) showed mean maximum imbibing of 60% (Figure 3 C-D).
Thumbnail
Figure 3
Cumulative water imbibition (±e.e.) by seeds of nine variants of four Opuntia species. Variants of (A) O. streptacantha Guanajuato (○) and Zacatecas (●), (B) O. hyptiacantha Zacatecas (▫ and ▪) (C) O. megacantha Estado de México (∆) and Zacatecas (▲) and (D) O. ficus-indica del Estado de (◊ and ⸎) and Zacatecas(♦).
Cumulative water imbibition (±e.e.) by seeds of nine variants of four Opuntia species. Variants of (A) O. streptacantha Guanajuato (○) and Zacatecas (●), (B) O. hyptiacantha Zacatecas (▫ and ▪) (C) O. megacantha Estado de México (∆) and Zacatecas (▲) and (D) O. ficus-indica del Estado de (◊ and ⸎) and Zacatecas(♦).
These results agree with those obtained by Monroy V.et al. (2016) in a seed germination study on O. streptacantha G, O. megacantha EM and O. ficus-indica EM1 and EM2. According to them, after 48 h the wild seeds imbibed the smallest amount (p≤0.05) of water (23%), those with intermediate degree of domestication (O. megacantha) embedded 28%, and the seeds of O. ficus-indica, with the highest degree of domestication, imbibed 31%. However, 160 h later, on average, the seeds of the three species had imbibed a similar amount of water (35%; p>0.05); at that time, 1% and 10% of the seeds of O. ficus-indica and O. streptacantha germinated; but, the germinated seeds had slightly higher humidity than the mean (47 and 55%).
-
Monroy V.et al. (2016Chemical scarification and ozone in seed dormancy alleviation of wild and domesticated Opuntia, CactaceaeOzone-Sci. Eng, 2016
Thus, in the assessment conditions, the seeds of the nine variants of O. streptacantha, O. hyptiacantha, O. megacantha and O. ficus-indica, imbibed quickly independently of the harvest year and obtained the highest weight during the first hours of imbibition. This coincided with the described by Orozco-Segovia et al. (2007), who report that seeds of O. tomentosa have the capacity to imbibe since their seed coat lacks layers of macroesclereides that could give impermeability to the seed coat. Besides, they document the formation of a germination valve and a water channel in the hilum-micropilar region during desiccation and seed storage, and they proposed that there is a low probability of physical latency due to the impermeability of the seed coat in the genus Opuntia. But the characteristics of various species of the genus Opuntia and their positive reaction to methods that eliminate dormancy are consistent with the FAO (1991) classification of seeds with combined or simultaneous dormancy of the seed coat and embryo.
-
Orozco-Segovia et al. (2007Seed anatomy and water uptake in relation to seed dormancy in Opuntia tomentosa (Cactaceae, Opuntioideae)Ann. Bot, 2007
The Pearson correlation analysis showed that the maximum seed imbibing was significantly associated with its initial moisture in any species. Furthermore, the maximum imbibition was not significantly related to the seed biomass of the wild species O. streptacantha. In contrast, in O. hyptiacantha which is near the wild species, O. megacantha which is fairly domesticated, and O. ficus-indica with the highest degree of domestication, the maximum imbibition was directly and significantly related to the biomass seeds (Table 2).
Thumbnail
Table 2
Pearson correlation coefficient, and probability, of mean seed weight and maximum seed imbibition of four species of Opuntia.
Pearson correlation coefficient, and probability, of mean seed weight and maximum seed imbibition of four species of Opuntia.
These results indicate that seed imbibition of Opuntia is modified by domestication (Table 2). Other seed characteristics in this genus that are proposed as changes promoted by domestication are width, length and hardness, which are lower in O. streptacantha respect to O. ficus-indica; moreover, these changes showed a direct gradient with the degree of domestication (López-Palacios et al., 2015).
-
López-Palacios et al., 2015Inter- and intra-specific variation in fruit biomass, number of seeds, and physical characteristics of seeds in Opuntia spp., CactaceaeGenet. Resour. Crop Ev, 2015
Viability and vigor
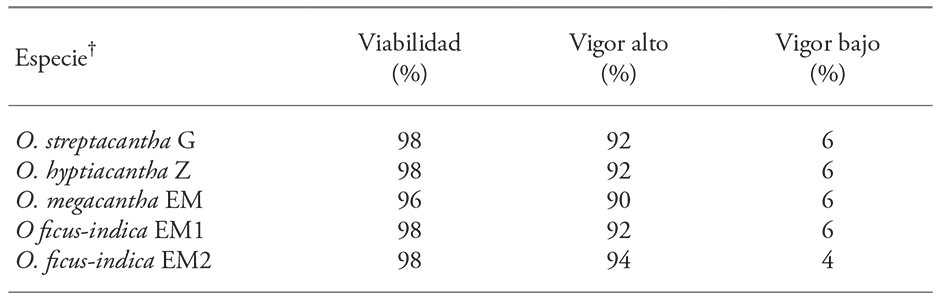
The tetrazolium test showed that the seeds of the four species, regardless of their harvest year, had a mean viability of 97.6% and had no significant differences between them (p>0.05) (Table 3). The vigor of the embryos, according to the pattern and staining level (Figure 4) was not different (p>0.05) between species and the mean high and low vigor was of 92 and 5.6% (Table 3).
Thumbnail
Table 3
Embryo viability and vigor of four Opuntia species.
Embryo viability and vigor of four Opuntia species.
Thumbnail
Figure 4
Typical colorations and no color change in Opuntia embryos, according to their viability and vigor, tetrazolium treated. This example corresponds to O. hyptiacantha variant Amarillo Olorosa embryos (A) viable with high vigor, (B) probably viable with low vigor and (C) not viable; mean lengths 3.75 mm (coefficient of variation: 9.30).
Typical colorations and no color change in Opuntia embryos, according to their viability and vigor, tetrazolium treated. This example corresponds to O. hyptiacantha variant Amarillo Olorosa embryos (A) viable with high vigor, (B) probably viable with low vigor and (C) not viable; mean lengths 3.75 mm (coefficient of variation: 9.30).
The tetrazolium (2,3,5,-triphenyl-tetrazolium chloride) test is used to assess seeds viability based on tissue respiratory activity (in the mitochondria); specifically, in the electron chain activity. The biochemical reaction of that test is a chemical reduction of the compound, in which living cells take the hydrogen released by dehydrogenase enzymes to form a red, stable and no diffusible compound, derivative of the 2,3,5,-triphenyl-tetrazolium chloride (colorless), known as triphenyl-formazan (Victoria et al., 2006). Thus, the intense red staining indicates the presence of living cells of the embryo (Figure 4A); the activity of the enzymatic systems decreases with seed deterioration, so that a pink or pale pink coloration indicates low viability (Figure 4B), and no staining (persistence of the original color) indicates that the embryonic tissue is dead (Figure 4C). Therefore, the proportion of stained areas is taken as a measure of seed vigor (Moreno M., 1996; International Seed Testing Association, 2010). Topological staining patterns in key regions such as the radicle, plumule, embryonic axis and cotyledons are related to a low or high probability of germination, as documented in embryos of species with commercial importance, Calendula officinalis (calendula) and Anethum graveolens (dill) (Victoria et al., 2006). These patterns can be established in cultivated species on a smaller scale, whose importance is recognized since pre-Hispanic times, as Malpighia mexicana and Byrsonima crassifolia (nanche) (Maldonado-Peralta et al., 2016). According to the above, the results of our study are reliable evidence that the seeds of the four species of Opuntia that rapidly imbibed contained viable and vigorous embryos, independent of their variant, species and harvesting year (Table 3).
-
Victoria et al., 2006Viabilidad en tetrazolio de semillas de caléndula y eneldoActa Agron, 2006
-
Moreno M., 1996Análisis Físico y Biológico de Semillas Agrícolas, 1996
-
International Seed Testing Association, 2010International Rules for Seed Testing, 2010
-
Victoria et al., 2006Viabilidad en tetrazolio de semillas de caléndula y eneldoActa Agron, 2006
-
Maldonado-Peralta et al., 2016Seed viability and vigour of two nanche species (Malpighia mexicana and Byrsonima crassifolia)Seed Sci. Technol, 2016
The information on the viability and vigor of the Opuntia genus is scarce. Out of the 188 species that comprises it (Anderson, 2001) only in few it has been documented, and their data cannot be generalized. But, the results of viability of our study were similar to those of O. maxima and O. stricta (97%) (Gimeno and Vilà, 2002) and O. ficus-indica seeds (100%) (Altare et al., 2006).
-
Anderson, 2001The Cactus Family, 2001
-
Gimeno and Vilà, 2002Recruitment of two Opuntia species invading abandoned olive grovesActa Oecol, 2002
-
Altare et al., 2006Stimulation and promotion of germination in Opuntia ficus-indica seedsJ. Prof. Assoc. Cactus Dev, 2006
The results in our study also agreed with those obtained by Monroy V.et al. (2016), who evaluated the effect of treatments on seeds of three Opuntia species, which, on average showed 97.5% viability. In spite of this viability percentage, seeds of O. streptacantha, O. megacantha and O. ficus-indica, under various scarification conditions, temperature, photoperiod and ozone presence (as an oxidizing agent), showed 3-76%, 0-38% and 0-48% germination. In our study, although it was not part of the objective, we considered appropriate to supplement the information on seed germination. After evaluating imbibition, it was observed that in 50 d, 23 and 33% of the O. streptacantha G and Z seeds germinated, along with 6.7% of O. hyptiacantha, but no seeds of the other six variants germinated during that time. These results showed a germination gradient related to that of domestication, in which a proportion of the seeds of wild and nearly wild species broke dormancy after a month and a half, in contrast to those recognized as with low and high domestication degree that did not show a break of their dormancy.
-
Monroy V.et al. (2016Chemical scarification and ozone in seed dormancy alleviation of wild and domesticated Opuntia, CactaceaeOzone-Sci. Eng, 2016
The intensity of embryo staining (viability), its relationship with the vigor and the high proportion of both values indicated that the tetrazolium test can be conclusive to evaluate these characteristics in Opuntia seeds. Other methods to assess vigor expression, such as deep planting in compact soil or limited soil moisture, and others (Copeland and McDonald, 2001), combined with the quantification of the emergency delay time, length of the stem and root, the accumulation of fresh and dry stem and root biomass can provide information on the ability of the seedling for its establishment (International Seed Testing Association, 2010). These methods are applied to seeds of wild and domesticated species in which scarification assures germination and seedling emergence (Celis-Velázquez et al., 2008a, 2008b; Celis-Velázquez et al., 2010). This is not the case for Opuntia seeds, as their dormancy and hardness of their seed coat (Orozco-Segovia et al., 2007) prevent the embryo to easily emerge. Therefore, the tetrazolium test seems to be the most appropriate method to assess the viability and vigor of Opuntia embryos.
-
Copeland and McDonald, 2001Principles of Seed Science Technology, 2001
-
International Seed Testing Association, 2010International Rules for Seed Testing, 2010
-
Celis-Velázquez et al., 2008aConsumo de reservas de la semilla de frijol para la emergencia y desarrollo inicial en diferentes profundidades de siembraAgron. Mesoam, 2008
-
2008bVariabilidad morfológica seminal y del vigor inicial de germoplasma mejorado de frijolAgron. Mesoam, 2008
-
Celis-Velázquez et al., 2010Caracterización morfológíca de las semillas y consumo de reservas durante la emergencia de plántulas de frijol (Phaseolus vulgaris L.) silvestre y domesticadoRev. Fac. Agron. (LUZ), 2010
-
Orozco-Segovia et al., 2007Seed anatomy and water uptake in relation to seed dormancy in Opuntia tomentosa (Cactaceae, Opuntioideae)Ann. Bot, 2007
The results of our study confirmed that the seeds of the four tested species were viable, vigorous and could imbibe moisture; these are all necessary elements for seed germination and the establishment of their seedlings. Difficulties for the seed germination of Opuntia were documented by Pilcher (1970) and Baskin and Baskin (1977), and they were attributed to seed dormancy (Rojas-Aréchiga and Vázquez-Yanes, 2000) and their seed coat hardness (Sánchez-Venegas, 1997; Mandujano et al., 2005; Olvera-Carrillo et al., 2009; Romo-Campos et al., 2010).
-
Pilcher (1970Germination of seeds of four species of OpuntiaCact. Suc. J. Am, 1970
-
Baskin and Baskin (1977Seed and seedling ecology of Opuntia compressa in Tennessee cedar gladesJ. Tennessee Acad. Sci, 1977
-
Rojas-Aréchiga and Vázquez-Yanes, 2000Cactus seed germination: a reviewJ. Arid Environ, 2000
-
Sánchez-Venegas, 1997Germinación, viabilidad y características distintivas de la semilla de Opuntia joconostle Weber, forma CuaresmeñoCact. Suc. Mex, 1997
-
Mandujano et al., 2005Breaking seed dormancy in Opuntia rastrera from the Chihuahuan desertJ. Arid Environ, 2005
-
Olvera-Carrillo et al., 2009Effect of burial on the germination of Opuntia tomentosa’s (Cactaceae, Opuntioideae) seedsJ. Arid Environ, 2009
-
Romo-Campos et al., 2010Seed germination of Opuntia species from an aridity gradient in Central MexicoJ. Prof. Assoc. Cactus Dev, 2010
Conclusions
The imbibition capacity of Opuntia seeds is modified by domestication. Viability and vigor of Opuntia embryos are significantly high and independent of the species
Agradecimientos
Los autores agradecen la donación de las semillas de cuatro variantes al Banco de Germoplasma del Centro Regional Universitario Centro-Norte, de la Universidad Autónoma Chapingo (CRUCEN-UACh).
Literatura Citada
- Aguilar-Estrada, A, J. A. Reyes-Agüero, y J. R. Aguirre R. 2003. Caracterización de la semilla de 403 variantes de nopal (Opuntia spp.). In: Esparza, F. G., L. M. A. Salas, C. J. Mena, y R. D. Valdez Z. (eds). Memoria del IX Congreso Nacional y VII Internacional sobre Conocimiento y Aprovechamiento del Nopal. Gobierno del Estado de Zacatecas, México, pp: 117-120. Links
- Altare, M., S. Trione, J. C. Guevara, and M. Cony. 2006. Stimulation and promotion of germination in Opuntia ficus-indica seeds. J. Prof. Assoc. Cactus Dev. 8: 91-100. Links
- Anderson, E. F. 2001. The Cactus Family. Timber, Portland, Or., USA. 776 p. Links
- Azcón-Bieto, J., y M. Talón. 2000. Fundamentos de Fisiología Vegetal. Mc Graw Hill Interamericana. Madrid, España. 522 p. Links
- Barbera, G., P. Inglese, and T. La-Mantia. 1994. Seed content and fruit characteristics in cactus pear (Opuntia ficus-indica Mill.). Sci. Hortic. Amsterdam 58: 161-165. Links
- Baskin, J. M., and C. C. Baskin. 1977. Seed and seedling ecology of Opuntia compressa in Tennessee cedar glades. J. Tennessee Acad. Sci. 52: 118-122. Links
- Bravo-Hollis, H., y H. Sánchez-Mejorada. 1991. Las Cactáceas de México. Vol. 3. Universidad Nacional Autónoma de México. D.F. México. 643 p. Links
- Callen, E. O. 1966. Analysis of the Tehuacan coprolites. In: Byers, D. S. (ed). The Prehistory in Tehuacan Valley. I. Environment and Subsistence. University of Texas, Austin. pp: 261-289. Links
- Celis-Velázquez, R., C. B. Peña-Valdivia, C. Trejo-López, J. R. Aguirre, L. Córdoba-Téllez, y A. Carballo-Carballo. 2008a. Consumo de reservas de la semilla de frijol para la emergencia y desarrollo inicial en diferentes profundidades de siembra. Agron. Mesoam. 19: 167-177. Links
- Celis-Velázquez, R ., C. B. Peña-Valdivia, M. Luna C., J. R. Aguirre R., A. Carballo, and C. Trejo. 2008b. Variabilidad morfológica seminal y del vigor inicial de germoplasma mejorado de frijol. Agron. Mesoam . 19: 179-193. Links
- Celis-Velázquez, R ., C. B. Peña-Valdivia, M. Luna C., and J. R. Aguirre R. 2010. Caracterización morfológíca de las semillas y consumo de reservas durante la emergencia de plántulas de frijol (Phaseolus vulgaris L.) silvestre y domesticado. Rev. Fac. Agron. (LUZ) 27: 61-87. Links
- Cerezal, P., y G. Duarte. 2004. Influencia sensorial de aditivos químicos en tunas (Opuntia ficus-indica (L.) Miller) peladas en almíbar conservada por métodos combinados. J. Prof. Assoc. Cactus Dev . 6: 102-119. Links
- Colunga-García, M. P., X. E. Hernández, y M. A. Castillo. 1986 Variación morfológica, manejo agrícola tradicional y grado de domesticación de Opuntia spp. en el Bajío Guanajuatense. Agrociencia 65: 7-49. Links
- Copeland, L. O., and M. B. McDonald. 2001. Principles of Seed Science Technology. 4 ed. Burguess Publishing Company. Mineapolis, Minnesota, USA. pp: 121-144. Links
- Delgado-Sánchez P., J. F. Jiménez-Bremont, M. L. Guerrero-González, and J. Flores. 2013. Effect of fungi and light on seed germination of three Opuntia species from semiarid lands of central Mexico. J. Plant Res. 126: 643-649. Links
- García, E. 2004. Modificaciones al Sistema de Clasificación Climática de Köppen. 4ª ed. Universidad Nacional Autónoma de México. México. 220 p. Links
- García-Nava, F., C. B. Peña-Valdivia, C. Trejo, R. García N., J. A. Reyes A., and J. R. Aguirre R. 2015. Biophysical and physiological characteristics of nopalitos (Opuntia spp. Cactaceae) as influenced by domestication. Genet. Resour. Crop Ev. 62: 927-938. Links
- Gimeno, I., and M. Vilà. 2002. Recruitment of two Opuntia species invading abandoned olive groves. Acta Oecol. 23: 239-246. Links
- Hamilton, M. 1970. Seedling development of Opuntia bradtiana (Cactaceae). Am. J. Bot. 57: 599-603. Links
- International Seed Testing Association. 2010. International Rules for Seed Testing. Basserdorf, CH Switzerland. 300 p. Links
- Lenzi, M, and A. I. Orth. 2012. Mixed reproduction systems in Opuntia monacantha (Cactaceae) in Southern Brazil. Braz. J. Bot. 35: 49-58. Links
- López-Palacios, C., C. B. Peña-Valdivia, J. A. Reyes-Agüero, and A. I. Rodríguez-Hernández. 2012. Effects of domestication on structural polysaccharides and dietary fiber in nopalitos (Opuntia spp.). Genet. Resour. Crop Ev . 59: 1015-1026. Links
- López-Palacios, C ., C. B. Peña-Valdivia, J. A. Reyes-Agüero, J. R. Aguirre-Rivera, H. M. Ramírez-Tobías, R. M. Soto-Hernández, and J. F. Jiménez-Bremont. 2015. Inter- and intra-specific variation in fruit biomass, number of seeds, and physical characteristics of seeds in Opuntia spp., Cactaceae. Genet. Resour. Crop Ev . 62: 1205-1223. Links
- Maldonado-Peralta, M. A. et al. 2016. Seed viability and vigour of two nanche species (Malpighia mexicana and Byrsonima crassifolia). Seed Sci. Technol. 44: 1-9. Links
- Mandujano, M. C., C. Montaña, and L. E. Eguiarte. 1996. Reproductive ecology and inbreeding depression in Opuntia rastrera (Cactaceae) in the Chihuahuan Desert: Why are sexually derived recruitments so rare? Am. J. Bot . 83: 63-70. Links
- Mandujano, M. C ., C. Montaña, and M. Rojas-Aréchiga. 2005. Breaking seed dormancy in Opuntia rastrera from the Chihuahuan desert. J. Arid Environ. 62: 15-21. Links
- Mandujano, M. C ., J. Golubov, and L. F. Huenneke. 2007. Effect of reproductive modes and environmental heterogeneity in the population dynamics of a geographically widespread clonal desert cactus. Popul. Ecol. 49: 141-153. Links
- Monroy V. et al. 2016. Chemical scarification and ozone in seed dormancy alleviation of wild and domesticated Opuntia, Cactaceae. Ozone-Sci. Eng. http://dx.doi.org/10.1080/01919512.2016.1261010. Links
- Moreno M., E. 1996. Análisis Físico y Biológico de Semillas Agrícolas. Universidad Nacional Autónoma de México. México DF. México. 393 p. Links
- Olvera-Carrillo, Y. et al. 2009. Effect of burial on the germination of Opuntia tomentosa’s (Cactaceae, Opuntioideae) seeds. J. Arid Environ. 73: 421-427. Links
- Orozco-Segovia, A. et al. 2007. Seed anatomy and water uptake in relation to seed dormancy in Opuntia tomentosa (Cactaceae, Opuntioideae). Ann. Bot. 99: 581-592. Links
- Pilcher, L. 1970. Germination of seeds of four species of Opuntia. Cact. Suc. J. Am. 42: 281-282. Links
- Reyes-Agüero J. A., J. R. Aguirre-Rivera, and J. A. Flores-Flores. 2005. Variación morfológica de Opuntia (Cactaceae) en relación con su domesticación en la Altiplanicie Meridional de México. Interciencia 30: 476-484. Links
- Roberts, M. J., S. P. Long, L. Tieszen L., y C. L. Beadle. 1988. Medición de la biomasa vegetal y de la producción primaria neta. In: Coombs, J., D. O. Hall, S. Long, y M. O. Scurlock. Técnicas en Fotosíntesis y Bioproductividad. Futura S. A. México. pp: 1-16. Links
- Rojas-Aréchiga, M., and C. Vázquez-Yanes. 2000. Cactus seed germination: a review. J. Arid Environ . 44: 85-104. Links
- Rojas-Aréchiga, M ., K. Aguilar, J. Gobulov, and M. Mandujano. 2011. Effect of gibberellic acid on germination of seeds of five species of cacti from the Chihuahuan Desert, Northern Mexico. Southwest. Nat. 56: 393-435. Links
- Romo-Campos, L., J. L. Flores-Flores, J. Flores, and G. Álvarez-Fuentes. 2010. Seed germination of Opuntia species from an aridity gradient in Central Mexico. J. Prof. Assoc. Cactus Dev . 12: 181-198. Links
- Sáenz, C. et al. 2006. Utilización agroindustrial del nopal. Boletín de Servicios Agrícolas de la FAO 162. Roma. 165 p. Links
- Sánchez-Venegas, G. 1997. Germinación, viabilidad y características distintivas de la semilla de Opuntia joconostle Weber, forma Cuaresmeño. Cact. Suc. Mex. 42: 16-21. Links
- Suárez, D., y L. M. Melgarejo . 2010. Biología y germinación de semillas. In: Melgarejo, L. M. (ed). Experimentos en Fisiología Vegetal. Universidad Nacional de Colombia. Bogotá, Colombia. pp: 13-24. Links
- Victoria T., J. A., C. R. Bonilla C., y M. S. Sánchez O. 2006. Viabilidad en tetrazolio de semillas de caléndula y eneldo. Acta Agron. 55: 1-15. Links