Introduction
There is well-documented evidence for atherosclerosis as a systemic disorder1-12. Postmortem studies have shown a strong correlation between carotid and coronary artery disease (CAD)1-3. In the same line, carotid intima-media thickness (CIMT) and/or the presence of plaque carotid plaque (CP) have been identified as independent predictors of myocardial infarction (MI), stroke and death in asymptomatic and presumably healthy patients4-12 improving the ability of risk functions to predict cardiovascular events13.
Conversely to people without previous cardiovascular disease, there are few studies correlating carotid disease and new cardiovascular events in patients with ischemic heart disease, some of them showing contradictory results14-20.
The aim of our study is to ascertain whether the carotid disease is a predictor of major adverse cardiac and cerebrovascular events (MACCE) in patients undergoing a coronary angiography regarding previous treadmill exercise stress echocardiography results.
Methods
Study population
This is a single-center retrospective study of patients submitted to coronary angiography after treadmill exercise stress echocardiography and carotid ultrasound were performed. From January 1, 2002, to December 31, 2013, 390 consecutive Caucasian patients older than 18 years with suspected CAD underwent coronary angiography in multiple projections using standard technique regarding exercise echocardiography results and physicians in charge of the patient criteria, mainly due to the persistence of symptoms. All patients signed an informed consent before testing. The study was approved by the Regional Ethics Committee.
Demographic, clinical, rest and exercise echocardiography, carotid, angiography, and treatment data were collected. Prior CAD was defined as previous MI21, coronary revascularization, or angiographic documentation of any coronary stenosis ≥50%. Prior vascular disease was defined as prior CAD, stroke, transient ischemic attack or peripheral artery disease.
Rest and treadmill exercise stress echocardiography
Rest and exercise echocardiography was carried out according to the current European and American guidelines at the time of their performance22-24.
Carotid ultrasonography
Carotid scans were performed immediately after stress test with the same exercise echocardiography ultrasound equipment using a high-resolution, non-harmonic B-mode ultrasound system (Philips Sonos 5500 between 2002 and 2005 and Philips iE33 after 2005; Philips Medical Systems) with a linear array (3-11 MHz) transducer. CP was defined as a focal structure encroaching into the arterial lumen by > 0.5 mm, a distinct area of CIMT > 50% than the adjacent wall or CIMT > 1.5 mm25-28. CIMT was assessed as stated by the atherosclerosis risk in communities study6 and current guidelines25-28 using a semiautomated edge detection algorithm (Qlab; Philips 110 Medical Systems, Andover, MA, USA).
CIMT age- and sex-specific percentile values were defined according to previously published data in our country29.
Carotid ultrasonography stored images were retrospectively analyzed by two imaging expert cardiologists blinded to MACCE. In case of disagreement, a third expert was consulted.
Coronary angiography
The significant angiographic disease was defined as stenosis ≥ 50% by visual assessment in any major epicardial arteries or their branches. CAD treatment was recorded as medical, balloon percutaneous coronary intervention (PCI), bare metal and drug-eluting stent PCI or coronary artery bypass grafting (CABG). Complete revascularization was defined as the treatment of any significant CAD in vessels ≥ 1.5 mm as estimated on the diagnostic angiogram during the local Heart Team conference.
Follow-up and end point
Follow-up data were obtained from the hospital database, medical records, and death certificates. In case of doubt, the Regional Mortality Registry was consulted. MACCE was defined as MI due to atherosclerosis progression, stroke and death due to MI, stroke, life-threating arrhythmias, cardiac arrest or unexpected and otherwise-unexplained sudden death. MI was defined as specified by the third universal definition of MI expert consensus document21. Patients with MI due to in-stent restenosis or thrombosis were censored at the time of the event. Stroke was defined as a loss of neurological function caused by an ischemic event, lasting for >24 h or leaving residual signs.
Statistical analysis
Categorical variables were reported as percentages and continuous variables as mean (standard deviation) when they are normally distributed or median (interquartile range) when their distribution departed from normal.
Cumulative events curves were calculated by Kaplan—Meier method and compared by log rank test. Coxs proportional hazards models were performed for univariate and multivariate analysis of the endpoint. In multivariate analysis, backward stepwise selection was used with an entry set at 0.2 significance level and a retention set of 0.1. p < 0.05 was considered statistically significant.
The statistical analysis was carried out with IBM SPSS Statistics for Windows, Version 20.0. (Armonk, NY).
Results
Of all patients, 2 (0.5%) were lost during follow-up. Baseline characteristics of subjects are summarized in tables 1 and 2.
Table 1 Clinical and biochemical baseline characteristics of enrolled patients
| Variable | All patients n=390 (%) |
|---|---|
| Age (years) | 66.0 (10.5) |
| Male sex (%) | 296 (75.9) |
| BMI (Kg/m2) | 28.4 (3.9) |
| Hypertension | 239 (61.3) |
| Hypercholesterolemia | 245 (62.8) |
| Diabetes mellitus | 131 (33.6) |
| Smoking habit | 191 (49.0) |
| Family history of premature CAD | 44 (11.3) |
| Obesity (BMI ≥ 30 Kg/m2) | 116 (29.7) |
| Atrial fibrillation | 37 (9.5) |
| Prior CAD | 205 (52.6) |
| Prior vascular disease | 234 (60.0) |
| Chest pain | 341 (87.4) |
| Fasting plasma glucose (mg/dL) | 114.1 (33.0) |
| GFR (ml/min/1.73 m2) | 77.0 (24.1) |
| Total Cholesterol (mg/dL) | 175.9 (44.5) |
| Low-density lipoprotein (mg/dL) | 104.7 (36.2) |
| High-density lipoprotein (mg/dL) | 41.6 (11.0) |
| Triglycerides | 152.9 (106.9) |
BMI: body mass index, CAD: coronary artery disease, GFR: glomerular filtration rate.
Table 2 Echocardiographic, carotid and angiographic baseline characteristics and medical treatment at discharge
| Variable | All patients n=390 (%) |
|---|---|
| Left ventricular ejection fraction (%) | 60.4 (8.49) |
| Left ventricular ejection fraction < 50% | 42 (10.8) |
| Mitral valve regurgitation | 239 (61.3) |
| Aortic valve stenosis | 20 (5.1) |
| Aortic valve regurgitation | 131 (33.6) |
| Positive stress echocardiography | 283 (72.6) |
| Metabolic equivalents | 7.46 (2.8) |
| Mean CIMT (mm) | 0.88 (0.21) |
| CIMT > 0.9mm | 171 (43.8) |
| Mean CIMT percentile ≥ 75th | 236 (60.5) |
| CP | 273 (70.0) |
| CAD ≥ 50% | 295 (75.6) |
| 1 vessel | 115 (29.5) |
| 2 vessels | 89 (22.8) |
| 3 vessels | 91 (23.4) |
| PCI | 165 (42.3) |
| CABG | 31 (10.5) |
| Incomplete/no revascularization | 191 (49.0) |
| Beta-blockers | 315 (80.8) |
| Calcium channel blockers | 89 (22.8) |
| Nitrates | 132 (33.8) |
| Statins | 349 (89.5) |
| Ezetimibe | 22 (5.6) |
| Antidiabetic drugs | 79 (20.3) |
| Insulin | 35 (9) |
| Antiplatelet drugs | 351 (90.0) |
| Oral anticoagulants drugs | 29 (7.4) |
CABG: coronary artery bypass grafting, CAD: coronary artery disease, CIMT: carotid intima-media thickness, CP: carotid plaque, PCI: percutaneous coronary intervention
Outcomes
During a mean follow up of 6.0 (2.9) years, 60 patients deceased (15.5%). The causes of death were non-cardiovascular events (fundamentally neoplasms) in 28 patients (46.7%), sudden death in 17 subjects (28.3%), MI in 5 patients (8.3%), heart failure in 5 patients (8.3%), arrhythmia in 3 patients (5%), and stroke in 2 patients (3.3%). MI was diagnosed in 42 patients (10.8%), 16 of them related to in-stent restenosis or thrombosis, and 12 patients suffered stroke (3.1%).
In the subgroup of 67 patients without significant CAD and previous vascular disease 29 subjects (43.28%) had CP in carotid ultrasonography. Neither AMI nor deaths were observed, just one stroke was reported in the subgroup of patients with CP.
MACCE was recorded in 52 subjects (13.4%). Mean annual event rate was 2.1%. Kaplan—Meier event-free survival was 96.4% (1.0) at 1 year, 88.72% (1.7) at 5 years, and 81.35% (2.8) at 10 years.
MACCE was higher in CIMT > 0.9 mm group with a mean annual event rate of 1.7% in the CIMT ≤ 0.9 mm compared to 2.7% in the CIMT > 0.9 mm group. Cumulative incidence of MACCE was 1.9%, 7.8%, and 13.3% versus 5.9%, 15.8, and 25.9% (p = 0.023) at 1, 5, and 10 years, respectively. CP presence was also predictor of MACCE with a mean annual event rate of 0.8% and cumulative incidence of 2.6%, 7.1 and 10.2% at 1, 5, and 10 years in the CP absence group and a mean annual event rate of 2.8% and cumulative incidence of MACCE at 1, 5, and 10 years of 4.1%, 13.2%, and 23.2% in the CP presence group (p = 0.013). Fig. 1 represents cumulative incidence of MACCE depending on carotid ultrasound characteristics.
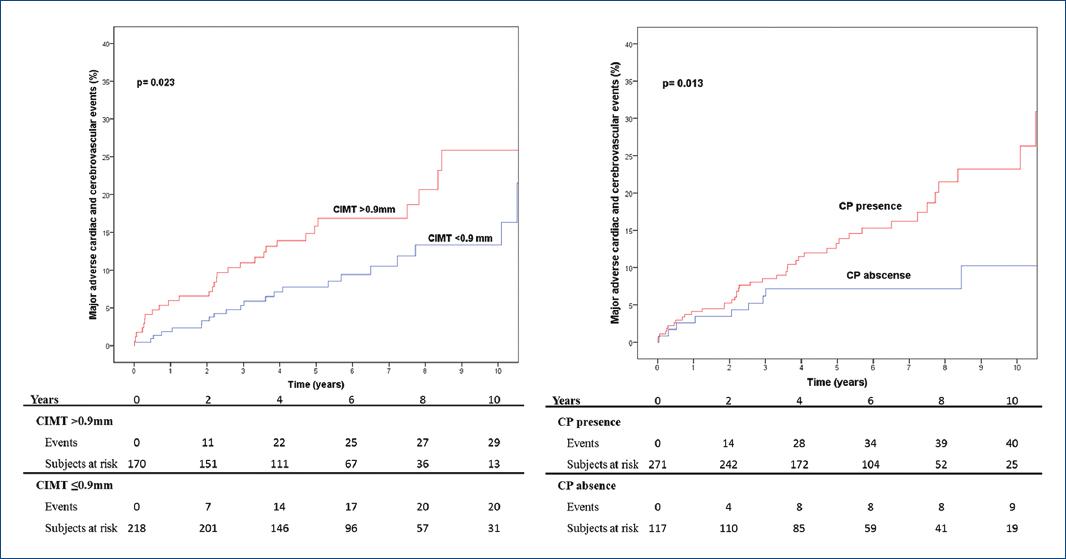
Figure 1 Cumulative incidence of major adverse cardiac and cerebrovascular events depending on carotid ultrasound characteristics (carotid plaque or carotid intima-media thickness > 0.9 mm).
Predictors of outcome
Univariate analysis showed age (Hazard Ratio [HR] 1.03, p = 0.040), diabetes mellitus (HR = 2.32, p = 0.002), smoking habit (HR = 1.87, p = 0.030), glomerular filtration rate (GFR) (HR = 0.98, p = 0.002), baseline left ventricular ejection fraction (HR = 0.96, p = 0.040), presence of aortic stenosis (HR 3.29, p = 0.007), metabolic equivalents in stress test (HR = 0.90, p = 0,046), CP (HR = 2.41, p = 0.017), CIMT > 0.9 mm (HR= 1.87, p = 0.025), number of coronary arteries affected (HR = 1.43, p = 0.006), incomplete revascularization (HR = 2.62, p = 0.001), and nitrates and insulin treatment (HR = 1.73, p = 0.049 and HR = 2.97, p = 0.001 respectively). Multivariate analysis of combined end point is represented in table 3.
Table 3 Multivariate analysis of MACCE
| Variable | HR | 95% CI | p value |
|---|---|---|---|
| Smoking habit | 2.51 | 1.36-4.62 | 0.003 |
| GFR | 0.98 | 0.97-0.99 | 0.043 |
| Aortic stenosis | 2.99 | 1.24-7.21 | 0.014 |
| Incomplete/no revascularization | 1.97 | 1.06-3.67 | 0.033 |
| Nitrates treatment | 1.73 | 0.95-3.18 | 0.075 |
| Insulin treatment | 2.63 | 1.30-5.31 | 0.006 |
| CP | 2.36 | 1.02-5.44 | 0.044 |
CI: confidence interval, HR: hazard ratio, rest of abbreviations as in table 1 and 2, MACCE: major adverse cardiac and cerebrovascular events, GFR: glomerular filtration rate, CP: carotid plaque.
Discussion
The present study shows that CP is an independent predictor of MACCE in patients undergoing coronary angiography.
Held et al. failed to demonstrate a significant association between CIMT or CP and cardiovascular events in patients with stable CAD; however, the diagnosis of angina was clinical14. Petersen et al. identified CP as a predictor of mortality in cardiological patients, but only 64% of them were ischemic and they did not take into account the approach done (invasive or medical treatment)15. Sirimarco et al. also identified CP as an independent predictor of coronary events in patients with atherosclerosis and/or CAD. CAD was defined as stable angina, previous MI or history of unstable angina, PCI or CABG but there was no angiographic assessment of CAD16.
Studies involving patients with CAD assessed by angiography showed consistent results; however, significant CAD and end points definitions were heterogeneous. Komorovsky identified echogenic or calcified CP as a predictor of cardiac death, nonfatal MI or unstable angina in patients with acute coronary syndrome. Both, selection criteria of the subjects and definition of significant CAD (≥ 70% luminal narrowing) were different from our study17. Zielinski et al. and colleagues showed CIMT as a predictor of death and MACCE, defined as death, stroke or MI, in hypertensive patients with CAD defined as ≥ 50% stenosis18. Park found CP as a predictor of cardiac death, stroke or MI in a cohort of patients with significant CAD (defined as stenosis > 50%). Contrary to our study all patients had significant CAD, they did not evaluate the impact of medical intervention and they included stent restenosis and target vessel revascularization in the end point, not only coronary atherosclerosis progression19. Finally, Steinvil et al. identified carotid atherosclerosis as a predictor of all-cause mortality, MI, stroke, and any CAD revascularization in patients with any coronary stenosis > 70% but not in patients without CAD. Similar to the park, medical treatment was not systematically registered, and significant CAD definition was different from ours20.
Pathological studies indicate that CIMT mainly represents medial hypertrophy, whereas CP probably represents a later atherogenesis stage1-3,25-27,30. According to this theory, CP might identify a subgroup of people with more diffuse and greater progression of atherosclerotic disease despite secondary prevention measures.
Besides CP, smoking habit, GFR, aortic stenosis, incomplete or no revascularization, and insulin treatment were MACCE predictors. Insulin treatment could reflect a subgroup of more advanced diabetes mellitus with severe organ damage or metabolic memory phenomenon31,32. It is not surprising to found aortic stenosis as a MACCE predictor. Aortic stenosis and CAD share physiopathological mechanism33 and furthermore, previous studies have shown similar results34. Several studies have correlated chronic kidney disease35,36 and smoking habit36-38 to cardiovascular events and incomplete revascularization to worse prognosis in patients with ischemic heart disease22,39. Although left ventricular ejection fraction has been well recognized as one of the most powerful indicators of adverse prognosis,22,40 we did not find significant association in our study. The reason could be because only 34 (8.7%) patients had mid-range and 8 (2.1%) reduced left ventricular ejection fraction. Finally, patients without CAD and previous vascular disease have an excellent prognosis.
The study has some drawbacks: it is a retrospective single institution study with low recruitment rate and therefore it is hampered by the use of different equipment and methods of image storage and new therapeutic devices or treatments that could have influenced in the final results. Due to the fact that the number of patients without previous vascular disease submitted to treadmill exercise stress echocardiography and coronary angiography yearly is low, one possible solution could be to perform a multicenter prospective study. Second, the CAD stenosis percentage was assessed visually and not using more accurate tools (intravascular ultrasound or optical coherence tomography) or by physiological assessment of CAD stenosis in the cardiac catheterization laboratory (fractional flow reserve). This is also a consequence of a retrospective study design (some techniques were not available at the time of the angiography performance) and reflects the usual clinical practice in catheterization laboratories where intermediate stenosis are treated if there is evidence of ischemia in previously performed stress test and the methods mentioned above are seldom used in case of negative stress test or at the interventional cardiologist criteria in case of no prior stress test available.
Conclusions
CP is an independent predictor of future MACCE in patients undergoing coronary angiography. The subgroup of patients without CAD and previous vascular disease has an excellent prognosis. CP presence could justify a more aggressive therapeutic approach in primary and secondary prevention.











 nueva página del texto (beta)
nueva página del texto (beta)


