Myasthenia gravis is an autoimmune disease that affects the neuromuscular junction and causes generalized skeletal muscle weakness. The catecholaminergic discharges that appear in the context of increased physical or emotional stress generated in a myasthenic crisis can act as triggers of stress cardiomyopathy or takotsubo syndrome.
A 78-year-old woman with a personal history of high blood pressure, diabetes mellitus, and paroxysmal atrial fibrillation. She had been recently diagnosed with myasthenia gravis with positive anti-acetylcholine receptor antibodies. She was on outpatient treatment with pyridostigmine (30 mg every 8 h) and prednisone (25 mg every 24 h).
The patient attended the emergency department with an acute respiratory infection. At admission, clinical examination showed a decrease in strength of facial predominance, together with dysphagia, and progressive partial respiratory failure (baseline arterial blood gas test: pH, 7.43; pO2, 57 mmHg; pCO2, 37 mmHg; and SatO2, 92%). Starting treatment with intravenous immunoglobulin was decided at a dose of 400 mg/kg for 5 days plus respiratory support with non-invasive mechanical ventilation, with good initial evolution.
After 7 days hospital stay, she had a new episode of acute respiratory failure with pulse oximetry desaturation of up to 80% despite high-flow oxygen therapy, which prompted orotracheal intubation and transferred to the Intensive Care Unit (ICU).
During the 1st day of ICU stay, slight crackles were documented in both lung bases, and T-wave inversion in the entire precordial series was observed on the surface electrocardiogram. In addition, myocardial damage markers (high-sensitivity troponin T, 154 ng/dL and creatine kinase, 43 U/L) elevation was detected, together with NT-proBNP increased values to up to 24,980 ng/L. Although the patient experienced no chest pain anytime, an urgent echocardiogram was performed, which revealed slight left ventricular hypertrophy with moderate left ventricular systolic dysfunction (calculated ejection fraction: 35-40%) due to akinesia of all apical and medial segments with basal segments hypercontractility (Fig. 1A). In view of these findings, an urgent coronary angiography was requested, which showed that epicardial coronary arteries were free of angiographic lesions (Figs. 2A and B).

Figure 1 A: Apical window four-chamber transthoracic echocardiography performed in systole in the acute phase; akinesia of all apical and medial segments with basal segments hyper-contractility is observed. B: Apical window four-chamber transthoracic echocardiography performed in systole in the subacute phase, where contractility segmental alterations are not observed.
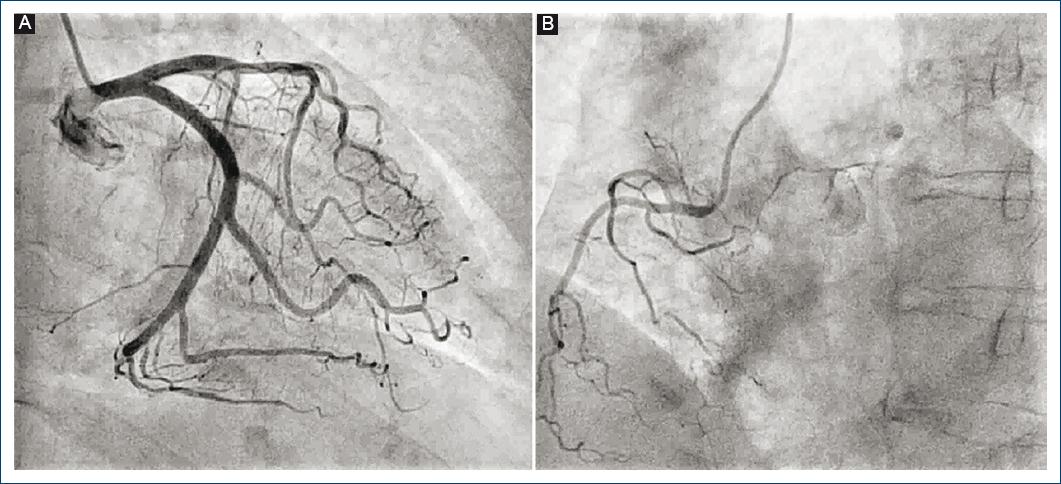
Figure 2 Diagnostic coronary angiography, where no epicardial artery filling defects consistent with ischemia, is observed.
Good respiratory evolution allowed early extubation at 36 h. Neurological workup was completed with a chest CT, which did not show thymus alterations that justified the episode. Control transthoracic echocardiography at 10 days indicated left ventricular systolic function normalization (75% ejection fraction) and absence of segmental contractile alterations (Fig. 1B).
In addition, she had no arrhythmic episodes anytime. The described findings meet all InterTAK classification criteria for Class IIb transient apical dyskinesia, secondary to a severe myasthenic crisis that required orotracheal intubation.
Myasthenic crises are a highly unusual triggering factor of transient apical cardiomyopathy or takotsubo syndrome. In the literature, from 1970 to the present day, only eight cases of takotsubo syndrome caused by the severe myasthenic crisis and requiring mechanical ventilation have been described in the western world.
Myasthenia gravis is an autoimmune disease characterized by fluctuating muscle weakness due to a neuromuscular junction transmission disturbance. Patients with this disease can present myasthenic crises due to various causes such as infections, drugs, or other concomitant autoimmune diseases1. Recently, the InterTAK classification has been published, which groups takotsubo syndromes based on their triggering factors2. Although the mechanisms are not yet are clear, a marked relationship has been observed between high blood catecholamine concentrations and stress cardiomyopathy. Elevated values of adrenaline, norepinephrine, and dopamine, which are released into the blood flow due to stress (whether physical or emotional), can trigger reversible ventricular dysfunction during a myasthenic crisis in patients affected by this anomaly3.
In the presented takotsubo syndrome case, the absence of other potentially triggering causes, such as pheochromocytoma or hyperthyroidism, and the concomitant presence of a myasthenic crisis, make of the latter the cardiomyopathy precipitating factor4.
The relationship between both these entities makes for treatment to be complex. In addition, it is very important not losing sight of which drugs that are commonly used in cardiology should be avoided in patients afflicted by myasthenia since they could aggravate symptoms, such as Class 1a antiarrhythmic drugs (sodium channel blockers), given that they exert an anticholinergic effect. Other drugs that should be avoided include β-blockers, calcium antagonists, and quinidine5. Due to the relatively low incidence of this syndrome and to its exceptional relationship with myasthenia gravis as clinical substrate, it is necessary to maintain a high suspicion index in the presence of non-specific symptoms or findings in these patients since they can compromise short-term prognosis, particularly in the acute phase6.











 nueva página del texto (beta)
nueva página del texto (beta)


