Introduction
Mitral valve prolapse (MVP) is the most common cause of mitral regurgitation in developed countries and affects approximately 2.4% of the population1,2. Recently, there has been increased interest in MVP. It especially has serious complications including endocarditis, arrhythmias, and death. As time has progressed, the prevalence of MVP has varied in the general population. This is partially because of different definitions of MVP in recent guidelines. The current imaging definition of MVP is billowing of any portion of mitral leaflets ≥2 mm above the annular plane3.
MVP is a multifactorial valvular disease caused by myxomatous degeneration of valvular tissue. Myxomatous changes are defined as the expansion of the middle spongiosa layer of the valve (due to accumulation of proteoglycans), structural alterations of collagen in all components of the leaflet, and by structurally abnormal chordae4,5. This process is triggered by valvular interstitial cell transformation. In general circumstances, it is inactively deployed in the spongiosa layer. Structural cells change into myofibroblasts and macrophages for unknown reasons6. Inflammation may play a role in this process, but it’s not known currently. Some new inflammatory markers have been identified in recent years and an association has been found with many cardiovascular diseases7-10. These inflammatory markers, obtained simply from a complete blood count (CBC), were evaluated to provide useful information for many different diseases and conditions. Although some research has been carried out on elevation of new inflammatory markers in valvular heart diseases11-13, no studies evaluating markers such as monocyte-to-high-density lipoprotein (HDL) ratio (MHR), lymphocyte-to-monocyte ratio (LMR), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-neutrophil ratio (PLR) in MVP patients. In this study, we aimed to investigate the above-mentioned inflammatory marker levels in MVP patients.
Methods
Patient selection
In this retrospective study, patients who applied to the cardiology clinic in Afyonkarahisar Health Sciences University, between October 2012 and 2019 were enrolled. First, we identified 512 patients diagnosed as having MVP. After taking into account exclusion criteria and availability of laboratory data, 461 patients were eligible. Second, 459 patients (have normal echocardiography and without MVP) who were age- and gender-matched were included randomly from the database as the control group. A total of 920 participants were examined for further analysis.
Patients under 18 and over 85 years old, chronic inflammatory disease (such as Crohn’s disease, rheumatoid arthritis, and vasculitis), heart failure, renal failure, hepatic disorders, malignancy, thyroid hormone disorders, acute coronary syndrome, developed MVP due to rheumatic valve disease, ischemic and infective endocarditis, presence of active infection, or lack of data were excluded from the study. Furthermore, the study was approved by the Institutional Local Ethics Committee (number of decision: 2019/261).
Transthoracic echocardiography (TTE)
All TTE examinations were performed using a Vivid 9 Pro System (General Electric Medical Systems, Milwaukee, Wisconsin, USA) with a 2.5 MHz phased array. Standard imaging measurements of patients were recorded, including left atrial, aortic annulus, ascending aorta, and left ventricular end-diastolic and systolic diameters. The diagnosis of MVP was based on echocardiographic criteria. Prolapse of the mitral valve is defined as an abnormal systolic displacement of one or both leaflets into the left atrium, and billowing of any portion of the mitral leaflets ≥2 mm above the annular plane in a long-axis view (parasternal or apical three chambers)3.
Laboratory analysis
Laboratory results of all participants were collected from patient records. Blood samples were collected after 12 h of fasting after admission to the hospital. Blood samples were centrifuged at a speed of 1600 rpm for 15 min. A Roche Cobas C501 autoanalyzer system (Roche, Rotkreuz, Switzerland) was used for all biochemical parameters (such as fasting blood glucose, serum creatinine, sodium, potassium, total cholesterol [TC], low-density lipoprotein cholesterol [LDL-C], HDL cholesterol [HDL-C], and triglyceride [TG]). Blood samples were taken into standardized tubes containing dipotassium ethylenedinitrilotetraacetic acid for CBC. An XN-2000 hematology system (Bornbarch, Norderstedt, Germany) was used for CBC analysis. Furthermore, MHR, LMR, NLR, and PLR were calculated by dividing.
Statistical analysis
Statistical analysis was conducted using SPSS software version 23.0 (SPSS, Inc., Chicago, Illinois). Variables were analyzed using visual and analytical methods to determine if they were normally distributed. Mean and standard deviation or median and interquartile ranges were used for descriptive statistics, also number and % were used for categorical variables. Chi-square test was used to compare nominal and categorical variables (gender, hypertension, diabetes, and hyperlipidemia). Parametric data were compared using t-test and non-parametric data with Mann–Whitney U-test. A two-sided p < 0.05 was considered statistically significant.
Univariate analysis to identify variables associated with MVP was also investigated using Chi-square, Student’s t, and Mann–Whitney U tests, when appropriate. For multivariate analysis, possible factors identified with univariate analyses were further entered into logistic regression analysis to determine independent predictors of MVP presence. Hosmer-Lemeshow goodness-of-fit statistics was used to assess model fit. A 5% type-1 error level was used to infer statistical significance. Furthermore, a receiver operating characteristics curve analysis was performed to determine a cutoff value of inflammation markers in MVP, but could not find an acceptable cutoff value.
Results
The study population consisted of 461 patients who had been diagnosed as having MVP and 459 patients in the control group. As shown from Table 1, there was no difference between the groups with respect to age, gender, hypertension, hyperlipidemia, diabetes mellitus, coronary artery disease, chronic pulmonary disease, or thyroid disease (p > 0.05). There were significant differences in echo findings (such as mitral regurgitation, LA size, AO diameter, and wall thickness) of the MVP and control group. All demographic and echocardiographic variables of the groups are given in Table 1.
Table 1 Baseline demographic and echocardiographic measurements of study groups
| Parameters | MVP (n = 461) | Control (n = 459) | p-value |
|---|---|---|---|
| Age, years | 33.75(±9.97) | 33.73(±12.10) | 0.980 |
| Male, n (%) | 162 (32.5) | 161 (32.3) | 0.945 |
| Hypertension, n (%) | 39 (4.2) | 44 (4.5) | 0.567 |
| Diabetes mellitus, n (%) | 20 (2.2) | 27 (2.9) | 0.299 |
| Hyperlipidemia, n (%) | 31 (3.4) | 32 (3.5) | 0.897 |
| CAD, n (%) | 31 (3.4) | 27 (2.9) | 0.684 |
| COPD, n (%) | 7 (0.8) | 13 (1.4) | 0.184 |
| Thyroid dysfunction, n (%) | 12 (1.3) | 18 (2.0) | 0.272 |
| LVEF (%) | 62.5 ± 4.83 | 62.1 ± 5.44 | 0.230 |
| LVDD, mm | 45.78 ± 4.69 | 45.95 ± 3.89 | 0.553 |
| LVSD, mm | 29.58 ± 4.03 | 29.77 ± 4.83 | 0.513 |
| IVS, mm | 9.80 ± 1.46 | 10.22 ± 1.13 | <0.001* |
| PW, mm | 9.53 ± 1.43 | 9.79 ± 0.98 | 0.002* |
| LA, mm | 35.21 ± 5.69 | 34.37 ± 5.42 | 0.021* |
| Ao, mm | 26.63 ± 3.70 | 27.79 ± 3.77 | <0.001* |
| Degree of mitral regurgitation 0/1/2/3/4, n | 169/186/75/16/15 | 432/16/11/0/0 | <0.001* |
*p < 0.05 was considered statistically significant. Ao: aortic root; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; IVS: interventricular septum; LA: left atrium diameter; LVDD: left ventricle diastolic diameter; LVEF: left ventricle ejection fraction; LVSD: left ventricle systolic diameter; MVP: mitral valve prolapse; n: number; PW: posterior wall.
As shown in Table 2, the MVP group had significantly more serum TC and LDL-C than the control group, whereas HDL-C level was lower (p < 0.05). Furthermore, neutrophil and lymphocyte count was significantly different in both groups. NLR (2.488 [1.72-4.51], 1.857 [1.49-2.38], p < 0.001), MHR (14.9 [11.9-18.6, 12.2 [9.4-17.3, p = 0.003), PLR (122.4 [85-171], 104.4 [85-130], p < 0.001), and C-reactive protein (0.71 ± 0.50, 0.67 ± 0.33 p < 0.001) were significantly higher in the MVP group than the control group, respectively. Furthermore, LMR (3.75 [2.75-5.09], 4.06 [3.12-4.83], p = 0.016) was significantly lower in the MVP group than the control group. According to our study results, the changes in inflammatory cells are summarized in figure 1. Furthermore, the boxplot graphic of the NLR, MHR, and LMR results between the two groups is summarized in figure 2.
Table 2 Comparing laboratory parameters between MVP and control groups
| Parameters | MVP (n = 461) | Contro (n = 459) | p-value |
|---|---|---|---|
| Fasting blood glucose, mg/dl | 90.9 (81-100) | 92.5 (83-103) | 0.124# |
| Serum creatinine, mg/dl | 0.65 (0.54-0.80) | 0.74 (0.6-0.9) | 0.062# |
| TC, mg/dl | 167.5 ± 30.9 | 161.4 ± 30.1 | 0.003* |
| HDL-C, mg/dl | 41 (37-45) | 43 (38-48) | <0.001*# |
| LDL-C, mg/dl | 115.6 ± 33.1 | 106.7 ± 29.4 | 0.016* |
| TG, mg/dl | 145 (121-168) | 135 (110-165) | 0.065*# |
| Hemoglobin, g/dl | 13.21 ± 1.39 | 13.19 ± 1.62 | 0.888 |
| Platelet, 10³/mm³ | 249 (186-283) | 231 (215-289) | <0.001*# |
| Mean platelet volume | 9.84 ± 1.51 | 9.80 ± 1.54 | 0.523 |
| White blood cell count, µl | 7.6 (6.8-8.5) | 7.4 (6.4-9.1) | 0.665# |
| Neutrophil count, µl | 4960 (3900-6780) | 4200 (3800-5600) | <0.001*# |
| Lymphocyte count, µl | 2030 (1530-2600) | 2400 (1950-2700) | <0.001*# |
| Monocyte count, µl | 600 (500-800) | 510 (400-700) | <0.001*# |
| MHR | 14.9 (11.9-18.6) | 12.2 (9.4-17.3) | 0.003*# |
| LMR | 3.75 (2.75-5.09) | 4.06 (3.12-4.83) | 0.016*# |
| C-reactive protein, mg/dl | 0.71 ± 0.50 | 0.67 ± 0.33 | <0.001* |
| NLR | 2.488 (1.72-4.51) | 1.857 (1.49-2.38) | <0.001*# |
| PLR | 122.4 (85-171) | 104.4 (85-130) | <0.001*# |
*p < 0.05 was considered statistically significant.
#Mann–Whitney U-test. MHR: monocyte-to-HDL ratio; NLR: neutrophil-to-lymphocyte ratio; LMR: lymphocyte-to-monocyte to ratio; PLR: platelet-to-lymphocyte ratio; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; TG: triglyceride.
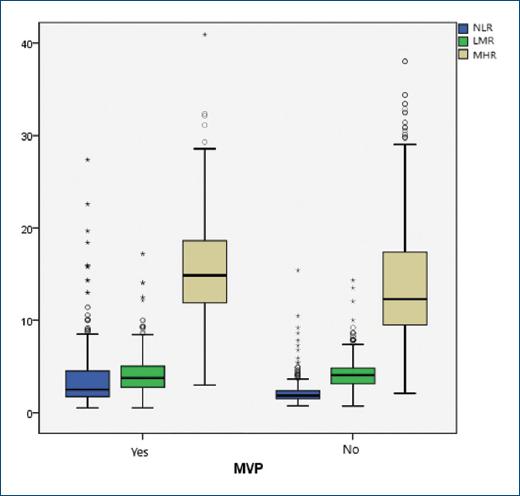
Figure 2 The box plot of the neutrophil/lymphocyte ratio, monocyte/high-density lipoprotein ratio, and lymphocyte/monocyte ratio results.
In logistic regression analysis, NLR (odds ratio [OR]: 1.058 [1.047-1.072]; p < 0.001), LMR (OR: 1.560 [1.211-2.522]; p = 0.027), and PLR (OR: 1.015 [1.012-1.019]; p = 0.003) were found to be independent predictors for MVP presence. The results of logistic regression analysis are presented in Table 3.
Table 3 Univariate and multivariate logistic regression analysis for the presence of MVP
| Variables | Univariate p-value OR (95% CI) | Multivariate p-value OR (95% CI) |
|---|---|---|
| Age | 0.981 1.00 (0.98-1.01) | 0.803 1.002 (0.989-1.014) |
| Gender (male) | 0.945 1.07 (0.92-1.12) | 0.662 0.936 (0.694-1.261) |
| White blood cell | 0.007 0.931 (0.88-0.98) | - |
| Neutrophil | <0.001 1.24 (1.15-1.33) | - |
| Lymphocyte | <0.001 −0.59 (0.49-0.705) | - |
| Platelet | 0.019 1.002 (1-1.004) | - |
| MHR | <0.001 −0.242 (0.12-0.48) | 0.070 0.182 (0.12-0.35) |
| NLR | <0.001 1.52 (1.37-1.69) | <0.001 1.058 (1.047-1.072)* |
| LMR | <0.001 0.242 (0.12-0.489) | 0.027 1.046 (1.021-1.062)* |
| PLR | <0.001 0.994 (0.992-0.997) | 0.003 1.015 (1.012-1.019)* |
*p < 0.05 was considered statistically significant.
−2 Log likelihood= 611.450, R2= 0.514 (Cox&Snell), 0.685 (Nagelkerke), X2(8)= 32.97, p < 0.001 (Hosmer&Lemeshow test).. OR: odds ratio; MHR: monocyte-to-HDL ratio; NLR: neutrophil-to-lymphocyte ratio; LMR: lymphocyte-to-monocyte ratio; PLR: platelet-to-lymphocyte ratio.
Discussion
MVP is a common valvular heart disease caused by myxomatosis transformation. Abnormal immune response and the inflammatory process can play an important role in MVP pathogenesis, but very little knowledge on the relationship of MVP with inflammatory markers has been found in the literature. This study was established to understand the underlying inflammatory aspect of MVP patients. According to our study findings, neutrophil count, NLR, PLR, and MHR were significantly higher, and LMR and lymphocyte count were significantly lower in MVP patients compared to the control group.
MVP predominantly affects middle-aged adults14. Using the currently accepted definition of MVP, the prevalence is nearly equal in men and women in some studies15,16. In a larger study, MVP was more common in women than in men17. When the results of our study are examined, it is seen that the average age of the MVP group was 33.7 years and the majority of the group was female (68%). These results are in line with those of the previous studies. In clinical practice, we often see patients with this profile. It is known that mitral regurgitation is the most frequent complication of MVP, and it is the most important parameter affecting the clinical course of the disease. As expected, we found much more mitral regurgitation in the MVP group compared to the control group. In addition, LA dimension was significantly higher in the MVP group in our study, which may be due to MVP and mitral regurgitation. Apart from this, there were differences in echo parameters that we had difficulty in explaining the clinical significance due to the large number of our patients.
As known from the previous studies, HDL-C has anti-inflammatory effects, stimulates nitric oxide, and promotes angiogenesis18,19. Chang et al. detected decreased HDL-C in valvular heart disease, which is consistent with our results20. We found lower HDL-C, and higher TG and LDL-C levels in the MVP group and these findings were consistent with earlier studies21,22. A possible explanation may be that TG-rich lipoproteins and LDL-C may promote an inflammatory response causing upregulation of TGF-b and metalloproteinase levels23, and in the end result in valve degeneration. TGF-b is an essential factor for developing cardiac and valvular fibrosis. Monocyte reproduction is promoted by TGF-b in non-syndromic MVP24 and infiltrated monocytes turn into macrophages provoking myxomatous degeneration25. In our study, monocytes were higher in the MVP group, but this was not statistically significant. Furthermore, platelet counts were higher in our MVP patients. This result may be explained by the fact that inflammatory mediators stimulate megakaryocytic proliferation and lead to relative thrombocytosis. Although endothelial deformation of the valve surface plays a role in platelet dysfunction, it can cause overexpression, especially in mitral regurgitation26.
Prior studies showed a correlation between 12 cardiovascular diseases and neutrophil count27. As is known, neutrophil levels increase with inflammation. We found a significant neutrophil increase in the MVP group. This finding correlated with previous observations7,28. In our study, we observed that the mean CRP value was significantly higher in MVP patients compared to the control group, as expected. In reviewing the literature, we found a strong association between NLR and inflammation in cardiac and non-cardiac disorders8,29-31. Several reports have shown that higher NLR levels are linked with increased inflammation in many cardiac diseases, such as coronary artery disease9 and rheumatic valvular disease32. In our study, we found significantly higher NLR in the MVP group and this significance persisted in logistic regression analysis. Although the odds ratio of NLR is not very high, we think that NLR is an independent predictor of MVP presence. To the best of our knowledge, this is the first study that evaluated a NLR association with MVP. NLR may be a predictor and prognostic determinant for MVP.
Furthermore, according to our results, PLR and MHR were elevated with MVP patients compared to the control group. Similar to our results, MHR was significantly higher in the Abacioğlu study conducted in our country and in which 82 MVP patients were evaluated recently33. In this study, we could not make a more detailed evaluation due to the lack of information about PLR, LMR, and NLR ratios, but perhaps similar results would have been seen if they were. PLR is a recent hematological inflammatory and prothrombotic marker. It was found to be associated with cardiovascular disorders and valve thrombosis7,34. The previous studies have demonstrated that MVP has an association with ischemic events35. This relationship may be partly explained by endothelial deformation and poor platelet function while increasing the count. PLR elevation may support this mechanism. In a recent study, MHR has been showed to be a marker of inflammation and oxidative stress. This new parameter has been found to be a poor prognostic marker in cardiac and non-cardiac diseases10,36,37. According to our study, MHR elevation was statistically significant in MVP patients compared to the control group. This finding corroborates the ideas of Demir et al.11 who found that MHR was significantly higher in rheumatic valvular disease. Our study provides the first data to consider the relationship between inflammatory parameters obtained from CBC and MVP. These findings may help with further implications in understanding the pathogenesis of MVP.
Conclusions
The main goal of the current study was to determine the association between MVP and frequently used inflammatory markers. From the results of our study, we found that MHR, NLR, and PLR were significantly higher, and LMR was significantly lower in MVP patients compared to the control group. To the best of our knowledge, these findings are the first data for these markers in MVP and represent compelling results. These parameters may be used as a simple, low-cost, reproducible tool to detect inflammation in MVP patients. Nonetheless, we need further prospective, randomized, large-scale studies involving other inflammatory biomarkers.
Limitations
The major limitation of this study was the retrospective and single-center design. It also lacks an evaluation of the prognostic value of NLR, PLR, and MHR on adverse outcomes. In addition, we have no data on other inflammatory markers such as interleukin-6 and tumor necrosis factor-a. MVP can be due to connective tissue disease, but there was no information about this etiology in our dataset. Another limitation was the lack of a classification of MVP (familial or syndromic), which was not analyzed. Moreover, no more detailed echocardiographic evaluation was done because our study is retrospective. Another limitation was the lack of the body mass index data of the participants.











 nueva página del texto (beta)
nueva página del texto (beta)



