Introduction
Carotenoids are non-polar pigments that are abundant in foods. Around 100 different carotenoids have been identified in fruits, vegetables, grains, tubers and bulbs, where they mainly accumulate as liquid-crystalline forms in tubular chromoplasts or solid crystals in crystalloid chromoplasts, although the occurrence of lipid-dissolved carotenoids within globular chromoplasts also occurs in some plant foods (e.g., peach palm fruit and tangerine tomato) (Schweiggert and Carle, 2017). Carotenoids are pigments that confer yellow, orange and red colorations to plant foods, although colorless carotenoids are also present (i.e., phytoene and phytofluene) (Schweiggert et al., 2012). Overall, carotenoids are commonly trior tetraterpenes (30 and 40 carbon atoms) with a system of conjugated double bonds, which is involved in their biological activities (Desmarchelier and Borel, 2017). They can be linear structures or contain cycled ends. They exist as free forms or esterified with fatty acids, sugars and proteins. They have been classified in carotenes and xanthophylls; carotenes are hydrocarbons and xanthophylls contain oxygenated groups (Britton, 2020).
Several beneficial effects on human nutrition and health have been attributed to carotenoids, including effects against several chronic diseases that are considered as public health problems, such as some cancer forms and cardiovascular diseases (Britton, 2020). These biological actions are performed by different mechanisms. Unfortunately, the beneficial effects of carotenoids are limited due to their low bioavailability, according to the low levels of circulating carotenoids commonly reported (Desmarchelier and Borel, 2017; Schweiggert and Carle, 2017). The absorption process for carotenoids is very complex and can be altered by various factors (Desmarchelier and Borel, 2017).
Plant foods rich in carotenoids
Although carotenoids can be found in foods of animal origin, algae and microorganisms, plant foods represent the main source of carotenoids in the human diet. Only 40 carotenoids are significantly consumed by humans, however, only five are the most abundant carotenoids in human plasma (Desmarchelier and Borel, 2017). The most abundant carotenoids in green leafy vegetables are lutein and β-carotene (Table 1). Carotenes in green leafy vegetables can be found as proteincarotenoid complexes. The roots (e.g. sweet potatoes and carrots) are commonly rich in α- and β-carotene, with carrots being the most important source of β-carotene (where it accumulates as crystals) in human diet (Schweiggert and Carle, 2017). Fruits are rich in xanthophyll esters (e.g., β-cryptoxanthin, zeaxanthin and lutein) and β-carotene, which are in non-crystalline form in the chromoplasts. Lycopene accumulates in tomato fruit as big crystals. The carotenoid content in commonly consumed fruits is shown in Table 1.
Tabla 1. Contenido de carotenoides (µg/g FW) en alimentos en función de diferentes factores.
| Food | Lyc | αC | βC | Lut | Vio | Zea | βCry |
|---|---|---|---|---|---|---|---|
| Processing/storage | |||||||
| Mango (Fresh) | 33 - 58* | 9 - 11* | 7 - 22* | ||||
| Mango (CD: 50 - 70 ºC) | 13 - 33* | 0 - 9* | 2 - 14* | ||||
| Orange Sweet Potato (Fresh) | 0.78* | 152* | 1 - 4* | 1 - 2* | |||
| Orange Sweet Potato (B, R, and S) | 0.6 - 0.8* | 19 - 1333* | 1 - 11* | 1 - 6* | |||
| Carrots (stored at - 15 ºC to 50 ºC) | 38-89 | 0.6 - 1.4 | 33 - 44 | 0.5 - 1.5 | |||
| Cauliflower (Raw) | 0.08-9.6 | 1 - 85 | 0.8 - 11 | 0.03 - 0.4 | 0.02 - 0.1 | ||
| Cauliflower (B, R, S, and MW) | 0.05 - 13 | 0.4 - 106 | 0.5 - 16 | 0.02 - 0.9 | 0.001 - 0.2 | ||
| Apricot (Fresh) | 150* | 200* | 250* | 120* | 250* | ||
| Apricot (H, F, and FD) | 70 - 200* | 100 - 240* | 120 - 280* | 110 - 250* | 120 - 260* | ||
| Cherries (Fresh) | 10* | 20* | 20* | 20* | 20* | ||
| Cherries (H, F, and FD) | 10* | 20* | 20* | 20* | 20* | ||
| Nectarines (Fresh) | 60* | 90* | 90* | 100* | 90* | ||
| Nectarines (H, F, and FD) | 20 - 60* | 30 - 90* | 30 - 80* | 40 - 90* | 40 - 90* | ||
| Peaches (Fresh) | 20* | 30* | 30* | 30* | 30* | ||
| Peaches (H, F, and FD) | 20-30* | 30 - 40* | 30 - 40* | 30 - 40* | 30 - 40* | ||
| Plums (Fresh) | 20* | 30* | 30* | 30* | 30* | ||
| Plums (H, F, and FD) | 10 - 20* | 20 - 30* | 30 - 40 | 30 - 40* | 30 -4 0* | ||
| Carrots (Fresh) | 280* | 380* | 440* | 420* | 450* | ||
| Carrots (H, F, and FD) | 220 - 300* | 310 - 360* | 370 - 440* | 360 - 900* | 380 - 470* | ||
| Peppers (Fresh) | 600* | 700* | 840* | 760* | 850* | ||
| Peppers (H, F, and FD) | 430 - 750* | 660 - 1020* | 720 - 1300* | 680 - 1310* | 720 - 1300* | ||
| Mango (Fresh) | 0.5 - 1.2 | 5.9 - 10 | 0.8 | 0.4 - 6.6 | 0.3 - 1.1 | 0.5 - 1.5 | |
| Mango (HHP: 592 MPa, 3 min) | 0.9 - 1.1 | 8.6 - 17 | 0.8 | 0.4 - 5.9 | 0.5 - 1.6 | 0.5 - 2.1 | |
| Papaya (Fresh) | 0.8 | 1.7 | 0 | 0.03 | 0 | 0.4 | |
| Papaya (HHP: 100 - 600 MPa, 5 min) | 0.6 - 0.9 | 2.0 - 6.5 | 0 - 2.7 | 0.2 - 1.2 | 0 - 2.3 | 0.9 - 3.9 | |
| Carrots (Fresh) | 2005 - 2132* | 3312 - 3449* | 242 - 253* | 210 - 269 | 271 - 336* | ||
| Carrots (HHP: 60 - 100 MPa, 5 min) | 1307 - 2180* | 2227 - 3983* | 237 - 289* | 327 - 392* | 394 - 480* | ||
| Avocado paste (Fresh) | 0.2 | 0.9 | 3.1 | 0.06 | 0.3 | ||
| Avocado paste (HHP: 600 MPa, 3 min) | 0.003 - 0.6 | 1.2 - 3.2 | 3.9 - 4.6 | 0 - 0.1 | 0.3 - 1.4 | ||
| Cape gooseberry juice (Fresh) | 1.5 | 2.1 | 3.3 | 3.4 | 4.0 | ||
| Cape gooseberry juice (HP: 80 ºC, 10 min) | 1.6 | 2.2 | 3.2 | 3.3 | 4.4 | ||
| Cape gooseberry juice (US: 10 - 40 W, 15 min) | 2-3.2 | 3 - 4.2 | 3.2 - 6.2 | 4.2 - 5.8 | 5.5 - 7.7 | ||
| Pumpkin juice (Fresh) | 0.8 - 0.9 | 0.9 - 1.2 | 1.1 - 1.3 | 1.1 - 1.4 | 1.1 - 1.3 | ||
| Pumpkin juice (US: 200 - 600 W) | 0.8 - 1.2 | 1.1 - 1.6 | 1.1 - 1.7 | 1.2 - 1.8 | 1.2 - 1.8 | ||
| Carrot juice (Fresh) | 30.1 | 76.6 | 2.9 | ||||
| Carrot juice (HPH: 100 - 150 MPa, 1.2 L/h) | 30 - 32 | 75 - 80 | 2.1 - 2.8 | ||||
| Carrots (Fresh) | 0.15 - 0.2 | 0.45 - 0.5 | 0.18 - 0.2 | ||||
| Carrots (PEF: 0.8 - 3.5 kV/cm-5 - 30 pulses) | 0.2 - 0.4 | 0.4 - 1.0 | 0.01 - 0.3 | ||||
| Cultivar/variety | |||||||
| Mango (16 varieties) | 1.1 - 4.6 | 0.4 - 13 | 0.03 - 1.4 | 0.01 - 12 | 0.2 - 0.6 | ||
| Carrots (10 varieties) | 41 - 60 | 72 - 84 | 2.1 - 4.1 | ||||
| Potato (72 cultivars) | 0 - 1400* | 0 - 1000* | 0 - 1400* | 3-5000* | |||
| 0.002 - 0.6 | 0.06 - 4.7 | 0.06 - 5.0 | 0 - 0.05 | ||||
| Corn (9 hybrids/cultivar) | 0.6 - 2.1 | 0.2 - 16 | 0.7 - 19 | 0.3 - 3.1 | |||
| Pumpkin (29 cultivars) | 0.01 - 0.18 | 0.06 - 0.50 | 0.3 - 1.2 | ||||
| 13 - 115* | 33 - 389 | 3 - 192* | |||||
| Food tissue | |||||||
| Mango (Peel) | 0 - 2.5 | 0 - 8.0 | 13 - 45 | 0.3 - 2.8 | 3.0 | 1.0 - 13 | 0.2 - 6.0 |
| Mango (Pulp) | 0.03 - 6.0 | 0.09 - 12 | 6.4 - 45 | 0.6 - 0.7 | 0.23 | 1.0 - 6.0 | 0.1 - 1.7 |
| Apricot (Peel) | 0 - 1.8 | 0.1 - 8.0 | 6.7 - 198 | 1.7 - 18 | 0.09 - 1.1 | 0.1 - 1.6 | 0.6 - 19 |
| Apricot (Flesh) | 0 - 1.7 | 0.002 - 73 | 0.69 - 123 | 0.03 - 1.4 | 0.01 - 0.08 | 0 - 0.5 | 0.02 - 12 |
| Peach (Peel) | 146 | 0.02 - 46 | 0.02 - 0.13 | 0.01 - 0.13 | 0.01 - 0.05 | ||
| Peach (Pulp) | 93 | 0 - 226 | 0.1 - 2.0 | 0 - 1.5 | 0.0.62 | ||
| Sweet potato (Peel) | 137 | 21 | |||||
| Sweet potato (Flesh) | 364 | 47 | |||||
| Tomato (Peel) | 83 | 144 | 17 | ||||
| Tomato (Flesh) | 113 | 84 | 25 | ||||
| Goldenberry (Pulp) | 70* | 3* | |||||
| Goldenberry (Peel) | 150* | 20* | |||||
| Ripening stage | |||||||
| Avocado at 5 ripening stages | 0.01 - 0.04 | 0.03 - 0.08 | 0.04 - 0.6 | 0.04 - 1.7 | |||
| Rosehip at 5 ripening stages | 4 - 136* | 0 - 13* | 15 - 186* | 14 - 112* | 1.9 - 2.4* | ||
| Mango at 6 ripening stages | 14 - 41 | 0 - 3 | 0 - 4 | 0.6 - 1.7 | |||
| Pre-harvest factors | |||||||
| Melon (5 rootstocks) | 5.7 - 11.2 | 6.3 - 117 | 0 - 13.7 | ||||
| Papaya (11 locations on the island of Hawaii) | 1.5 - 3.2 | 2.4 - 7.4 | |||||
| Brassica vegetables (bed, pot, field and tunnel) | 0 - 68 | 0 - 105 | |||||
| Leafy kale from Italy, Portugal, and Turkey | 385 - 64* | 536 - 615* | |||||
| Baby leaf lettuce (led light and blue led light) | 0.2 - 2.9 | 0.09 - 2.8 |
*Values expressed in dry weight; FW: flesh weigh; Lyc: lycopene; αC: α-carotene; βC: β-carotene; Lut: lutein; Vio: violaxanthin; Zea: zeaxanthin; βCry: β-cryptoxanthin; CD: convective draying; B: boiling; R: roasted; S: steaming; MW: microwaving; H: heating; F: freezing; FD: freeze drying; HHP: high hydrostatic pressure; US: ultrasound treatment; HP: heat pasteurization; W: watts; PEF: pulsed electric fields; HPH: high-pressure homogenization. Sources: Condurso et al., 2012; Dhliwayo et al., 2014; Donado-Pestana et al., 2012; Jacobo-Velazquez and Hernandez-Brenes, 2012; Leong and Oey, 2012; Fernandez-Orozco et al., 2013; Ferioli et al., 2013; Reif et al., 2013, Samuolienė et al., 2013; Zhao et al., 2013; Kljak and Grbeša, 2015; Ma et al., 2015; Behsnilian and Mayer-Miebach, 2017; Cao et al., 2017; Ordonez-Santos et al., 2017; De Andrade Lima et al., 2019; Kourouma et al., 2019; Ranganath et al., 2018; Kulczyński and Gramza-Michałowska, 2019; Elizondo-Montemayor et al., 2020; Etzbach et al., 2020; Fratianni et al., 2020; Liang et al., 2020; Zhou et al., 2020; Cervantes-Paz et al., 2021; Diamante et al., 2021; Hu et al., 2021; Lara-Abia et al., 2021; Laurora et al., 2021; Lebaka et al., 2021; Lopez-Gamez, et al., 2021a; Viacava et al., 2021; Zhang et al., 2021; Suo et al., 2022; Szczepanska et al., 2022.
The carotenoid content in plant foods depends on many factors, especially on the ripening process. Ethylene, the ripening hormone, triggers carotenoid biosynthesis, causing in many cases, exponential increases in carotenoid content (Cervantes-Paz et al., 2012). This process involves the degradation of chlorophylls and the transformation of chloroplasts in chromoplasts rich in carotenoids (Cervantes-Paz et al., 2012). Any postharvest technology applied to preserve plant foods in postharvest may retard or compromise the biosynthesis of carotenoids. This is the case of the use of low temperatures, the modification of the gas composition surrounding the food (modified atmosphere packaging and controlled atmosphere storage), application of ripening inhibitors (e.g., KMnO4 and 1-methylcyclopropene) or application of phytosanitary treatments with heat or ionizing energy (Table 1) (Ornelas-Paz et al., 2017; Yahia et al., 2018).
Carotenoids do not accumulate homogeneously in plant foods, and their content also depends on genotype (Kljak et al., 2015). The geographical origin of plant foods also alters the carotenoid content in foods, since it is influenced by environment temperature, exposition to light, and rain (Laurora et al., 2021). Processing also alters significantly the carotenoid content in plant foods because carotenoids are prone to degradation, transformation or isomerization under light exposition, heating and alkaline or acid conditions. New processing techniques (e.g., pulsed electric fields, ultrasound, high hydrostatic pressures, high pressure homogenization, etc.) reduce the negative effects of conventional processing techniques on carotenoid content (López-Gámez et al., 2021), however, mincing, chopping or homogenizing are enough to alter the carotenoid content in fruits and vegetables (Yahia et al., 2018). Undoubtedly, other factors can alter the content of carotenoids in plant foods (Table 1).
The carotenoid absorption process
The first step of the carotenoid absorption process implies the release of these compounds from foods during digestion (Figure 1) (Cervantes-Paz et al., 2017). This step largely depends on the mechanical and chemical disruption of food by mastication, movements of the gastrointestinal tract, gastric acid, alkaline medium in the intestine, and digestive enzymes. Due to their lipophilic nature, the released carote-noids must then be incorporated into the lipids co-consumed with the carotenoidrich food (Yahia et al., 2018). The carotenoid-lipidic phase tends to emulsify with the aqueous content of the gastrointestinal tract. The lipid droplet size in this emulsion is large in the gastric phase, but it decreases in the intestinal phase of digestion due to the emulsifying action of bile salts secreted by gall bladder (Victoria-Campos et al., 2013; Desmarchelier and Borel, 2017). This reduction of lipid droplet size is very important for lipid digestion, since lipases are hydrosoluble and only exert their action on lipid droplet surface (Cervantes-Paz et al., 2017).
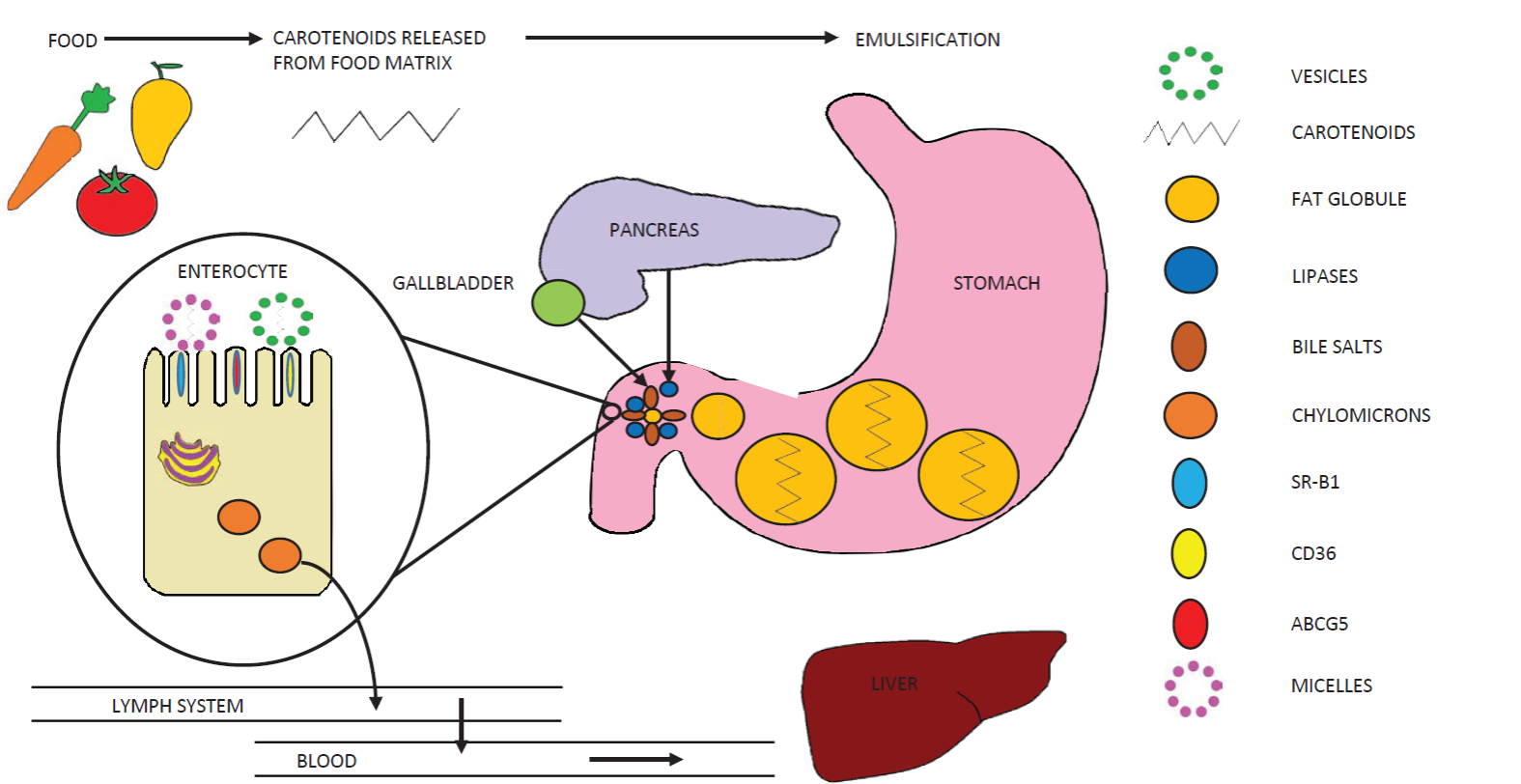
Figure 1 Overview of the carotenoid absorption process. Figura 1. Diagrama general del proceso de absorcion de carotenoides.
Lipid digestion influences the formation of micelles, which are structured by the products of lipid digestion (free fatty acids, diglycerides, monoglycerides, etc.), bile salts, phospholipids and cholesterol (Yahia et al., 2018). Micelles are required for transportation of carotenoids from the chime to the enterocyte. Only micellarized carotenoids can be absorbed by intestinal cells (Cervantes-Paz et al., 2017; Desmarchelier and Borel, 2017).
It must be stated that the distribution of carotenoids in lipid droplets depends on their polarity, with xanthophylls being located on droplet surface and carotenes in the core of the lipid droplets (Yahia et al., 2018). Thus, the lipid digestion starts on the surface of the lipid droplet and therefore xanthophylls are more efficiently released and micellarized than carotenes. Micellarized carotenoids are considered as bioaccessible and represent the amount of carotenoids available in the required form to be absorbed by intestinal cells (Victoria-Campos et al., 2013; Cervantes-Paz et al., 2017). Micellarized carotenoids are transported to the brush border of enterocytes through the aqueous medium. The acidic medium of the unstirred water layer adjacent to the brush border of the enterocytes causes the dissociation of micelles and the liberation of carotenoids, which are passively taken by the enterocytes and facilitated diffusion and unilamellar or multilamellar vesicles of phospholipids (Reboul, 2013).
Currently, the quantity of micellarized carotenoids (bioaccessible carotenoids) is used as a measure of carotenoid absorption (bioavailability). The passive diffusion process is quickly saturated causing that most carotenoids are incorporated into the enterocytes by protein transporters (e.g. SRBI, CD36, NPC1L1) (Reboul, 2013; Desmarchelier and Borel, 2017). Table 2 shows the bioaccessibility of carotenoids from several plant foods. In the enterocytes, the provitamin A carotenoids are converted in vitamin A esters. Then, the non-provitamin A carotenoids, vitamin A and other com-pounds are packed in chylomicrons, which are evacuated to the lymphatic system and then to the bloodstream. Several transporters are involved in the intracellular flux of carotenoi-ds, including CD36, NPC1L1, SR-BI, GSTP1, HR-LPB and fatty acid-binding proteins (Reboul, 2013). Chylomicrons exposed to the action of endothelial lipoprotein lipases in the bloods-tream, lead to chylomicrons remnants, which are taken by the liver (Schweiggert and Carle, 2017; Desmarchelier and Borel, 2017).
Tabla 2. Bioaccesibilidad (%) de carotenoides en frutas y hortalizas comúnmente consumidas.
| Food | Phy and Phytf | Lut | Zea | Neox | Viol | βCry | αC | βC | Lyc | Carotenes | Xantophyllsester | TC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tomatoes | 5-43 | 9-59 | 0.5 - 57 | 0.3-4 | 0.5 - 18 | 2-12 | ||||||
| Carrots | 17-64 | 0-41 | 0 | 3 - 7 | 4-22 | 39 | 2 - 7 | |||||
| Spinach | 4-38 | 22 | 3 | 20 | 3-47 | 19 | ||||||
| Kale | 8-100 | 1-10 | 0.1 | |||||||||
| Lettuce | 11-18 | 6-16 | ||||||||||
| Broccoli | 1-38 | 0 | 7-54 | 0.1 | ||||||||
| Pepper | 17-98 | 73-77 | 8 | 4-39 | 30-45 | 1 | 7-21 | 0-41 | ||||
| Pumpkin | 10-14 | 25-35 | ||||||||||
| Butternut squash | 16 | 4 | 18 | 17 | ||||||||
| Sweet potato | 14 | |||||||||||
| Avocado | 0.4 - 2 | 0.4 - 1 | 1 - 15 | 9 - 11 | ||||||||
| Mango | 14 | 19 | 4 - 32 | |||||||||
| Papaya | 2 | 0.6 | 3 - 9 | 0.6 - 6 | 0.3 | 0 - 20 | 0.3 | |||||
| Melon | 34 | 50 | 7 | |||||||||
| Watermelon | 64 | 0 | 30 | 3 | ||||||||
| Orange juice | 8 - 10 | 103 | 9 | 2.7 | 98 | 6 - 34 | 100 | 6 - 7 | 23 | |||
| Mandarins | 10 - 72 | 33 - 42 | 16 - 36 | 8 - 36 | 0 - 37 |
Abbreviations: TC = total carotenoids; βC = β - carotene; αC = α - carotene; δC = δ - carotene; ϒC = ϒ - carotene; Lut = lutein; Lyc = lycopene; Zea= zeaxanthin; βCry = β - cryptoxanthin; Viol = violaxanthin; Phy = phytoene; Phyt = phytofluene; Carotenes includes αC, βC, Lyc. Sources: Ornelas - Paz et al., 2010; Jeffery et al., 2012; Schweiggert et al., 2012; Victoria-Campos et al., 2013; Li et al., 2016; Petry and Mercadante, 2017; Bergantin et al., 2018; Gonzalez-Casado et al., 2018; Mapelli-Brahm et al., 2018; Zhang et al., 2018; de la Fuente et al., 2019; Liu et al. 2019; Zhong et al., 2019; De Oliveira et al., 2020; Etzbach et al., 2020; Hayes et al., 2020; Cervantes-Paz et al., 2021; Iddir et al., 2021; Lara-Abia et al., 2021; Laurora et al., 2021; Lopez-Gamez et al., 2021a; Lopez-Gamez et al., 2021b; Schmidt et al., 2021.
Carotenoids are exported from liver to different tissues by lipoproteins. Carotenes (e.g., β-carotene and lycopene) are transported by low-density lipoproteins (LDL) and very low-density lipoproteins, while xanthophylls (e.g., lutein, zeaxanthin and β- cryptoxanthin) are transported by high-density lipoproteins and LDL (Desmarchelier and Borel, 2017; Meléndez-Martínez et al., 2017). Carotenoids that are being absorbed, metabolized, stored and/or employed by the organism are considered as bioavailable (Desmarchelier and Borel, 2017). The general process for carotenoids absorption is shown in Figure 1.
Factors affecting the carotenoid absorption process
The micellarization of carotenoids, which is a key step for carotenoid bioavailability, is affected by many factors (Tables 3 and 4). The food matrix is the most important factor affecting the bioaccessibility/bioavailability of carotenoids (Victoria-Campos et al., 2013; Cervantes-Paz et al., 2017). The term “food matrix” refers to the combined effects of all factors associated with a food that improve or reduce the bioavailability of carotenoids (Cervantes-Paz et al., 2017). This explain why the bioaccessibility/bioavailability of carotenoids from simple foods (e.g., supplements) is higher than that of complex plant foods. The form in which carotenoids are present in foods determines their bioaccessibility/bioavailability.
Tabla 3. Efecto del procesamiento térmico y no térmico en la bioaccesibilidad (BA) de carotenoides.
| Food source | Treatment | Bioaccessibiliy (BA) |
|---|---|---|
| Cooking treatments | ||
| Spinach | Raw and steamed (100 °C/3 min) | BA of Lut and βC was higher with steamed (16.8 - 21.7 % and 5.6 - 6.2 %, respectively) than with raw (5.3 - 6.4 % and 2.0 - 3.5 %, respectively) tissues |
| Lettuce | Raw and boled (98 °C/ 20 min) | BA of βC and Lut from raw lettuce was 8.5 - 13 % and 5 - 11 % higher than with cooked samples |
| Tomatoes | Boiled (100 °C/10 min) and digested in absence and presence of an emulsion containing 4 % of olive oil | BA of TC was higher in boiled samples with fat than in raw tissues without fat. Boiling ↑ the BA of TC (45 - 72 %) in digestions with and without fat |
| Jalapeno peppers | Raw, boiled (94 °C /12.5 min) and grilled (210 °C /13.2 min) | BA of free xanthophylls from red peppers followed the order of raw > grilled > boiled. Cooking methods did not influence clearly the BA of carotenoids from green peppers. |
| Industrial thermal-treatments | ||
| Orange-fleshed sweet potato (OFSP) based baby puree and pumpkin products | Homemade steaming (HM-100 °C), PAST (100 °C/15 min), blanching (BP- 90 °C/3 min+PAST), blanching and sterilization (BS) and sterilization at 123 °C/30 min (S) and commercial baby food (CBF-OFSP) | BA of βC ↑ with the intensity of the thermal treatment (BS and CBF-OFSP (5 - 6 %) > BP and S (3 - 4 %) > HM and PAST (0.5 - 1 %)). BA of cis-βC also was higher in S, BS and CBF-OFSP samples that in BP (29 %), HM (6 %) and P (0 %) treated samples |
| Mature pinalate orange juice | Fresh, UF and thawed at room temperature (UF-RT), in the fridge (UF-FG), or in MW (UF-MW) and PAST | Thermal processing ↑ the BA of TC in the order of PAST (26 %) > UF-MW (18 %) > UF-RT (14 %) > UF-FG (12 %) > Fresh (8 %). All treatments ↑ the BA of carotenes (~ 238 %), but only 49 % for UF - FG samples. The effect of treatments was variable in the BA of xanthophylls |
| Tomato and Kale juices | Raw and treated with TT (90 °C/30 s), OH (13 V/cm/ 60 Hz/ 90 °C/30 s) and PEF (35 kV/cm) | In tomato juices, PEF significantly ↓ and ↑ the BA of βC (50 %) and Lyc (2.5 times), respectively. OH and TT did not influence the BA of βC and Lyc. The BA of βC and Lut from kale juices was not significantly influenced by treatments |
| Fresh carrot, sweet potato, yellow bell pepper and broccoli florets | Fresh and HAD (60, 70, 80 °C) samples with or without 5 % (w/w) of olive oil | In carrots, HAD ↑ the BA of Lut, αC and βC (33 - 42 %, 62 - 141 % and 217 - 256 %, respectively). HAD ↑ 1.4 - 23 times the BA of Lut, αC and βC from bell peppers. For sweet potato and broccoli florets, HAD ↑ the BA of αC and βC (9 - 136 %) with olive oil. HAD ↓ the BA of Lut, αC and βC (9 - 74 %) in absence of oil |
| Non-thermal emerging technologies | ||
| Sweet Mary papaya | Cubes (1 cm3) treated with HHP (100, 350 and 600 MPa) at 26.2 °C for come-up time (CUT) and holding time (HT) of 5 min | The highest BA of carotenoids was observed for samples treated with HHP at 350 MPa/CUT and 350 MPA/5 min (1.4 % and 1.1 %, respectively). The lowest BA was observed at 100 MPa/5min (0.4 %) and 600 MPa/5 min (0.6 %) |
| Persimmon | Control (freeze-dried pulverized fruit), PAST (85 °C/15 min) and HHP (200 MPa/25 °C/6 min) | Carotenoids were only bioaccessible in samples treated with HPP and PAST. The most bioaccessible were βCry - laurate (23.9 - 36.9 %), βCry (11.6 - 54.2 %), Ant (18.4 - 30.1 %) and Lyc (17.2 - 27.2 %) and HPP caused the highest bioaccesibility. In HPP treated samples, all-trans and 13-cis βC were highly bioaccessible (19.3 % and 16.4 %, respectively) |
| Valencia orange juice | Untreated and treated juices with HPP (600 MPa/3 min), PEF (12.7 kV/cm / 107.4 kJ/L /61 Hz), Low PAST (LP - 73.9 °C/ 30 s), Conventional PAST (CPAST- 92.2 °C/31 s) and Hot filling (HF) (CP + filling at 82.3 °C). All treatments with/ without US (65.7 - 68. 3 W/cm2/29.5 - 30.7 °C) | BA of carotenoids was higher (41 - 51 %) in juices treated with US than in non-sonicated samples (19 - 24 %). PAST techniques did not affect significantly the BA of TC, however, treatments at higher temperatures (CPAST and HF) ↓ BA, especially that of TC, epoxy carotenoids, diepoxy carotenoids and esterified carotenoids |
| Tomato puree | Untreated and treated with PEF (0.4, 1.2 and 2 kV cm-1; 5, 18 and 30 pulses) in presence of olive oil (5 %) | The highest BA of TC (17.1 %), βC (21.6 %), ϒC (34.5 %), Lut (21.4 %), δC (18.4 %) was observed with puree treated with 5 pulses at 2 kV m-1. Lyc BA was equally enhanced by treatments at 1.2 and 2 kV cm-1 (9.7 % and 9.5 %, respectively). The BA of more polar carotenoids, Phyt and Phy was higher in untreated tomatoes. The number of pulses did not affect significantly the carotenoid BA |
| Carrots | Untreated puree and treate with PEF (5 pulses of 3.5 kV cm-1), TT (70 °C/10 min), PEF and TT (PEF/TT). Samples were digested in presence of olive oil (5 %) | All treatments ↑ carotenoid BA (PEF/TT (245.5 - 296 %), PEF (231.8 - 256 %) and TT (66.7 - 100 %). The impact of treatments was similar for all carotenoids |
| Mango (peels and paste) | Untreated and treated with US (300 W/cm2) | US ↑ BA of βCry (44 - 47 %), Lut (35 - 46 %) and βC (33 - 44 %) from peels. The impact of US on carotenoid BA was higher for paste than for peels. |
| Kale | Untreated samples and treated with HPP (200, 400 and 600 MPa for 5, 10, 40 min) | Higher pressures and extended holding periods ↑ the BA of individual carotenoids from 28.5 up to 78.6 % (600 MPa/40 min) |
| Tomato juice | Fresh and treated with HPH (200, 300, 400, 500 bar) and US (200, 400, 600, 800 W for 20 min, 50 μm of amplitude and 10 s interval) | BA of total all-trans and cis isomers of Lyc was higher for juice treated with HPH at 500 bar (6.9 and 13.4 %, respectively) and US at 800 W (9.7 and 15.8 %, respectively). BA of total Lyc was higher with HPH at 500 bar and US at 800 W (1.4 and 1.8 times higher than with fresh samples, respectively). BA of ζC was not affected by treatments |
Abbreviations: UF = ultrafrozen; MW= microwave oven; PAST = Pasteurized; OH = Ohmic heating; PEF= Pulsed electric field; HPP= high pressure proces-sing; HHP = High hydrostatic pressurization; US = ultrasonication; HPH = high-pressure homogenization; HADϒ = hotϒ air drying; TT = thermally-treated; BA = bioaccessibility; TC = total carotenoids; βC = β-carotene; αC = α-carotene; ζC = ζ-carotene; δC = δ-carotene; C = -carotene; Lut = lutein; Lyc = lycopene; βCry = β-cryptoxanthin; Viol = violaxanthin; Ant = antheraxanthin; Phy = phytoene; Phyt = phytofluene. Sources: Victoria-Campos et al., 2013; Li et al., 2016; Eriksen et al., 2017; Mercado-Mercado et al., 2017; Dhuique-Mayer et al., 2018; González-Casado et al., 2018; Mapelli-Brahm et al., 2018; Zhang et al., 2018; Cano et al., 2019; Zhong et al., 2019; Zhang et al., 2019; de Oliveira et al., 2020; Etzbach et al., 2020; Lara-Abia et al., 2021; López-Gámez et al., 2021.
Tabla 4. Modificadores de la bioaccesibilidad (BA) de carotenoides.
| Digested food | Modifier | Bioaccessibiliy (BA) |
|---|---|---|
| Goji and spinach | Coconut oil (1 %) | Coconut oil ↑ the BA of Zea from 6.7 % to 13.3 % |
| Whole, peel and flesh persimmon | Whole-fat (WFM) and skimmed (SM) milks | BA of TC was higher with WFM than with SM, especially in digestions with whole fruit (2.74 times) than with flesh and peels (0.05 - 0.26 times higher) |
| Frozen Caja pulp - based beverages | Water (W), SM or WFM, with/without sugarcane (7 %) | Sugar enhanced the BA of all carotenoids, in the order W (27 %) > SM (22 %) > WFM (15 %) |
| Commercial milk - fruit juices | Skimmed milk, vitamins (C, D, A, E, B6), sugars, and gelling agents | Beverages containing pectin showed greater BA values (40.7 %) than those containing arabic, xanthan or guar gums (7.4 %) |
| B. gasipaes fruits | Lyophilized peach palm, oil-in-water (O/W) food emulsion | BA of TC, all - trans - βC, all - trans - Lyc and all - trans - γC was 11 - 21 times higher in digestions with food emulsion than with the lyophilized fruit |
| Tomato products | 3 ripening stages (mature-green, pink and red), 5 % oil (coconut, olive and sunflower oils) and two disruption levels (puree and cubes) | BA of TC and Lyc followed the order red > pink > mature-green (0 %). Olive oil ↑ 11 - 15 times the BA of TC and Lyc and sunflower and coconut oils between 7- and 11-times vs digestions without oil. BA of TC and Lyc was higher (46 - 251 %) from tomato puree than from cubes |
| Spinach | Mg (0, 200, 400 mg/mL), canola oil, coffee creamer with 10 % fat and bile extract (1 or 8 mM) | Mg ↓ the BA of TC, Lut and βC. Coffee creamer ↑ more the BA of carotenoids than canola oil (0.1 - 4.3 % vs 0 - 1.9 % respectively), especially with bile extract at 8 mM |
| Pure Lyc, βC and Lut | WPI, SPI, SC, GEL (0, 10, 25, and 50 % of the protein RDA) | WPI ↑ the BA of βC and Lyc, but ↓ the BA of Lut. SPI ↓ the BA of Lut and Lyc (up to 41.0 and 14.3 %, respectively) and ↑ the BA of βC (35 - 37 % at 10 and 25 % of the RDA). SC and GEL ↓ the BA of Lut (up to 62.9 % - 63.4 %) and ↑ that of βC (36.6 - 49.8 %) |
| Spinach, tomato juice and carrot juice | WPI, SPI, SC, GEL, turkey, and cod (0, 10, 25 and 50 % of the protein RDA) | Proteins ↑ the BA of TC (1.1 %) from tomato juice but ↓ that of TC from carrot juice and spinach (2.1 - 2.8 %). Proteins ↓ the BA of Lut and Zea from all foods. Proteins ↑ the BA of βC from juices (at 25 and 50 % of the RDA) |
| Carrot juice (CJ), apricot nectar (AN), tomato juice (TJ), frozen spinach (FS), field salad (FSD) | Ca (0, 250, 500, 1000 mg/L), Mg (0, 100, 200, 300 mg/mL), Na (0, 375, 750, 1500 mg/L) and Zn (0, 12.5, 25, 50, 100, 200 mg/L) | The highest concentrations of Ca and Mg inhibited the BA of carotenoids. Na ↑ the BA of carotenoids. Zn ↑ the BA of βC from FSD and FS but ↓ the BA of βC from AN, CJ and TJ. Na ↓ the BA of xanthophylls from FSD but Zn ↑ their BA. Na and Zn did not affect the BA of xanthophylls from FS. BA of Lyc, Phyt and Phyt from TJ ↓ as the concentration of Ca and Mg ↑, while the highest level of Na and Zn ↑ slightly their BA |
Abbreviations: BA = bioaccessibility; TC = total carotenoids; βC = β-carotene; αC = α-carotene; Lut = lutein; Lyc = lycopene; Zea = zeaxanthin; βCry = β-cryptoxanthin; Phy = phytoene; Phy t = phytofluene; RDA = recommended dietary allowance; WPI = Whey protein isolate; SPI = soy protein isolate; SC = sodium caseinate; GEL = gelatin. Sources: Corte-Real et al., 2017; da Costa and Mercadante 2017; García-Cayuela et al., 2017; Hempel et al., 2017; Corte-Real et al., 2018; González-Casado et al., 2018; Stinco et al., 2019; De Souza et al., 2020; Iddir et al., 2021.
Carotenoids present in foods as lipid-based forms, are readily released from the food and then incorporated into the oily phase of chime, as compared to carotenoid crystals or carotenoid-protein complexes (Schweiggert and Carle, 2017). That is the reason why β-carotene from some fruits (e.g., tomatoes and mango) is more bioavailable than β-carotene from carrots or spinach (Ornelas-Paz et al., 2010).
Food processing alters the effect of food matrix. Cooking and mincing favor carotenoid release from food during digestion, increasing the micellarization and absorption of these compounds (Table 3) (Li et al., 2016; Eriksen et al., 2017; De Oliveira et al., 2020). Industrial processing also increases the bioaccessibility of food carotenoids (Dhuique-Mayer et al., 2018; Mapelli-Brahm et al., 2018; Zhong et al., 2019). Non-thermic technologies (e.g., high hydrostatic pressurization, high pressure homogenization, pulsed electric fields and ultrasound) also increase the bioaccessibility of carotenoids (Lara-Abia et al., 2021; López-Gámez et al., 2021). The increase of carotenoid bioaccessibility is a consequence of the processing-mediated softening of food (cellular disruption). Ripening also causes softening of fruits, favoring the release of carotenoids during digestion. However, ripening also favors the esterification of carotenoids with fatty acids, reducing their polarity and, consequently, their micellarization rate and bioavailability (Cervantes-Paz, et al., 2012; Victoria-Campos et al. 2013).
On the other hand, the fiber from carotenoid-rich food or co-consumed foods alters the carotenoids micellarization and bioavailability (Table 4). Overall, fiber reduces the absorption of carotenoids by altering of the macro- and microviscosity of chime. This alteration reduces the emulsification of lipid droplets favoring the formation of large ones, and reduces the activity of lipase, formation of micelles, the transference of carotenoids from lipid droplets to micelles and the diffusion of carotenoidrich micelles to the enterocyte (Cervantes-Paz et al., 2016). Low concentrations of pectin in the chime (~ 2 %) can cause high reductions in carotenoid bioavailability (20 - 50 %) (Rock and Swendseid, 1992). This effect depends on the physicochemical characteristics of fibers (solubility, molecular weight, degree of esterification, etc.), with soluble fibers exerting the most negative effect on carotenoid absorption (Cervantes-Paz et al., 2016; Cano et al., 2019).
The amount and type of fat consumed with carotenoids alter the absorption rate of carotenoids (Table 4), since the latter are poorly absorbed in absence of fat. The minimum amount of fat required for carotenoid absorption is unknown. Some studies have suggested that at least 3-5 g of fat per meal are required for good carotenoid absorption, although higher quantities of fat per meal (e.g., 20 g) have also been proposed (Goltz et al., 2012). The positive effect of fat on carotenoid absorption depends on carotenoid type, with zeaxanthin, α -carotene, and β-carotene requiring more fat than lutein to reach the highest micellarization values (Hempel et al., 2017; González- Casado et al., 2018; De Souza et al., 2020). Fat consumption can increase the absorption of carotenoids by 2-3 times or even more, depending on carotenoid type. The beneficial effect of fat on carotenoid micellarization and absorption also depends on fat type, with monounsaturated and polyunsaturated fatty acids best promoting the bioaccessibility of carotenoids than saturated fatty acids (Goltz et al., 2012; Victoria-Campos et al., 2013).
Protein improves the bioaccessibility of dietary carotenoids due to its emulsifying properties (Iddir et al., 2021). The carotenoid dose also influences the carotenoid absorption rate, with low doses resulting in a higher carotenoid absorption than large doses (Yahia et al., 2018). Under gastrointestinal conditions, there is a competence among carotenoids for absorption, thus the inclusion of a new carotenoid in the diet will cause a reduction in the absorption of other carotenoids. In some cases, the inclusion of xanthophylls causes a decrease in carotene absorption while in other cases the opposite has been observed (Kopec and Failla, 2018). The effect of some modifiers on carotenoid bioaccessibility is shown in Table 4.
Beneficial effects of carotenoids on human health
Carotenoids are able to neutralize free radicals, whichcan damage molecules in cells and cause degenerative diseases in humans. Abnormal levels of free radicals in the human body cause oxidative stress, which has been associated with the pathogenesis of more than 100 different diseases (Yahia et al., 2018). Carotenoids can neutralize several oxygen reactive species, including singlet oxygen and peroxyl radicals (Britton, 2020). This antioxidant activity depends on their number of conjugated double bonds and oxygenated substituents (Yahia et al., 2018), and involves the binding of carotenoids to free radicals and the transference of energy from the free radical to the carotenoid (Swapnil et al., 2021). Lycopene, canthaxanthin and astaxanthin show a higher antioxidant activity than β-carotene and zeaxanthin. Several studies have demonstrated that the antioxidant activity of carotenoid mixtures is higher than that of individual carotenoids, demonstrating the synergistic effects of carotenoids (Rowles and Erdman, 2020).
The consumption of some carotenoids has been associated with a reduced risk of some forms of cancer (Table 5). The carotenoid with the highest anticancer effect seems to be lycopene, independently if it is consumed in purified form or from tomato products. Lycopene is highly effective in the prevention of prostate cancer, although consumption of lycopene has also been related to the protection against other cancer forms (digestive tract, pancreatic, cervical, etc.) (Lu et al., 2015; Bakker et al., 2016; Van Hoang et al., 2018; Kim et al., 2018; Swapnil et al., 2021).
Tabla 5. Carotenoides y salud.
| Disease / type of study | Population or studied subjects | Effect* |
|---|---|---|
| Gastric cancer (GC) / casecontrol study | 415 cases and 830 controls | Case group consumed significantly less TC, βC, βCry, Lyc, tomato, and ketchup than control group. TC intake in women and an overall intake of Lyc were inversely associated with GC risk |
| Breast cancer (BC) / casecontrol study | 1502 cases (BC), 462 estrogen receptornegative (ER-) and 1502 controls (C) | The highest αC (198 - 1520 vs 14 - 57 nmol/L) and βC (1067 - 7699 vs 29 - 348) plasma levels were inversely associated with the risk of ER - BC |
| Colorectal cancer (CC) / case control study | 845 cases (CC) and 845 controls | The intake of carotenoids was inversely related with the risk of CC in the order of βCry (110 - 167 vs 42 - 68 μg/day) > Lyc (841 - 1278 vs 223 - 379 μg/day) and αC (770 - 1065 vs 207 - 355 μg/day) > βC (7856 - 9164 vs 3818 - 4299 μg/day). The effect of βC was only observed in males |
| Prostate cancer (PC) / case control study | 244 cases PC and 408 controls | The intake of Lyc (>1200 vs <648 μg/day), tomatoes (> 16.5 vs < 7.1 g/day) and carrots (> 3.2 vs < 1 g/day) was inversely related with the risk of PC |
| Head and neck cancer (HNC) / Cohort Study | 120,852 participants, 3898 subcohort members | Inverse association between the intake of vitamin E and carotenoids and incidences of HNC and HNC subtypes |
| Congestive heart failure (CHF) / Longitudinal population-based study | Data from 1031 participants in the Kuopio Ischemic Heart Disease Risk Factor Study from Finland - followed prospectively for > 17 y | The lowest serum βC concentration increased the hazard ratio of CHF (≤ 0.22 vs > 0.46 μmol/L). The lowest levels of serum βC and Lyc increased the risk of death for coronary heart disease |
| Cardiovascular diseases (CDVs) / cross-sectional study | 1350 healthy adults from Japan | Serum levels of TC were inversely associated with baPWV, SBP, DBP, HOMA-IR, blood insulin, FBG, TGs and cholesterol in males, and with seven biomarkers (BMI, baPWV, SBP, HOMA-IR, blood glucose, FBG, TGs and cholesterol) in females. In both sex, TC had a positively association with HDL while Lyc was inversely associated with baPWV and positively associated with HDL. In males, serum Lut was negatively associated with HOMA-IR and insulin |
| Cardiovascular diseases (CDVs) / Meditation analyses using US national data from the NHANES | Data from 1312 men and 1544 women participating in the NHANES 2003-2006 | CRP and tHcy were inversely associated with serum concentration of TC. LDL were inversely associated with Lut/Zea. HDL cholesterol had a positive association with serum αC, βCry, Lut/Zea and TC. βC was inversely associated with the CRP levels |
| Type 2 Diabetes (T2D) / Cohort study | 37,846 participants of the European Prospective Investigation from Netherlands | βC (2.8 - 4.3 vs 1.1 - 1.7 mg/day) and αC (0.4 - 1.1 vs 0.2 - 0.4 mg/day) were inversely associated with the risk of T2D |
| Alzheimer dementia (AD) / A community-based cohort | 927 older adults (81 y) participanting in the Rush Memory and Aging Project followed prospectively for 7 y | TC (24.8 vs 6.7 mg/day) and Lut/Zea (0.37, 8.1 vs 1.2 mg/day) were inversely related with the risk of AD. βC and βCry were inversely related with the HR of AD. TC, Lut and Lyc were inversely related with global AD pathology and individual disease indicators |
*All effects in the table were reported as statistically significant trends (p < 0.05) in original papers. Abbreviations. TC = total carotenoids; βC = β-carotene; αC = α-carotene; Lut = lutein; Lyc = lycopene; Zea = zeaxanthin; βCry = β-cryptoxanthin; BMI = body mass index; baPWV = brachial ankle pulse wave velo-city; SBP = systolic blood pressure; DBP = diastolic blood pressure; HOMA−IR = Homeostatic Model Assessment for Insulin Resistance; FBG = fasting blood glucose; TG = triglyceride; HDL = high-density lipoprotein; LDL = low density lipoprotein; CRP = C-reactive protein; tHcy = total homocysteine. Sources: Karppi et al., 2013; Wang et al., 2014; Curhan et al., 2015; Lu et al., 2015; Sluijs et al., 2015; Munter et al., 2015; Bakker et al., 2016; Van Hoang et al., 2018; Kim et al., 2019; Matsumoto et al., 2020; Yuan et al., 2021.
The effects of other carotenoids in cancer prevention are less clear. There is some evidence that lycopene, β-carotene and β-cryptoxanthin prevent lung cancer (Iskandar et al., 2016). β-Carotene, α -carotene and lutein have shown protective effects against breast cancer (Bakker et al., 2016). β-Carotene, lycopene, β-cryptoxanthin, lutein and zeaxanthin prevent pharyngeal, laryngeal and pancreas cancers (Rowles and Erdman, 2020). Lycopene reduces the risk of gastric and prostate cancer (Van Hoang et al., 2018; Kim et al., 2018). β- cryptoxanthin, lycopene, α-carotene and β-Carotene reduce the risk of colorectal cancer (Lu et al., 2015). The anticancer effects of carotenoids have been attributed to their antioxidant properties, the alteration of genes expression involved in the pathogenesis, alterations in the levels of signaling molecules and other effects (Rowles and Erdman, 2020).
The effect on carotenoid consumption on cardiovascular diseases is unclear, as both positive and negative effects have been reported (Table 5). There are some evidences suggesting that β-carotene, lycopene, lutein, zeaxanthin, astaxanthin, among others, might prevent the development of cardiovascular diseases (Karppi et al., 2013; Wang et al., 2014; Kulczyński et al., 2017; Matsumoto et al., 2020). The effects of lycopene and β -carotene on cardiovascular diseases has been highly studied. The antioxidant activity of carotenoids seems to be associated with their beneficial effect on prevention of cardiovascular diseases, however, other mechanisms seem to be involved, including the carotenoid-related reduction of the inflammatory response caused by the tumor necrosis factor-α (TNF-α), maintaining endothelial nitric oxide bioavailability, altering the expression of some genes, inhibiting leucocyte adhesion and migration, reducing apoptosis, inhibiting macrophages activation, regulating cholesterol synthesis, inhibiting platelet activation, regulating the levels of lipoproteins, among others (Kulczyński et al., 2017). The consumption of β-carotene and α-carotene also is inversely related to type 2 diabetes risk (Sluijs et al., 2015). A neuro-protective effect was recently associated with the intake of total carotenoids, lutein, zeaxanthin, β-cryptoxanthin, and β-Carotene (Yuan et al., 2021).
Lutein and zeaxanthin are important components of macula and retina and their levels in these tissues have positively been associated with visual functions (García-Romera et al., 2022). These effects have been clearly demonstrated in clinical trials. Lutein and zeaxanthin absorb blue light, exert antioxidant effects, improve vision and prevent the development of cataracts and agerelated macular degeneration (Yahia et al., 2018). Interestingly, the levels of these carotenoids in the macule can be used as markers of their concentrations in the brain and cognitive functions (García-Romera et al., 2022).
β-Carotene, β-cryptoxanthin, zeaxanthin, lycopene, siphonaxanthin, fucoxanthin, astaxanthin, crocetin and crocin, and some of their conversion products, are able to reduce the adiposity in animals and humans (Bonet et al., 2020), by influencing adipocyte differentiation, oxidation rate of fatty acids and thermogenesis (Bonet et al., 2015).
Also, carotenoids can reduce liver damage, due to their antioxidant and anti-inflammatory effects as well as the modulation of the expression of genes (Yahia et al., 2018).
Other beneficial effects of carotenoids include the protection of photodamage of skin, cytoprotection against mycotoxins, human growth, reproduction and immune function, among other beneficial effects (Yahia et al., 2018; Swapnil et al., 2021). Table 5 summarizes the results of some recent epidemiological studies on the effect of carotenoids on human health.
Conclusions
Fruits and vegetables represent the most important source of carotenoids in the human diet. These compounds are able to exert many protective effects on human health, which are limited by the low absorption of these compounds in the intestinal tract. Food processing can increase the bioavailability of carotenoids, although processing causes a negative effect on carotenoid content. Further research is needed in order to clarify the beneficial effects of carotenoids on human health and the mechanisms involved in such effects.











 nueva página del texto (beta)
nueva página del texto (beta)


