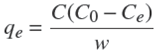1 Introduction
Fluoride is considered beneficial in drinking water at levels about 0.7 mg/L, but hazardous if it exceeds 1.5 mg/L which is the maximum permissible limit proposed by the World Health Organization (WHO, 1984). Some health damage caused by water intake with a high fluoride concentrations are: dental cavities, dental and skeletal fluorosis (Mohapatra et al., 2009). The methods used to remove excessive fluoride from drinking water are ion exchange resins, coagulation, electrochemical methods, unfortunately the high cost of these technologies make them unviable in developing countries (Miretzky and Cirelli, 2011).Many technologies have been developed to remove fluoride from water, that are based on the adsorption process using different adsorbents materials such as activated alumina, double layered hydroxides, carbon nanotubes, bone char, clays, among others (Leyva Ramos, et al, 2001; Medellin Castillo et al., 2007; Viswanathan and Meenakshi, 2010). In recent years, there has been special attention to adsorbents containing natural polymers such as chitosan, which is the second most abundant natural polymer on earth. Chitosan a cationic copolymer consisting of 2-glucosamine and N-acetyl- glucosamine-2 (Fig. 1), which is derived from chitin by demineralization processes, deproteinization and deacetylation (Navarro et al., 2010). This polymer is characterized by its high content of amino groups. Which are responsible of the interaction among metal ions by chelation mechanisms (Guibal et al., 1999; Kawamura et al., 1993). Chitosan can be found with the shape of flakes and powder, this has been a drawback for application in studies of columns, as it presents low mechanical strength. Thus, it has been looking for alternatives to overcome this disadvantage. Chitosan based microspheres have been prepared by using different type of support material that can give a greater mechanical strength. For example clay minerals, hydroxyapatite, and double layered hydroxide (Boddu et al., 2008; Dalida et al., 2011; Sairam Sundaram et al., 2008; Viswanathan and Meenakshi, 2010). Furthermore, chitosan has a pk ≈ 6.5, therefore, it is soluble in acid medium. Chitosan shows a tendency to form a gel in acetic acid and this gel may be precipitated in an alkaline solution to obtain microspheres (Salazar Leyva, 2014). The natural zeolites are microporous crystalline aluminosilicates, with structures formed by tetrahedrons of [SiO4 ]4− and [AlO4 ]5− that are linked through oxygen atoms, which characteristics provide high mechanical resistance. In addition to the property of having cation exchange because the surface is negatively charged (Leyva Ramos et al., 2001). There are many techniques that exist for the modification of zeolites and that make them more selective for anions such as fluoride. For example, it the surface of the zeolite can be modified by exchanging the Na+ bound with Al3+ or La3+ ions, and these latter has an affinity with fluoride anions (Onyango et al., 2004). Furthermore, seaweed has also been used to study the removal of fluoride. Some species of the genus Ulva are considered as natural sources of alginate. Therefore, they are presented as functional carboxylic acid groups (Ghimire et al., 2007), and various amino groups, which are known to have affinity in the removal of fluoride (Paudyal et al., 2013). Seaweed is available in seas and oceans, especially in nutrient-rich waters. Sometimes the seaweed can begin to form massive algal blooms (Pin ̃ón Gimate et al., 2008), which cause an excessive seaweed which have been observed commonly on the beaches of the coastal areas causing an unpleasant appearance. This excessive of seaweed is consider as waste material. Seaweed has the tendency to disintegrate, causing operational problems, principally in adsorption columns; hence, the seaweed can be immobilized in various polymeric materials in order to increase stability and mechanical strength. Many studies have used some natural and synthetic polymers as polyvinyl alcohol and polyurethane (Chu and Hashim, 2007; Zhang and Banks, 2006). Furthermore, literature has reported studies on the removal of fluoride with different synthesized composites based in chitosan and different materials such as alumina composites, hydrotalcite (Viswanathan and Meenakshi, 2010), hydroxyapatite (Sairam Sundaram et al., 2008), mixed oxides (Muthu Prabhu and Meenakshi, 2014), bentonite (Zhang et al., 2013). The objective of this study is to synthesize composites based on a natural polymer such as chitosan and low cost materials such as zeolites and waste materials like seaweed and to evaluate their fluoride adsorption capacity.
2 Experimental
2.1 Materials
Zeolite-clinoptilolite samples (Ca3 (Si30 Al6 )O72 · 20H2O) from Sonora Mexico and, seaweed Ulva lactuca samples from lagoon Santa Maria-La Reforma, Sinaloa Mexico were used in the adsorption experiments.
All chemicals used were of analytical grade: chitosan (75-85% deacetylated) low molecular weight (Sigma-Aldrich), NaF (Fermont), acetic acid (J.T. Baker), 0.1 N NaOH (Hycel), 0.1 N HCl (Hycel), and NaCl (Caledon).
A fluoride stock solution of 100 mg L−1 was prepared by dissolving 221 mg of sodium fluoride in 1000 mL of deionized water. From the previous stock, solutions at different concentration in the range of 10 -100 mg L−1 were prepared for the adsorption experiments.
2.2 Seaweeds pretreatment
The seaweeds were washed with deionized water to remove impurities and were lyophilized at temperature of -36 °C. Then, they were ground to obtain a uniform particle size less than 100 microns. Subsequently, they were treated according to the methodology from Venkata Mohan, et al., (2007) in a solution 0.1 N HCl for 8 hours. Finally, the seaweed was washed with deionized water and dried at 60 °C for 24 hours.
2.3 Synthesis of chitosan-zeolite and chitosan-algae composites
Two types of composites were synthesized with different proportions chitosan-zeolite (ch-z) and chitosan-algae (ch-a). For the preparation of ch-z and ch-a composites, 3.0 g of chitosan were dissolved in 50 mL of acetic acid 5% (v/v) and maintained under constant stirring, then 2.7 g of zeolite of the type clinoptilolite-Ca were added to 20 mL of deionized water, afterward it was incorporated into the chitosan solution and maintained under stirring for 4 hours at 200 rpm. The formation of composites is obtained by supplying the chitosan-zeolite solution through a drip system in the solution of 25% NaOH, then washed with deionized water and, the pH was adjusted to neutral pH and dried at room temperature for 48 hours (Ennajih et al., 2012; Fajardo et al., 2005). The same methodology was followed to prepare ch-a composite, where the zeolite was replaced by pretreated seaweed with 0.1 N HCl.
2.4 Adsorption experiment
Adsorption experiments in batch were carried out to compare the fluoride adsorption capacity of the different materials (chitosan, zeolite, seaweeds, ch- z and ch-a composites) at different pH (5 and 7). Adsorption experiments were carried out as follows: 0.1 g of each material was added into polyethylene tubes that contained 50 ml of fluoride solution at different concentrations (10, 20, 40, 60, 80 and 100 mg L−1). Subsequently, the samples were placed in an orbital incubator at 25 °C y 75 rpp, until equilibrium was reached. The pH was adjusted by adding 0.1N NaOH or HCl. Finally, the fluoride was determined by an ion selective electrode (SM 4500-F) and the amount of fluoride adsorbed was calculated using the following equation:
were: qe is the adsorption capacity (mg g−1) at equilibrium, C0 and Ce are the initial and equilibrium fluoride concentrations (mg L−1) respectively, V is the volume (L) of solution and w is the mass (g) of adsorbent.
2.5 Materials characterization
Thermogravimetric Analysis (TGA)
Thermogravimetric analysis was carried out to determine the percentage of chitosan present in the ch-z and ch-a composites with a Thermo Cahn Versa Therm at a temperature range of 30-800 °C with a temperature ramp of 10 °C/minute in air.
FT-IR analysis
FT-IR analysis were performed to identify the functional groups, which may be involved in the fluoride adsorption and thus to determine the possible adsorption mechanisms with a Nicolet 67000 spectrophotometer (Thermo Scientific), using the ATR technique (Attenuated Total Reflection).
Point of zero charge
The point of zero charge of materials was performed by the method of Slurry pH: 0.1 g of sample was contacted with 5 mL of 0.1 M NaCl previously purged with nitrogen during 10 minutes to eliminate carbon dioxide from the solution. Subsequently, the solution was constantly stirred for 24 hours, finally the pH of each sample was recorded, being the point of zero charge (Davila et al, 2009).
3 Results and discussion
3.1 Characterization of the different materials and composites
Thermogravimetric Analysis (TGA)
The result of thermogravimetric analysis of 5 materials is shown in Fig. 2. The analysis of the derivative of the TGA in general for all materials showed a weight loss between 50 and 100°C corresponding water loss. In the case of chitosan, ch-z and ch-a may be seen a second range of temperatures between 200 and 400°C with a weight loss of 60% which is attributed to degradation of chitosan (Ge and Wang, 2014). As for the decomposition of algae the degradation of carbohydrates (loss 13% wt) occurred at temperatures between 180-270°C, while in a range of 270-390°C the cellulose is volatilized (Kebelmann et al., 2013). Finally the percentage of chitosan present in ch-z and ch-a was 43% and 20%, respectively.
Infrared Spectroscopy Fourier Transform analysis (FT-IR)
The FT-IR spectra resulting from analyzing the various materials before and after adsorption of fluoride is shown in Fig. 3 and Fig. 4. The spectra of chitosan (see Fig. 3b) showed a bond at 3368 cm−1 which is attributed to -NH2 groups. This is because the principal groups in the chitosan are primary amino. The vibration of C-H bonds is reported at 2876 cm−1 and finally the bond 1053 cm−1 corresponds to the stretching vibration of C-O (Jagtap et al., 2011). The spectra of zeolite (see Fig. 3c) shows bands at 768, 1620 and 3600 cm−1, which correspond to stretching vibrations of the Si-O, H-O and stretching -OH attached to aluminum, respectively (Corma et al., 2008; Karge, 1998; Quintero et al, 2012). Finally in the case of U. lactuca, bands at 1100 and 1200 cm−1 are due to vibrations of C-O bonds, while the band at 3290 cm−1 is attributed to the stretching vibrations of -OH and -NH (He and Chen, 2014). The spectra of ch-a composite (Fig. 4a) showed a bond at 1133 cm−1 which belongs to C-O vibrations, the bonds at 2861, 3645 and 3563 cm−1 are attributed to C-H, -OH and -NH vibrations, respectively (Ata et al., 2012). The presence of -NH2 groups of chitosan and -OH and - NH groups of seaweed is due to the synthesizing of ch-a composite. When the fluoride was adsorbed in the ch-a composite (Fig. 4a) the bond attributed to C-H and -OH showed a lower intensity. In the ch-z composite peaks of 1367 cm−1 belongs to stretching -CH and -OH vibrations, 1645 cm−1 is attributed to - NH2 groups vibrations, and finally the band of 2960 cm−1 corresponds to the stretching vibrations of type C-H (-CH and -CH2). Also the absorption band 2960 cm−1 indicates the interaction of chitosan and zeolite by the CH groups, since this functional group is not present in zeolite.
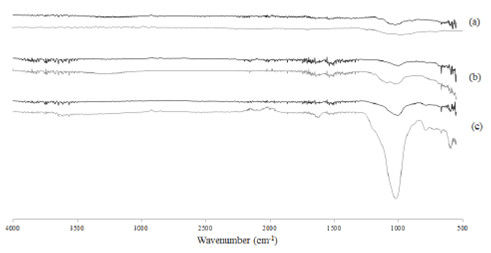
Fig. 3 FT-IR spectra for: (a) algae, (b) chitosan and (c) zeolite before and after the fluoride adsorption.
3.2 Adsorption isotherms
Experimental data of fluoride adsorption isotherms for the different materials were fitted by using the Langmuir (1) and Freundlich (2) isotherm models, which are represented mathematically as:
The constants values of both isotherms and the percentages of standard deviation are shown in Table 1. The experimental data of all materials were reasonably fitted by both Langmuir and Freundlich models, based on the correlation coefficient (r 2) and the standard deviation. This indicates that the materials presented a heterogeneous surface, and also the amount of adsorbed solute exponentially increased as the fluoride concentration increased.
3.3 Effect of pH and point of zero charge in the fluoride adsorption capacity in the different materials
The pH is considered one of the most important factors in the fluoride adsorption process, which depends on the specific characteristics of each adsorbent material. The F- adsorption isotherms at pH 5 and 7 are shown in Fig. 5 and 6. The results demonstrated that chitosan has a greater adsorption capacity compared to zeolite and algae. Moreover, the fluoride adsorption capacity for the chitosan adsorbents, zeolite and algae were of 1.62, 1.20 and 1.31 mg g−1, respectively, at pH 5 and equilibrium concentration of 40 mg L−1, when the pH was raised at pH 7 the adsorption capacity of chitosan, algae and zeolites decreases to 1.08, 1.06 and 1.01 mg g−1, respectively. In both cases it can be seen that chitosan has a higher adsorption capacity than zeolite and algae, because it removes a 13% average of fluoride from water. However, we can observe that chitosan removed 1.5 times of fluorides at pH 5, respect to pH 7. The high adsorption capacity of chitosan can be attributed to its cationic behavior in acidic conditions, the protonation of amino groups promotes the adsorption anions such as fluoride, also according to the PZC (chitosan 7.7, zeolite 7.8 and algae 6.8) it is expected that the surface of the materials present positive charges at lower pHPZC, which favors the adsorption of fluoride (Loganathan et al., 2013). These results are comparable with those obtained by (Menkouchi et al., 2007) in a study on natural chitosan, where they obtained a maximum adsorption capacity of 1.39 mg L−1.
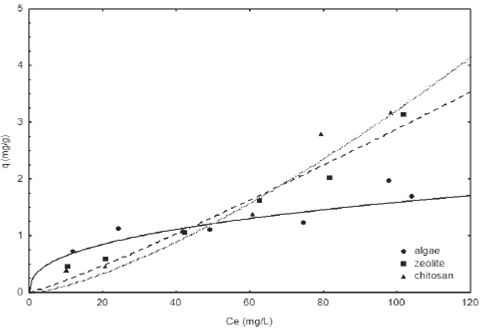
Fig. 5 Fluoride adsorption isotherm of different materials at pH 7 and 25°C. The lines represent the Freundlich model.
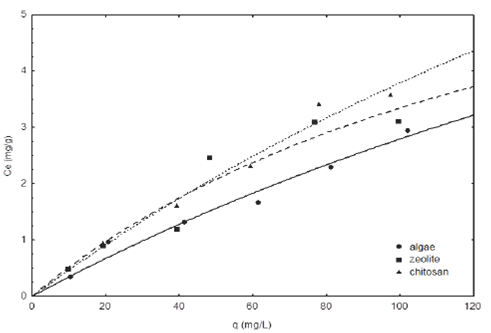
Fig. 6 Fluoride adsorption isotherm of different materials at pH 5 and 25°C. The lines represent the Freundlich model.
Zeolite has a low adsorption capacity, because, it is an excellent cationic exchanger according to the literature, but it can have a low anions adsorption capacity (Bosch and Schifter, 1997). On the other hand, algae are known as excellent adsorbent material on removing cations and there are few studies on their use in the anions adsorption as fluorides. However, the functional groups that are present in their cell wall confer a negative surface charge, but when they are at low pH, the functional groups such as hydroxyls, carbonyls and amines confer positive charges to the surface due to the increased concentration of H+ ions, thus increasing the capacity of anion exchange (Park et al., 2010). Although this capacity can be increased with various treatments for modifying the surface of adsorbents, as was performed in this study.
3.4 Effect of pH and point of zero charge in the adsorption capacity of fluoride in the ch-z and ch-a composites
Adsorption isotherms of fluoride at pH 5 and 7 on the two chitosan-zeolite (ch-z) and chitosan-algae (ch- a) composites are shown in Figures 7 and 8. In accordance to the results we can see that for a pH of 5 the ch-z composite shows a better adsorption capacity compared to the ch-a composite, since for low and high concentrations of fluoride, 20 and 100 mg L−1, this removed 0.90 and 3.34 mg g−1, respectively, while the ch-a composite had a removal capacity of 0.76 and 2.58 mg g−1 for the same equilibrium concentrations. This represents for both cases that the ch-z composite has an adsorption capacity of 1.2 times in contrast to the ch-a composite. Whereas for a pH of 7 the adsorption of fluorides is greater in the ch-a composite, because for these same concentrations (20 and 100 mg L−1) this had an adsorption capacity of 1.47 and 3.33 mg g−1: the ch-z composite removed only 1.21 and2.64mgg−1. That is to say to a pH of 7 the ch-a composite removed 1.2 times more fluoride than ch-z composite. These results are comparable with those of other authors such as Sairam et al., 2008 who synthesized a composite based on nanohidroxiapatite- chitosan which showed a capacity of 1.56 mg g−1 and a point of zero charge of 6.97. The pHPZC for both ch- z and ch-a composites was 8 and 7 respectively. Based on literature, pH 7 is optimized for the adsorption of fluorides on chitosan based composites (Viswanathan et al., 2009).
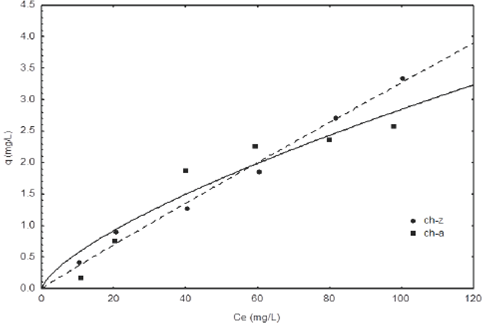
Fig. 7 Fluoride adsorption isotherm of different composites at pH 5 and 25°C. The lines represent the Freundlich model.
3.5 Fluoride adsorption mechanism in composites
Through on FT-IR and PZC analysis we can elucidate the possible mechanism of adsorption of fluoride on the different composites. According to these results, the adsorption mechanism is accomplished by electrostatic interaction between the amino and hydroxyl groups present in the composite. This is because the working pH is below of the pHPZC of adsorbent material. Therefore, the surface of the absorbent material is positive, favoring the adsorption of fluoride ions.
Conclusions
With the exception of the zeolite, all the adsorption experimental data were best adjusted by the Freundlich adsorption isotherm. The adsorption capacity of all materials and composites is affected by the solution pH, so that the range of optimal performance of composites is at pH 5 in the case of ch-z composite, as this removes 1.2 times more fluoride respect to ch-a composite. In the case of ch-a composite, its optimum performance was observed at pH 7 as it increased its adsorption capacity 1.2 times compared to ch-z composite. The modified algae could be considered as an adsorbent material since showed the capacity to remove fluoride. The zeolite had a very low adsorption capacity, but its role in the composite is as a mechanical support for chitosan so that it could be used as an adsorbent material. The adsorption mechanism is by electrostatic attraction. Therefore both composites can be considered as good adsorbents for fluoride removal from water as well as being biodegradable and friendly with the environment.











 nueva página del texto (beta)
nueva página del texto (beta)