Introduction
Cellulose is a polydisperse linear polymer of β-(1, 4)-D-glucose, which is considered the most abundant biopolymer in the world [1]. Their monomer units are linked through ether linkages intermediates C-1 of a monomeric unit and C-4 of the adjacent monomeric unit. Cellulose structure is lineal due to the presence of covalent and hydrogen bonds as well as Van der Waals forces. The monomeric units forming cellulose microfibrils and monocrystalline domains are stabilized laterally by inter- and intramolecular hydrogen bonding inducing an order or disorder in the system, according its structural regularity and amorphous domains [2,3]. For instance, monocrystalline cellulose has been obtained with length ranges from 100 to 300 nm and diameters between 5 and 20 nm under certain process conditions. A transverse cleavage of the cellulose happens primarily in the amorphous zone of the fiber, releasing needle-like monocrystals, which are referred as cellulose nanowhiskers. In general, whisker dimensions depend on both the origin of the cellulose and reaction conditions employed [4-7]. Microfibers and cellulose nanowhiskers (CNW) have excellent mechanical properties, low density and biodegradability, which enable it to be used as reinforcing material in polymer composites [8-11]. Recently, CNW have been combined with polymers in an effort to give it better mechanical properties.
The molecular interactions among CNW and elastomeric polymers have a significant impact on the adhesion and mechanical properties of the resultant material. For instance, nanowhiskers improved the overall tensile properties polymers. Thus, the aim of this work was to study the intra- and intermolecular interactions between the CNW and poly(butyl acrylate) (PBA) by molecular mechanical (MM) simulations. Additionally, cellulose, CNW and PBA were analyzed by using FTIR
Materials and methods
Materials
All reagents used in the study were purchased from Sigma-Aldrich Co.: methanol, toluene. Butyl acrylate of Mw = 128.2 g/gmol (BA, 99 %) was purified by treating it with 5 wt% aqueous NaOH to remove the inhibitor and then washed with ion-free water until a pH = 7 was achieved. Later, it was dried using sodium sulphate. The initiator, 2-2' Azobisisobutyronitrile (AIBN) was purified with methanol by recrystallization. Sulfuric acid (98 %) was used for acid hydrolysis of cellulose. Cellulose (C6H10O5)n was obtained by traditional kraft pulping process from Eucalyptus saligna wood.
Simulation Details
Molecular mechanical simulations were performed on the blend cellulose/PBA at room temperature (25 °C) using the software Materials Studio 8.0 (Accelrys, Inc.). Initially, the monomeric units CNW and PBA were built in the Polymer Builder Module. The polymerization degree (DP) of cellulose and PBA were of 10 and 50, respectively. Each molecule was optimized geometry at lowest potential energy level by using the PCFF force field. The following steps were taken to realize the simulation: Smart algorithm (cascade of steepest descent, conjugate gradient, and quasi-Newton methods) with 50,000 steps [12-13].
The cellulose and PBA analysis to determine the hydrogen and Van der Waals interactions was performed using a parameter in H-bond Geometry of 2.5 Å. The interactions between PBA and cellulose groups were analyzed by two methods: a) Considering a lateral coupling between the two chains and b) Using a unique end molecular interaction of a cellulose chain on a PBA molecule.
Preparation of cellulose nanowhiskers
Cellulose was modified by sulfuric acid hydrolysis, which was completed in three stages; 1) Initially, 15.0 g of cellulose was dispersed into 67.57 mL deionized water; the suspension was placed in a bath sonicator and stirred for 20 min, afterward sulfuric acid was added drop by drop until the desired concentration of 65 % was reached, the suspension was stirred at 44 °C for 130 min [14]. The obtained suspension was centrifuged at 5000 rpm for 20 min and washed repeatedly with deionized water [15]. The sediment was dialyzed against an aqueous solution at room temperature for 7 days using a semipermeable membrane (MEMBRA-BEL, 12,000 - 14,000 MWCO) to remove the acid remained in the solution. Then, sonication was used for 30 min to disperse the CNW. Finally, the resulting samples were dried under vacuum oven at 60 °C for 24 h [16].
Synthesis of poly(butyl acrylate)
PBA was prepared using the following procedure: BA (4.8×10-1 moles) and AIBN (9.6×10-4 moles) were dissolved in toluene (100 mL). After that, the mixture was degassed by several freeze-thaw cycles under high vacuum, and then polymerized for 2 h under nitrogen gas at 70 °C. Finally, the solution was diluted in toluene and precipitated in methanol to purify the polymer [17-18].
Composite of cellulose nanowhiskers with poly(butyl acrylate)
A solution containing 0.01 g CNW was mixed with 0.99 g of acetone (1.0 wt% CNW). Then, 0.99 g of PBA were added gradually to the CNW solution. This solution was kept stirring for 4 h, at ambient temperature, until to obtain a homogeneous CNW/PBA solution. Finally, from the previous solution, the acetone was eliminated under vacuum at 70 °C.
Results and discussion
Molecular analysis of cellulose
Figure 1a and 1b show hydrogen bonds and Van der Waals forces of cellulose molecule containing 10 monomelic units. Figure 1a allows to observe the O-H···O-C bonds, which promote the crystallinity of cellulose. Additionally, it can be observed several free OH groups. Moreover, Figure 1b shows the H···H bonds and H···O-C of Van der Waals interactions.
Molecular analysis of poly(butyl acrylate)
Figure 2 shows the molecular configuration of PBA (containing 50 monomers). Fractions of PBA can see in Figures 2b and 2c which correspond to the short-distance hydrogen interactions and Van der Waals forces respectively. Figure 2b shows the hydrogen bond between C-H ··O=C, which contributes to stabilize the PBA molecule. In this figure, it can also see some free polar groups as C=O. While, Figure 2c shows the Van der Waals forces along the molecular structure of the PBA, as consequence of intra molecular bonds. Thus, hydrogen bond and Van der Waals forces generated an extended chain configuration of PBA, where the almost all functional groups were able to interact strongly with each other.
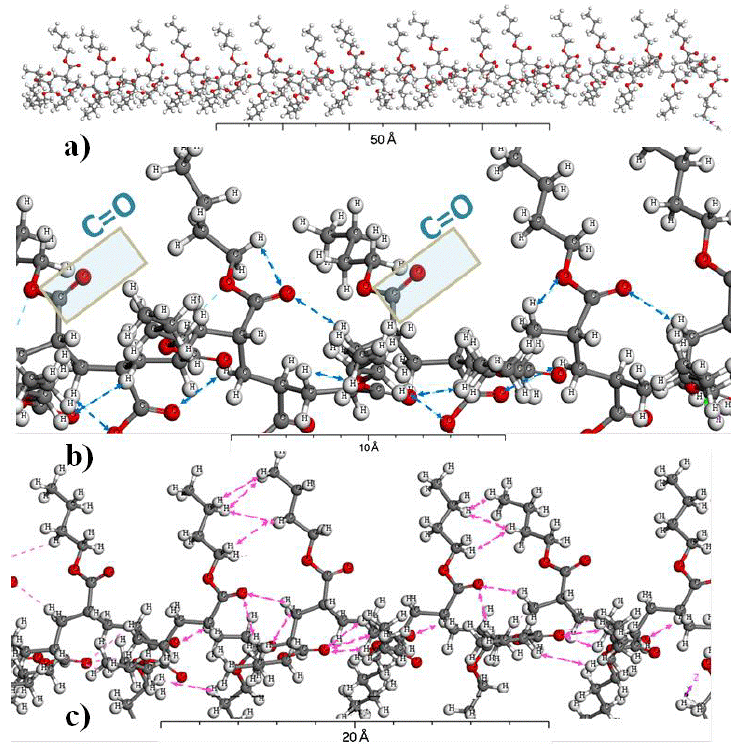
Figure 2 Geometrically optimized molecule of PBA, a) the full image of PBA, b) hydrogen bonds and c) Van der Waals interactions.
As has been seen, Cellulose and PBA show -OH and C=O groups respectively, which do not participate in intramolecular hydrogen bonding. It is favorable, because they could interact with each other to form a molecular hydrogen bond complex.
Molecular simulation of cellulose/PBA
Figures 3a and 3b show the cellulose/PBA blend with intermolecular interactions due to hydrogen bonds and Van der Waals forces under parallel and perpendicular approaches respectively.

Figure 3 Molecular simulation NCW/PBA analysis by hydrogen bonds and by Van der Waals: a) parallel and b) perpendicular coupling.
The parallel molecular arrangement generated the highest interactions as a consequence of more surface area available for interactions, being more intensive for the hydrogen bonds.
The cellulose/PBA association is more intensive when the molecules are aligned with each other.
Although, the Van der Waals bonds are relatively weak compared to hydrogen bonds; they are particularly in polymers due to the cumulative effect of thousands of bonds.
Structural analysis by FTIR
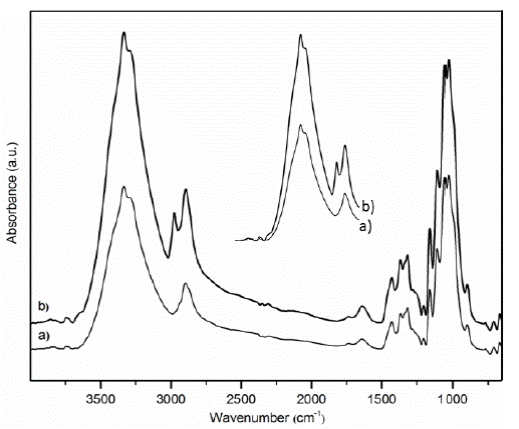
Figure 4 shows the IR spectra of cellulose and CNW, where the characteristics signals of cellulose are showed.
For both samples a signal at 3335 cm-1 and another signal at 2896 cm-1 were attributed to stretching vibrations of -OH and C-H bonds respectively [19-20]. While a peak at 1638 cm-1 was assigned to bending OH bond of adsorbed water [21]. The band at 1159 cm-1 was assigned to the C-O-C asymmetric stretching mode. Moreover, the peaks near 1105, 1055 y 1029 cm-1 corresponding to the respective -C-O stretch bending.
An enlarged area of Fig.4 (from 3500 to 2700 cm-1) was inserted in it (in the center above) to attract the attention about the CNW obtained by acid hydrolysis process. The CNW showed a more intense absorption around 3335 cm-1 region (-OH stretching) compared to cellulose. This suggest the presence of higher number of hydroxyl groups which were most likely formed as consequence of the attack to the β-1,4- glucosidic linkage [22-24].
Additionally, the CNW showed an absorption near 3010 cm-1 which is referred to an asymmetrical bending vibration out-of-plane bending of C-H groups [25]. This signal could be attributed to the intense movements of C1 and C4.
According to the chain scissions on the glyosidic linkage of the cellulose, it is more likely to obtain fibrillar aggregates, ending with the nanowhiskers formation.
Figure 5 shows the IR spectrum of PBA. It presented the characteristic peaks at 2957 and 2869 cm-1 corresponding to -CH3 and -CH2- groups respectively. Meanwhile the peaks at 1728, 1158, 1117 cm-1 and 1116 cm-1 were assigned to C=O, -O-C-, C-C and C-H (of alkyl chain) groups. Moreover, the peak near 1242 cm-1 was characteristic of -CH2 groups [26].
Comparative spectra of PBA, CNW and CNW/PBA system were analyzed in Figure 5. The signal of hydrogen bond (at 3335 cm-1) revealed in the CNW sample was not showed in the comparative spectra CNW/PBA. However, simply by subtracting the infrared spectrum of PBA from the CNW/PBA spectrum allowed to identify the CNW spectrum. This new spectrum (inserted left above) exhibited the effect of hydrogen bonding on an O-H stretching vibration at 3306 cm-1; the change in wavenumber to a lower value (about 29 cm-1) was due to an intermolecular hydrogen interaction. Additionally, the signal corresponding to C-O group presented a slight shift to the right (1064 cm-1), this confirms the presence of the above-mentioned interactions.
Notable shifts of the stretching vibration at 2960 cm-1, 2876 cm-1, 1453 cm-1 and 1242 cm-1 were observed, which are assigned to intermolecular interactions given to C-H bonds.
Conclusions
In order to explain the CNW/PBA associations, molecular simulation and experimental work were carried out. Thus, in the present study, CNW, PBA and CNW/PBA samples were prepared. Derived of the molecular simulation analysis, the preliminary study determined hydrogen bonding interactions between cellulose and PBA due to -OH/C=O groups which did not participate in intramolecular bonding. Particularly, the highest intermolecular hydrogen bonding was identified mainly in parallel molecular arrangement between cellulose and PBA. Meanwhile, FTIR analysis confirmed the intermolecular hydrogen interactions between CNW and PBA. Then, considering both results, it is possible conclude there is a strong molecular association between CNW and PBA.











 nueva página del texto (beta)
nueva página del texto (beta)