Introduction
Worldwide, stroke is responsible for approximately 5.5 million deaths and 116 disability-adjusted life years1. The estimated incidence of this disease will keep on increasing, in part due to the ever-expanding population with metabolic disease. Until 2015, the only acute treatment available to patients with acute stroke was intravenous thrombolysis with recombinant tissue plasminogen activator (rtPA), and only when it could be administered in the first 4.5 h from symptom onset2. Over the past few years, new therapies for acute ischemic stroke (AIS) have had a major impact on the functional outcome and survival of stroke patients2. AIS caused by large vessel occlusion (LVO), which 10 years ago had a somber prognosis, is today an important therapeutic target of interventional vascular neurology3. LVO ischemic strokes are more likely to have a poor functional outcome when compared to those occurring due to distal occlusion and are responsible for up to 90% of the mortality and 60% of dependence in patients with ischemic stroke4,5.
In 2015, based on the evidence of five clinical trials, the American Heart Association/American Stroke Association (AHA/ASA) included mechanical thrombectomy (MT) within 6 h of symptom onset as a therapy with level IA evidence, for patients with AIS due to a LVO6. After several trials showed that this therapy could be used for selected patients in even longer time windows, the 2019 update of these guidelines adopted an extended therapeutic window7. MT requires a selection of patients based on clinical and imaging criteria, including the need for vessel imaging with computed tomography angiography (CTA) or magnetic resonance angiography (MRA) in the 6-h window and an evaluation of mismatch between the stroke core and the area of tissue at risk using magnetic resonance imaging (MRI), computed tomography perfusion (CTP) and/or perfusion-weighted imaging MRI (Magnetic resonance perfusion [MRP])8,9.
Despite their important impact on AIS outcome, rtPA and MT are only available in two thirds of countries worldwide, but are underused in low- and middle-income countries10. To effectively employ these new therapies, healthcare systems must implement policies to ensure access to diagnostic and therapeutic interventions for patients with AIS. In Mexico, few of these processes have been implemented, and there are significant delays in accessing such health system and few hospitals have the necessary processes to deploy these new treatment modalities, although the use of rtPA has increased since 200511,12. In a recent study done at four Mexican hospitals with AIS protocols that included data from 500 patients, the time from symptom onset to arrival at the hospital was approximately 11 h; for 7.6% of patients who were treated with rtPA, the door-to-needle time was 82 ± 51 min. The proportion of patients who lived independently 6 months later was 68.4% among those treated with rtPA and 41.7% in those not treated11.
Here, we review the principles of neuroimaging, how to interpret data and summarize the imaging criteria for AIS treatment. We also discuss how the new techniques can help in clinical practice, the different treatment options, as well as the inclusion and exclusion criteria for each of them, according to the available evidence.
This article was created through a systematic qualitative search in DATABASES, PUBMED, MEDLINE, LILACS, and EMBASE using the following Medical Subject Headings terms: (AIS) + (neuroinmaging/techniques) + (thrombolytic therapy) + (MT). Then, we selected the main papers and trials regarding the current treatment and selection criteria for the management of AIS, as well as the most recent and used criteria for the different neuroimaging modalities. All papers were required to have been published in a good quality journal with a good impact factor. The most widely used guidelines were added and reviewed. Finally, our team discussed all the information that was included, with the senior researcher (Alcoholics Anonymous) having the final word on any disagreements among the junior researchers.
What are the important concepts related to AIS imaging?
The aim of the most novel neuroimaging techniques in the context of acute cerebral ischemia is to distinguish likely infarcted and unsalvageable areas of the brain (ischemic core) from potentially salvageable tissue (penumbra). The ischemic core is key to determine the outcome. It is time-sensitive and could be assessed using diffusion-weighted imaging (DWI) or CTP. Penumbra is the target of reperfusion therapy and, while it is not a novel concept, it is now measured with neuroimaging techniques. Perfusion imaging has helped proof-of-concept studies, and reperfusion has been observed to correlate with a better clinical outcome in patients with a considerable amount of tissue at risk of infarction. Below, we review the most important concepts in the AIS treatment and its correlation with neuroimaging13.
Cerebral blood flow (CBF)
− CBF is defined as the blood volume that flows per unit mass per unit time in brain tissue and is typically expressed in units of mlblood/100 gtissue/min14. When discussing neuroimaging studies, CBF has a very similar definition, with the tissue being represented as a voxel15.
− Normal: 60-100 ml/100 g/min
-
− Hypoperfusion refers to CBF < 60 ml/100 g/min. When CBF ranges from 22 to 60 ml/100 g/min, the tissue is considered oligemic and may or may not spontaneously recover without reperfusion therapy, and thus vigilance is warranted. Ischemia develops when CBF < 22 ml/100 g/min. If reperfusion is re-established quickly, the tissue may recover; otherwise, it will die. Hypoperfusion is further classified in the following:
Oligemia: asymptomatic hypoperfusion with spontaneous recovery and no need for reperfusion therapy. CBF is 22-60 ml/100 g/min.
Ischemia: symptomatic hypoperfusion that may or may not respond to reperfusion therapies, depending on stroke severity. CBF is < 22 ml/100 g/min.
Penumbra: the tissue surrounding the ischemic core, and by definition, can recover from ischemia. Penumbra may be sustained by collateral circulation, and the time it will remain viable will differ from patient to patient. This condition warrants reperfusion therapy. CBF is 22-10 ml/100 g/min.
Ischemic core: dead, non-recoverable tissue. CBF is < 10 ml/100 g/min14.
The mismatch may have different meanings, depending on the imaging method; it is used differently when talking about perfusion imaging, MRI, and the relationship between symptoms and imaging. Mainly, it is used to describe the presence of salvageable tissue. Several studies (EPITHET16, DEFUSE17, and DEFUSE-218) have shown an association between the degree of reperfusion and clinical outcomes in patients with mismatch; this confirms that reperfusion relates to better clinical outcomes in patients with mismatch. A mismatch can be defined according to the study with which it is assessed.
− CTP mismatch: we talk about a mismatch when there is a difference in the volume of the area with a severe cerebral blood volume (CBV) decrease and delayed CBF; it is a way to operationalize the concept of ischemic core and penumbra19.
− DWI-perfusion weighted imaging (DWI-PWI) MRI mismatch: the mismatch between these two imaging modalities is thought to be a hallmark of ischemic penumbra19 and can be used to estimate the extent of the penumbra20. Some of the DWI lesion is potentially reversible, and some of the peripheral regions of the perfusion abnormality will not progress to infarction, and thus may benefit from intravenous thrombolysis20.
− Fluid attenuation inversion recovery-DWI (FLAIR-DWI) MRI mismatch: related to the differences in DWI and FLAIR or T2 sequence; ischemic injuries will appear at different times in each of the previously mentioned sequences: DWI will show hyperintensities in the 1st few min of a stroke, while FLAIR will manifest hyperintensities (in the same regions as DWI) from 6 h onward. A mismatch in this context refers to DWI that shows hyperintensities, but FLAIR and/or T2 has no such changes, which suggests that the lesion is acute, and thus may warrant reperfusion therapy. On the other hand, if the same hyperintensity appears both in DWI and FLAIR, there is no mismatch, which means that the ischemic injury is already established, and thus, this tissue is not salvageable21.
− Clinical-radiological mismatch refers to patients that may present with clinical features that do not seem to correlate to the neuroimaging findings (severe deficit and mild imaging features, and vice versa). Such is the case with posterior circulation injuries, in which small lesions may cause a very striking clinical picture; this is because structures in the posterior fossa and brainstem are very small and tend to be packed together9.
Vessel occlusion
In imaging studies with an angiographical sequence, an occlusion can be appreciated as an amputation, interruption, or reduction in vessel continuity or width. CTA and MRA identify LVOs involving the internal carotid artery (ICA), middle cerebral artery (MCA), and anterior cerebral artery (ACA) in the anterior circulation, and the basilar and vertebral arteries in the posterior circulation. By location, the LVO can be classified as22:
- Proximal occlusion: the occlusion is at the level of the first portion of ACA, MCA, and the final portion of the ICA.
- Distal occlusion: the occlusion is beyond the established limits for a proximal occlusion, or in vessels other than those mentioned for proximal occlusion.
LVOs can be identified with a neuroimaging study of the intracranial vessels. Because of their availability and diagnostic precision, CTA is usually the preferred method, and it should include the supra-aortic vessels. In the acute period (< 24 h), patients with a confirmed National Institutes of Health Stroke Scale (NIHSS) > 6 may warrant neuroimaging with angiography to determine the level of the occlusion23, depending on the patient's characteristics.
Clinical severity
The most widely used tool to clinically quantify AIS severity is the NIHSS, in which a higher score means greater stroke severity24. While the definition of minor stroke has changed over time, the accepted definition by the AHA/ASA guidelines is NIHSS < 57.
According to a trial that analyzed various clinical scales for AIS, a NIHSS ≥ 11 may yield good accuracy (79%) and for LVO diagnosis, with the drawback of 27% false negatives. NIHSS ≥ 6 has a greater sensibility (87%) for LVO diagnosis, at the expense of 40% false positives and an accuracy of 69%25.
Which are the imaging methods to evaluate AIS patients?
The timely detection of an AIS, and correct identification of candidates for rtPA or MT (or a combination of both), depends on clinical and neuroimaging findings. The different neuroimaging methods depend on the availability in each center, and all the techniques are useful to predict which patients with AIS will benefit from acute treatment, and which ones have a higher risk of hemorrhagic complications. The time criterion is still the most widely used marker for patient selection for reperfusion therapies, especially for rtPA7.
Nowadays, neuroimaging protocols are being developed to optimize patient selection, with a renewed interest in perfusion studies. In this line of thought, some trials have begun to focus on collaterals, which may be a valuable biomarker in the future9. Ideally, in neuroimaging studies, we should evaluate the parenchyma, cerebral vessels, cerebral perfusion, ischemic core, and penumbra. Although there are multiple imaging techniques for patient selection, a non-contrast computed tomography (NCCT) may be sufficient for the management with rtPA, which remains the standard treatment7.
NCCT
NCCT alone is used in the initial assessment of patients with the clinical diagnosis of AIS. NCCT has three uses in the acute period: ruling out hemorrhage, detection of non-acute lesions, and detecting hyperacute changes that may suggest acute ischemia. In most stroke centers, the first study in the evaluation of suspected AIS is a NCCT, as it answers the first question one should ask themselves when dealing with an acute, sudden neurological deficit: "does this patient have an intracranial hemorrhage?"9 If no bleeding is detected, and, depending on clinical variables, rtPA could be initiated if inclusion criteria were met. The classic early ischemic signs seen in NCCT (Fig. 1) are obscuration of the lentiform nucleus, loss of gray-white matter differentiation at the insula (insular ribbon sign), loss of gray-white matter differentiation at the surface cortex, and hyperdense artery sing (may be indicative of a thrombus within the MCA)22. The Alberta Stroke Program Early CT Score (ASPECTS, Fig. 2)26 is the most widely used method to assess the extent of early ischemic changes27. ASPECTS is a validated, prospective score, that allows us to have an idea of the infarct's extension. This method divides the MCA's territory into ten regions, and for each hypodensity, in each of these regions we will subtract a point (the abnormality must appear on at least two consecutive 5 mm cuts); a score of < 6 is considered to suggest extensive, established injury, and a lower probability of a good outcome27. In general, an ASPECTS of 6-10 was used as an inclusion criterion in endovascular trials, such as ESCAPE28, SWIFT PRIME29, and REVASCAT30, to select patients with a relatively small core infarct. ASPECTS assessment is affected by CT quality. It is important for the grayscale of NCCT to be properly adjusted for brain parenchyma so that the gray-white matter differentiation is readily distinguished9.
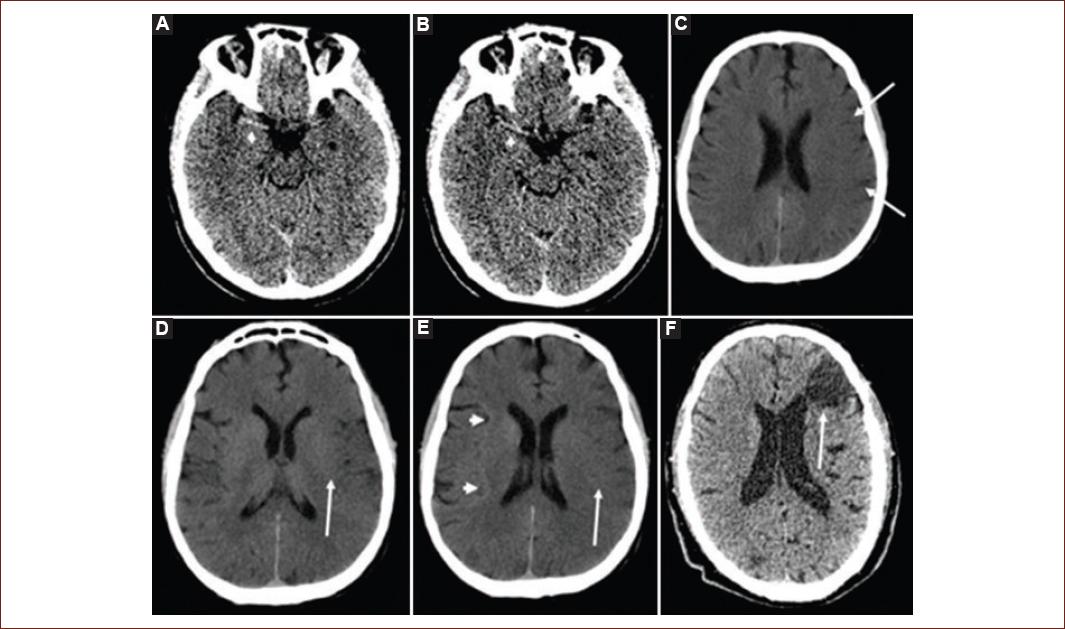
Figure 1 Classic early ischemic signs in a non-contrast computed tomography: A and B: show a hyperdense right middle cerebral artery sign (white arrow head). C: shows the loss of gray-white matter differentiation at the surface cortex (white arrows). D: hypoattenuation of the lentiform nucleus (white arrow). E: loss of gray-white matter differentiation at the left insula (insular ribbon sign, white arrow) compared to a normal right insular ribbon (white arrowheads). F: shows a chronic cortical infarct (white arrow) with ventricular deforming due to gliosis.

Figure 2 Middle cerebral artery territories according to ASPECTS in a NCCT. Caudate nucleus (C), insular ribbon (I), posterior arm of the internal capsule (IC), lenticular nucleus (L), anterior MCA cortex (M1), MCA cortex lateral to the insular ribbon (M2), posterior MCA cortex (M3), superior-anterior MCA cortex (ACA-MCA watershed territory; M4), frontal posterior MCA cortex (M5), parietal cortex (M6). It is necessary to have at least one cut at the level of the basal ganglia and thalamus and another one just above the basal ganglia. ACA: anterior cerebral artery; ASPECTS: Alberta stroke program early CT score; MCA: middle cerebral artery; NCCT: non-contrast computed tomography.
NCCT is considered suboptimal to evaluate the posterior fossa due to artifacts caused by bony structures. Since it is the most basic study for AIS, it does not allow us to evaluate other relevant variables, such as the site of occlusion or collaterals22. Still, there may be times when the clinician will have to evaluate the posterior fossa with only a NCCT, and it is for these situations that pc-ASPECTS31 was developed; it is used in the same manner as ASPECTS and accounts for the territories supplied by the vertebrobasilar system. As of now, it has not seen much use in the clinical field.
NCCT is also useful to discard any stroke mimics that could otherwise lead to unnecessary reperfusion therapy. Mimics are non-stroke disorders with a presentation that could suggest AIS, such as an intracranial hemorrhage or tumors32.
CTA
The primary modality used to assess blood vessels is CTA, which is increasingly used as part of the initial imaging protocol to identify patients with LVO. CTA is also the most frequently used vascular imaging modality in clinical trials and clinical practice. This sequence can be obtained on all modern CT scanners and can be easily incorporated into an AIS imaging protocol. It requires intravenous iodinated contrast and, depending on the equipment that is used, is usually completed within 2 min. There may be concern for the use of contrast, but the 2019 update to the AHA guidelines states that the risk of contrast-induced nephropathy is relatively low, and waiting for the laboratory results may delay treatment; this is especially true in patients with no known history of kidney disease7, and even then, according to a systematic review, neither CTA nor CTP increase the risk of acute kidney injury in patients with known chronic kidney disease33. CTA allows us to pinpoint the location, extension of the vessel occlusion, and identifying thrombi in large proximal intracranial arteries. Determining the location of the thrombus helps predict the likelihood that thrombus will respond to rtPA or MT, especially in tandem lesions (occlusions with both intracranial and extracranial components)34. An LVO will appear as an amputation (Fig. 3) or reduction of the affected vessel's caliber. It is also possible to measure thrombus length, which will influence recanalization potential, which is to say that clot length has been reported to be inversely proportional to rtPA effectiveness, especially if said clot is > 8 mm35. This is not normally used in the clinical setting and is currently not included in any guidelines.

Figure 3 Computed tomography angiography with occlusion of the left middle cerebral artery (white arrow).
CTA also allows us to qualify collaterals using a less standardized scale than the one used for digital subtraction angiography (DSA), characterizing findings as: robust collaterals (collateral circulation is symmetric between the affected and the healthy hemispheres); intermediate collaterals (collateral circulation is appreciated in 50-99% of the tissue at risk); poor collaterals (30-50% in the tissue at risk). Patients with better collaterals seem to not only fare better but also appear to have a lower bleeding risk by reperfusion injury36. As of now, this information is still under research, there are no standardized CTA scales for collaterals, and collateral grading is not used in guidelines, so it is not a parameter to exclude patients from reperfusion therapy.
CTA is also useful to determine the etiology of an ischemic stroke and to identify underlying pathologies, such as carotid atherosclerosis (artery-to-artery embolism), intracranial atherosclerosis, or arterial dissection22. Figure 3 shows a CTA with occlusion of the left MCA.
Computed tomography (CT) perfusion
Perfusion imaging, using either CT or MRI, has been used to select patients for treatment outside the recommended time window. Perfusion studies use contrast to measure the amount and time it takes for intravenous contrast to pass through certain areas of the brain and can help identify the ischemic core and penumbra. Recent studies (DAWN37 and DEFUSE 338) showed improved outcomes after MT for patients selected by specific parameters or perfusion. Its primary role in AIS is determining whether brain tissue is hypoperfused and, therefore, at risk of infarction. Penumbra (viable tissue) is the target for reperfusion therapy. CTP can be performed in a few minutes and gives us a number of measures, especially CBF and CBV. During acquisition for a CTP, the brain is repeatedly scanned during the passing of intravenous contrast throughout the brain parenchyma. As the contrast flows, the relative increase, peak, and the decrease in radiodensity create an attenuation-time curve. These curves are calculated for an arterial and venous input function and outflow, allowing to measure perfusion for each voxel. Unlike multiphase CTA, CTP involves acquiring many such images, which results in a higher radiation dose than CTA. This repeated scanning of the brain allows the generation of estimates for CBF, blood volume, median transit time, time to peak (TTP), time to drain, and tissue permeability. These measurements are shown in color-coded parametric maps, representing each variable. The ischemic core is identified by markedly reduced CBF and reduced CBV, with a marked delay in TTP and mean transit time. By contrast, the ischemic penumbra, which usually surrounds the ischemic core, has prolonged mean transit time but has only moderately reduced CBF and near-normal or even increased CBV (Fig. 4)17. It is now established that its use does not delay rtPA application, nor intervention through MT when compared to NCCT39, and it is associated with more reperfusion therapy use40. A systematic review of AIS diagnosis with CTP showed high sensitivity (80-82%) and very high specificity (95%). Its limitation in AIS includes that it can miss lacunar infarcts and has a relatively low sensitivity for posterior circulation infarcts41. CTP may also cause significant delays in workflow due to the longer acquisition and processing times and does not invariably provide accurate information. Several studies have shown that automated processing of CTP can provide a quantitative mismatch classification. Recently, DAWN37 and DEFUSE 338 used automated software (RAPID) to determine the ischemic core and showed excellent clinical outcomes in patients treated up to 24 h from symptom onset.

Figure 4 CTP with mismatch due to a large region of ischemic penumbra (salvageable hypoperfused tissue), compared to the ischemic core (unsalvageable tissue). A: CBF map shows a region of decreased perfusion within the right MCA territory (white arrowheads). B: median transit time map shows an increased blood contrast time that matches the same region as (white arrowheads). C: CBV map demonstrates no abnormality. D: large ischemic penumbra (green) with a small ischemic core (purple), representing a CTP mismatch. CBF: cerebral blood flow; CBV: cerebral blood volume; CTP: computed tomography perfusion; MCA: middle cerebral artery; MTT: median transit time.
MRI
MRI can provide estimates of penumbral tissue through the combination of diffusion and perfusion imaging, but in practice, this is less available and more difficult to interpret. MRI sequences considered to be essential for AIS are apparent diffusion coefficient (ADC), DWI (ischemic findings appear from the 1st few min after symptom onset), susceptibility-weighted imaging (SWI, so as to detect any hemorrhage that may not be evident in other sequences), and FLAIR (ischemic changes will appear after 6 h from symptom onset). ADC is used in conjunction with DWI to distinguish truly ischemic lesions. DWI is highly sensible (88-100%), specific (95-100%), and accurate (95%) to detect and delimitate parenchyma at risk of infarction9. This sequence can demonstrate hyperintensities in oligemic tissue and may overestimate infarct size13, so information obtained with his method must be interpreted with caution. Lesion volume determined by DWI and its pattern seems to be related to collateral flow grade in AIS42. Supratentorial infarcts with a great volume (> 100 ml) are related to higher bleeding risk and lower possibilities of benefit from MT; those of intermediate size (70 - 100 ml) have an uncertain prognosis regarding reperfusion therapy43. MRI is more useful than NCCT to evaluate the posterior fossa and brainstem, as MRI presents no bone artifact. Both NCCT and MRI have distinct and clearly defined roles in decision-making. Figure 5A and B shows a DWI-FLAIR mismatch, while figure 5C-D shows no mismatch.

Figure 5 Magnetic resonance imaging in patients with acute ischemic stroke. Lesion shown on diffusion-weighted imaging (DWI) (A) but not on fluid attenuation inversion recovery (FLAIR) imaging (B); thus, it is considered a DWI-FLAIR mismatch. Meanwhile, the lesion on DWI (C) has a corresponding parenchymal hyperintensity on FLAIR (D), so there is no mismatch.
MRA
MRA is useful when a patient in whom LVO is suspected and cannot receive iodinated contrast for CTA. The main limitation lies in that MRA may overestimate the degree of stenosis and may be inaccurate to detect a distal occlusion, as MRI is dependent on flow and does not adequately represent intraluminal anatomy, unlike CTA9.
MR
MRP can create similar maps as CTP, so findings and definitions are the same for both studies. The role of this modality is still debated. MRP has the advantage of not using ionizing radiation, unlike CTP9. MRP can opt-out of using contrast with arterial spin labeling, a sequence with the capacity of proving flow information, with the drawback of not providing volume measures, so penumbra cannot be delimited. It is mostly used to study tumoral lesions22,44.
DSA
DSA is considered the gold standard to evaluate vessel morphology, level, and occlusion percentage. The thrombolysis in cerebral infarction (TICI) scale was developed for DSA studies with the objective of quantifying response to reperfusion therapies45; nowadays, a modified version (mTICI, Table 1) is used, which reflects the increasing use of MT46. DSA also allows us to evaluate collaterals using the American Society of Interventional and Therapeutic Neuroradiology/Society of interventional Radiology scale (Table 2), which is useful to characterize collaterals quality, with very important implications for clinical outcome47. The use of DSA (outside MT) in AIS is decreasing, although it is still used when in doubt, even after CTA or MRA43. Figure 6 shows a DSA before and after the retrieval of a thrombus that was occluding the proximal right MCA.
Table 1 Reperfusion of large vessel occlusion (LVO) defined by modified Thrombolysis in Cerebral Ischemia (mTICI)
| Grade | Definitions |
|---|---|
| 0 | No perfusion |
| 1 | Antegrade reperfusion past the initial occlusion, with limited distal branch filling with little or slow distal reperfusion |
| 2a | Antegrade reperfusion of less than half of the ischemic territory |
| 2b | Antegrade reperfusion of more than half of the ischemic territory |
| 3 | Complete antegrade reperfusion, with no occlusion at the distal branches |
Table 2 American Society of Interventional and Therapeutic Neuroradiology/Society of interventional Radiology (ASITN/SIR) collateral grade scale
| Grade | Angiographic collaterals (Digital subtraction angiography) |
|---|---|
| 0 | No collaterals visible at the ischemic region |
| 1 | Slow collaterals to the periphery of the ischemic region with the persistence of some of the defect |
| 2 | Rapid collaterals to the periphery of the ischemic region, with the persistence of some of the defect and to only a portion of the ischemic territory |
| 3 | Collaterals with slow but complete angiographic blood flow of the ischemic bed by the late venous phase |
| 4 | Complete and rapid collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion |
What are the current therapies and how to select patients for each of them?
Clinical decisions for treatment in acute stroke include a brief history and examination followed by imaging, leading to quick action (Fig. 7). Clinical evaluation based on NIHSS is the first step inpatient evaluation. It is clear, according to different studies, that there is a correlation between the NIHSS score and outcome48. Seminal MT trials DAWN35 and DEFUSE 336 included patients with a median NIHSS of 16-17 points, and DEFUSE 338 included patients with a mean ASPECTS of 8, which implies that in extended therapeutic windows, patients with severe stroke could be excluded.
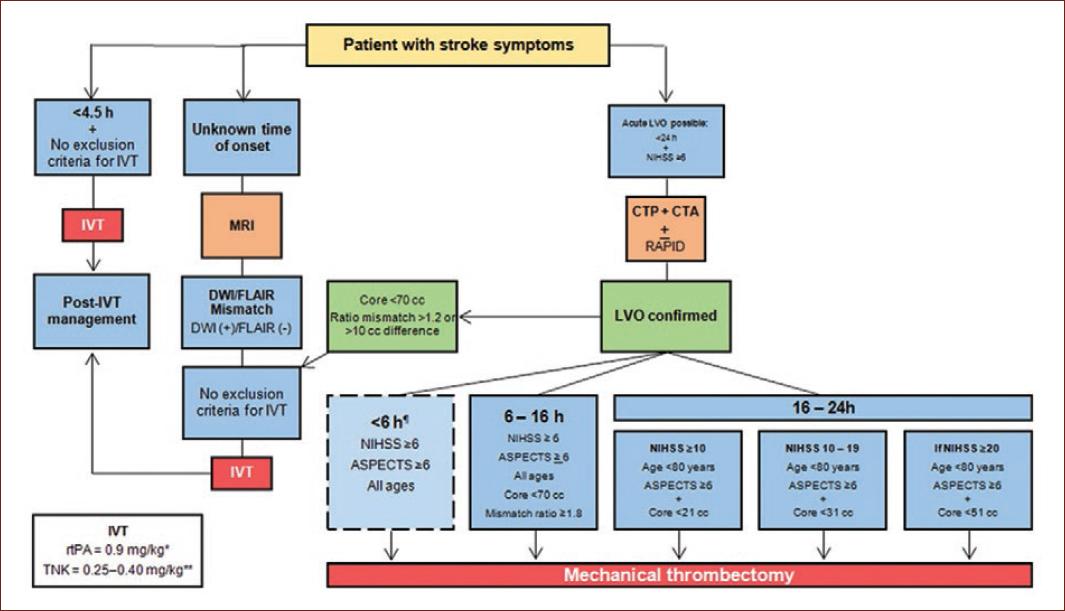
Figure 7 Treatment algorithm in acute ischemic stroke. CTA: computed tomography angiography; CTP: computed tomography perfusion; DWI: diffusion-weighted imaging; FLAIR: fluid attenuation inversion recovery; IVT: intravenous thrombolysis; LVO: large vessel occlusion; MRI: magnetic resonance imaging; NIHSS: National Institutes of Health Stroke Scale; RAPID is an automated software for CTP; rtPA: recombinant tissue plasminogen activator; TNK: tenecteplase; w/o: without. No brain perfusion imaging needed before 6 h for thrombectomy. *Maximum dose: 90 mg. **Depending if the patient will receive MT. Maximum dose: 25 mg.
Current reperfusion therapies are rtPA and MT, or a combination of both of them. rtPA is the first-line treatment for AIS, as long as it can be administered in < 4.5 h from symptom onset, and no exclusion criteria are met (Table 3); it is within this timeframe that mismatch criteria are not needed, and the only advanced neurimaging technique that could be used is CTA, if the patient were suspected to be a candidate for MT. These first 4.5 h are divided into a classical window (0-3 h after symptom onset) and an extended window (3-4.5 h); the latter has some specific criteria that must be met before initiating rtPA7. It must be stressed that some of the criteria that were previously considered to be contraindications for rtPA (age > 80 years-old, NIHSS > 25, previous stroke in a diabetic patient, etc.) are now considered merely warnings for rtPA during the extended window and are in no way a reason for the physician to refrain from administering rtPA7.
Table 3 Inclusion and exclusion criteria for the treatment of acute ischemic stroke with alteplase, with additional warnings for the extended therapeutic window
| Inclusion criteria − Clinical diagnosis of IS causing the measurable neurologic deficit − Onset of symptoms < 4.5 h before beginning treatment; if the exact time of stroke onset is not known, it is defined as the last time the patient was known to be normal or at neurologic baseline − Age ≥ 18 years Exclusion criteria − IS or severe head trauma in the previous 3 months − Previous ICH − Intra-axial intracranial neoplasm − Gastrointestinal malignancy or hemorrhage in the previous 21 days − Intracranial or intraspinal surgery within the prior 3 months − Symptoms suggestive of SAH − Persistent BP elevation (systolic ≥ 185 mmHg or diastolic ≥ 110 mmHg) − Active internal bleeding − Presentation consistent with infective endocarditis − Stroke known or suspected to be associated with aortic arch dissection − Acute bleeding diathesis − Platelet count < 100,000/mm3 − Current anticoagulant use with an INR > 1.7 or PT > 15 s or aPTT > 40 s or PT > 15 s − Therapeutic doses of LMWH within 24 h (e.g., to treat VTE and ACS); this exclusion does not apply to prophylactic doses − Current use of a direct thrombin inhibitor or direct factor Xa inhibitor with evidence of anticoagulant effect by laboratory tests such as aPTT, INR, ECT, TT, or appropriate factor Xa activity assays − Head CT: evidence of hemorrhage, or extensive regions of obvious hypodensity suggestive or irreversible injury |
Warnings − Only minor and isolated neurologic signs or rapidly improving symptoms − Serum glucose < 50 mg/dL (< 2.8 mmol/L) − Serious trauma in the previous 14 days − Major surgery in the previous 14 days − History of gastrointestinal bleeding (remote) or genitourinary bleeding − Seizure at the onset of stroke with postictal neurologic impairments − Pregnancy − Arterial puncture at a non-compressible site in the previous 7 days − Large (≥ 10 mm), untreated, unruptured intracranial aneurysm − Untreated intracranial vascular malformation Additional warnings for treatment from 3 to 4.5 h from symptom onset − Age > 80 years − Oral anticoagulant use regardless of INR − Severe stroke (NIHSS score > 25) − Combination of both previous ischemic stroke and diabetes mellitus |
ACS: acute coronary syndrome; aPTT: activated partial thromboplastin time; ECT: ecarin clotting time; INR: international normalized ratio; PT: prothrombin time;
NIHSS: National Institutes of Health Stroke Scale; tPA: intravenous alteplase; TT: thrombin time; VTE: venous thromboembolism; ISL: ischemic stroke; ICH: intracranial hemorrhage; SBP: systolic blood pressure; SAH: subaracnoid hemorrhage; LMWH: low molecular weight heparin; BP: blood pressure.
The recanalization rate for patients with a distal occlusion who are treated with rtPA goes from 38% to 50%49,50. rtPA is a drug with a number of benefits that depend on the speediness with which it is applied. It is estimated that for every 30 min of treatment delay, chances of a good clinical outcome decrease by 10-15%51. The therapeutic windows are determined by the time from symptom onset. Inclusion and exclusion criteria, as well as additional warnings for rtPA have not been modified since 2008. rtPA is the most used drug for intravenous thrombolysis, although there is a growing body of evidence that supports tenecteplase (TNK) as either and safe alternative or even as a potential replacement for rtPA. rtPA binds to the fibrin in a thrombus and causes the plasminogen within the clot to convert to plasmin, which will break down the thrombus46.
Candidates for MT must receive rtPA as soon as possible. The treating physician must keep in mind that MT is not a contraindication for rtPA and that under no circumstances, one should wait for rtPA response in a patient with LVO to decide if they should be taken to MT23. During MT, every time a device is directed at the thrombus with the objective or removing it, it is called a "pass." With second-generation devices, MT may achieve a complete or near-complete (TICI 2B/3) recanalization rate of up to 84%52. In a trial that included 330 patients, the investigators tried to determine which was the minimal necessary number of attempt for MT; there was a median of 1 pass, with an achieved TICI 2B or 3 in 46.8% of patients; after three passes, the goal was met in 67.9%. Better clinical outcomes were observed in those with 1-3 passes53.
After 4.5 h of symptom onset, the only treatment option is MT. Although the therapeutic window has extended up to 24 h, the more time passes, the selection of patients becomes more rigorous and requires more elements to demonstrate viable brain tissue7,35. The criteria on which treatments are based beyond 6 h of evolution, wake-up stroke, minor stroke, and in some specific situations are described below.
Thrombolysis in minor stroke
Minor stroke is one of the most commonly cited reasons for nonuse of rtPA within the classical and extended time windows, and benefits regarding reperfusion therapy with rtPA in this context remains unclear. Prospective data suggest that 30% of patients with a minor stroke will have a functional disability (modified Rankin Scale [mRS] > 2) at 90 days after stroke54. The most recent recommendations establish that for patients with mild but disabling stroke symptoms (NIHSS 0-5 that prevents the patient from performing basic daily activities or returning to work)54, rtPA should be administered within 3 h from symptoms onset or from last known well (Strength of recommendation Class I, Level of Evidence B) and its use may be reasonable in the group of patients within the extended window (Strength of recommendation Class IIb, Level of evidence B)7.
Thrombolysis and anticoagulation
Some patients, particularly those with known non-valvular atrial fibrillation (NVAF), are treated with direct oral anticoagulants. Even with these drugs, approximately 1-2% of these patients will have a stroke55. Active anticoagulation for patients with NVAF can be achieved with either vitamin K-dependent (e.g., acenocumarin), or with direct oral anticoagulants (factor Xa inhibitors such as apixaban or rivaroxaban, and direct thrombin inhibitors, e.g., dabigatran) is a contraindication for rtPA (Table 3). Factor Xa inhibitors and direct thrombin inhibitors have an additional warning if the last intake was within 48 h from symptom onset, or if the patient has a known history of renal impairment56. Indeed, either International Normalized Ratio or serum creatinine are needed to consider rtPA in patients with Vitamin K-dependent or direct oral anticoagulants, respectively56. Idarucizumab, a humanized monoclonal antibody, has been available since 201557; it binds to dabigatran, and it is capable of reversing its effects within a few minutes, with no hypercoagulable state afterward58. Its efficacy was proven in the RE-VERSE AD trial59. It is currently indicated for life-threatening bleeding, emergency surgery, and other urgent procedures55, but, as of now, there are no indications in the most widely-known guidelines regarding its use in AIS, although this might change in a future revision of the major guidelines. Even if the patient does not meet the criteria for rtPA due to oral anticoagulation, they can still be candidates for MT7. The clinician must keep in mind that hemodialysis can be used to reverse the effect of any anticoagulant if the patient was otherwise a candidate for rtPA56.
Thrombolysis and head trauma
Trauma, as a contraindication for rtPA, must be classified as either major non-intracranial trauma or as severe intracranial trauma. In AIS, patients with recent (14 days or less) major trauma that does not involve the head, may be carefully considered for rtPA, weighing the risk of bleeding from trauma-related injuries against the severity and potential disability due to a stroke (Class IIb, level of evidence C). Meanwhile, rtPA is contraindicated for patients with recent (within 3 months) severe head trauma and AIS (Class III, level of evidence C)7.
Thrombolysis and recurrent stroke
The previous guidelines had a contraindication for rtPA within 4.5 h after symptom onset in patients with prior stroke in the previous 3 months, due to the alleged increased risk of intracranial hemorrhage7. However, recent studies have reported that rtPA for patients with small infarct volumes and functional independence might be considered even within 3 months of the previous stroke, with a low risk of symptomatic intracranial hemorrhage60,61.
Stroke beyond 4.5 h
MT is indicated in those with proximal LVO of anterior circulation and, with a lower level of evidence, LVO in the posterior circulation. There is evidence of the benefits of MT in selected patients62. HERMES3 is a meta-analysis of five clinical trials (MR CLEAN63, ESCAPE28, REVASCAT30, SWIFT-PRIME29, and EXTEND-IA64) that demonstrated the safety and efficacy of MT for LVO. In this analysis, 46% of selected patients treated with MT + rtPA achieved a mRS of < 2 at 90 days, when compared the 27% of those who only received rtPA (odds ratio [OR]: 2.49, confidence interval [CI] 95% 1.76-3.53; p < 0.0001). This is how the current criteria for the "classic" window of 6 h for MT came to be. Table 4 summarizes the criteria for MT in these patients, in accordance with the 2019 update of the North American guidelines7.
Table 4 Criteria for treatment with mechanical thrombectomy in < 6 h
| 1. Pre-stroke modified Rankin scale 0-1 2. Internal carotid artery oclussion, or at the M1 segment of the middle cerebral artery 3. Age > 18 years old 4. NIHSS ≥ 6 5. ASPECTS ≥ 6 Treatment can be initiated (groin puncture) within 6 h of symptom onset |
NIHSS: National Institute of Health Stroke Scale, ASPECTS: Alberta Stroke Program Early CT Score.
Reperfusion therapy from 6 to 24 h
Two clinical trials published in 2018 changed the paradigm of the treatment of AIS. Both of these studies are based on the concept of mismatch and had to be stopped early due to benefit from thrombectomy. DEFUSE 338 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) demonstrated that if a patient with LVO has NIHSS > 6, and a small ischemic core (< 70 ml) with an extensive penumbra (penumbra: core ratio > 1.8), there is a great possibility of benefit with MT from 6 to 16 h from symptom onset. DAWN37 (Thrombectomy 6-24 h after stroke with a mismatch between deficit and infarct) required LVO with NIHSS > 10, small core (stratified in different age groups, from < 21 ml to < 51 ml), which would cause a clinical-radiological mismatch. Both studies required the patient to be previously independent (mRS < 2 for DEFUSE 3, and mRS < 1 for DAWN) and used PWI. In DEFUSE 3, patients treated with MT at a median of 11 h, had a 28% increase in functional independence and an additional 20% absolute reduction in death or severe disability. These two trials used a proprietary automated software (RAPID) to determine the ischemic core and penumbra. The median time from symptoms to enrollment was 12.5 h in DAWN and 11 h in DEFUSE 3, and core volumes were < 10 mL; it is worth noting that the patients in both studies were very slow progressors. Effectiveness for late window thrombectomy was maintained across all subgroups of patients, including those defined by time, age, mode of presentation, and ASPECTS score. Undoubtedly, the results of DAWN and DEFUSE 3 have shown great benefit from MT in selected patients with AIS within a therapeutic window of 6-24 h. However, as suggested by the large treatment effect size observed in both trials, the clinical and imaging criteria were probably too strict35,36. In addition, not all hospitals that treat AIS have this specific software required in the inclusion criteria of both studies. However, physicians treating AIS patients should be familiar with this technique and its inclusion criteria, so as to offer the treatment to as many patients as possible, when appropriate.
Wake-up strokes and patients with an unknown time of symptom onset
Wake-up stroke includes patients that wake up with a focal deficit attributed to an AIS, while those that are simply found with an established deficit, with an unknown time from symptom onset, are deemed to have an AIS with unknown time of symptom onset. There are fundamental differences with therapeutic implications in these apparently all-too-similar stroke definitions. WAKE UP (MRI-Guided thrombolysis for stroke with unknown time of onset, 2018)21 trial demonstrated the benefit of rtPA in both of these situations, with a median mRS of 1 after 90 days in the alteplase group, and 2 in the placebo group (adjusted common OR, 1.62; 95% CI, 1.17-2.23; p = 0.003); the trial used a DWI-FLAIR mismatch in the ischemic region to determine candidates for rtPA. The trial was stopped early due to funding issues, but the benefit was clear among those who received rtPA, as opposed to placebo.
EXTEND65 (Thrombolysis Guided by Perfusion Imaging up to 9 h After Onset of Stroke) was published in May 2019, which assessed rtPA in a period defined by the researchers as the late window (4.5-9 h from symptom onset) in 225 patients. The primary outcome (mRS 0-1 at 90 days) occurred in 35.4% of the patients in the alteplase group and in 29.5% of the placebo group (adjusted risk ratio, 1.44; 95% CI, 1.01-2.06; p = 0.04). This trial evaluated cerebral perfusion using CTP. They concluded that these imaging modalities increased rtPA use, with the risk of more symptomatic hemorrhages (6.2% in the rtPA group and 0.9% in the placebo arm).
In patients with AIS on awakening or with a known time of onset and who have MRI DWI/FLAIR mismatch, the European guidelines suggest rtPA over no rtPA, even when the quality of evidence is moderate, and the strength of recommendation is weak66.
Stroke units
Stroke units are equipped with continuous non-invasive monitorization, with trained personnel, and coordinated by neurologists who lead a multidisciplinary team. The AHA/ASA has demonstrated, with a level of evidence I, a reduction in mortality or dependency of 18%, compared to the hospitalization in general wards23,67. A British study reported that physicians working at stroke units are more likely to treat patients based on up-to-date evidence, with better functional outcomes68. There is also a Cochrane69 review with similar results, with independent outcomes for age, gender, and stroke severity. To date, there are few stroke units in our country, and, even in developed countries70, the characteristics of stroke units are still not standardized, leading to varying results.
Future prospects
TNK is a new-generation fibrinolytic agent. This drug represents a promising alternative to rtPA as a thrombolytic treatment for patients with AIS. An important advantage of TNK over rtPA is the manner of administration, as TNK is applied in a single bolus, compared to rtPA that requires a bolus and a 60-min infusion71. EXTEND-IA TNK72 (TNK vs. rtPA before Thrombectomy for Ischemic Stroke) randomized 202 patients with AIS who candidates for intravenous thrombolysis (either rtPA or TNK) were followed by MT. The primary endpoint was the demonstration of reperfusion greater than 50% of the ischemic territory, or absence of any retrievable thrombus. This outcome was reached in 22% of the TNK arm, and in 10% of the rtPA group, with no differences in symptomatic intracranial hemorrhage. The accepted dose for TNK to treat AIS depends on whether or not the patient will receive MT: 0.25 mg/kg if the patient will receive MT, and 0.40 mg/kg if the patient will not receive MT, with a maximum dose of 25 mg in a single intravenous bolus7.
A number of trials mean to establish whether patients with less favorable imaging findings (i.e., with no DAWN or DEFUSE-3 criteria) could receive some degree of benefit from MT. Ongoing studies TESLA, SELECT 2, TENSION, and IN EXTREMIS, all use ASPECTS to define if MT is reasonable and safe for moderate to large infarcts, defined as ASPECTS 2-573. Depending on the results, the number of candidates for reperfusion therapy could be greatly increased.
Conclusions
Intravenous thrombolysis continues to be the cornerstone of reperfusion therapy for AIS. LVOs are to be rapidly diagnosed and may have an even greater benefit with a combined therapy (rtPA + MT). Neurologists have a fundamental role in suspecting of LVO and confirming this suspicion using advanced neuroimaging; if proximal LVO is confirmed and the patient is within the therapeutic window from rtPA, the infusion must begin as soon as possible, while simultaneously alerting the team responsible for MT. The main function of the neurologist in the context of AIS is to not delay rtPA and to recognize LVO in a timely manner, so as to offer MT to all possible candidates, to achieve the maximum possible benefit. The implementation of algorithms to act according to the reality of each one of our hospitals is central for the proper diagnosis and treatment of patients with AIS caused or not by LVO.
Lessons to take home
Only 10-20% of AIS is due to LVO, but these are responsible for the greatest morbidity and mortality burden.
For patients with symptom onset between 3 and 4.5 h, one must mind the additional warning criteria for rtPA
In a patient with more than 4.5 h and LVO, must consider MT
In patients with symptom onset of < 4.5 h, no perfusion imaging is required
In a patient with 6-24 h of symptom onset, perfusion imaging should be performed to assess core and penumbra, so as to determine if the patient is a candidate to MT by DAWN or DEFUSE 3 criteria.
Wake-up stroke patients can receive rtPA only if they have a DWI-FLAIR mismatch, according to the WAKE-UP criteria
TNK is a promising alternative to rtPA and seems to have a better safety profile and to be easier to administrate. Its use in the clinical field is gradually increasing
Lessons to take home for physicians in Latin America
8.. For patients with either wake-up stroke or stroke of undetermined time, should the receiving hospital only have NCCT, patients with ASPECTS 7-10 would benefit from a referral to a third-level hospital, where advanced neuroimaging may be implemented
9.. Within the first 4.5 h from symptom onset, only NCCT with ASPECTS 7-10 is required to start rtPA
10.. Given the possibility, the physician should obtain at least NCCT and CTA before referring a patient to a third-level hospital. CTP is required for AIS > 4.5 h, and it should be obtained if possible
11.. If the physician detects a high NIHSS (≥ 6), they should consider referring the patient to a center with MT. rtPA must not be withheld if the patient meets criteria for thrombolysis











 nueva página del texto (beta)
nueva página del texto (beta)



