Introduction
Ketosis is a state of metabolism where the body uses fats as a source of energy1. Prolonged fasting (> 72 h) and ketogenic diets1, which can be characterized by ingesting large amounts of fat and restricting carbohydrates. Ketosis should be differentiated from ketoacidosis characterized by metabolic acidosis1,2. In general, we can describe three main ketone bodies, acetoacetate (AcAc), β-hydroxybutyrate (βOHB), and acetone. In typical conditions, they range between 0.05 mmol/L and 0.4 mmol/L1, but they can reach 1-2 mM after 2 days with a ketogenic diet, and range between 6 and 8 mM during prolonged fasting.
Neonates usually present high levels of βOHB ranging between 3 and 4 mM during the first days of birth3. Recently, several studies on the applications of ketone bodies in different pathologies have been published, but their applications come from years ago4. Possibly, the first ketogenic therapies were used to treat epilepsy and came from the Greeks4.
Between 1911 and 1921 continued fasting treatments in adults and children, and in 1921 in the Emergency Center in Chicago, Ronald T. Woodyatt discovered fasting and high-fat diets can increase acetone and βOHB levels4. In 1970, Robert C. Atkins developed a diet and a treatment for a young girl with epilepsy, showing a decrease over 3 years5.
In 2001, doctors Kristopher J. Bough and Douglas pointed out the importance of treating epilepsy with ketogenic diets since their biggest advantage over drugs was that they did not have neurotoxic effects, unlike valproic acid and phenytoin4. Finally, in 2006, the ketogenic diet was formalized and given a name5.
Ketogenesis
Ketogenesis is one of the metabolic pathways in the body that provides an alternative source of energy using fatty acids6. We know that most of the ATP production is carried out in the mitochondria and for each glucose molecule, 32 ATP molecules are formed7. When glucose is scarce, ketone bodies are produced in the liver and transported into the cell by monocarboxylate transporters (MCT's) also known as proton MCT's. Ketone bodies can cross the blood-brain barrier and be used as energy, especially when the levels of body ketones are high, they can provide energy to the brain and meet 60% of its requirements8. When the body cannot use glucose for energy, either due to insulin resistance or a decrease in bloodstream glucose, the liver breaks down free fatty acids and produces acetoacetate and βOHB9. The ketone body that predominates in the blood and the urine when ketosis occurs is βOHB9,10 (Fig. 1).
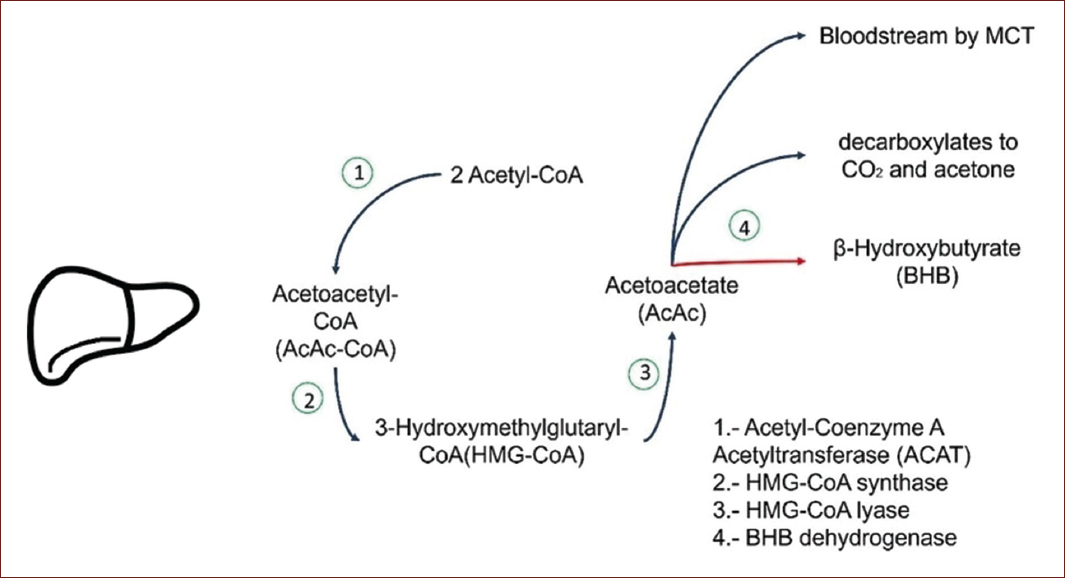
Figure 1 Ketogenesis. The two acetyl-CoA molecules condense to form acetoacetyl-CoA by a thiolase reversal reaction, acetoacetyl-CoA with acetyl-CoA by 3-hydroxy-3-methylglutaryl-CoA synthase forms 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). After this reaction, acetyl-CoA is cleaved from HMG-CoA by 3-hydroxy-3-methylglutaryl-CoA lyase, leaving acetoacetate free11,12.
Even though ketone bodies are produced in the liver, this organ is not able to use them as it lacks succinyl-CoA: 3-CoA transferase (SCOT) which is essential12. Pathological concentrations of ketonic bodies are presented in the diabetic population with poor glucose control or partial insulin resistance11,13.
Once this process is completed in the liver, the pathway continues in extrahepatic tissues (Fig. 2). Once the ketone bodies are produced, they will be carried by MCTs14. The family of MCTs is formed by 14 members, with MCT1-4 being the ones responsible for proton-monocarboxylate transport, as L-lactate, pyruvate, and short-chain fatty acids across the plasma membrane14. MCT1 and MCT4 are the two main lactate transporters under physiological and pathological conditions. MCT1 is expressed in almost all tissues of the body and its main physiological function is to facilitate the entry or exit of lactate from cells, the difference with MCT4, which is more specific since it is found in glycolytic tissues such as white skeletal muscle fibers, astrocytes, white blood cells, and chondrocytes14.
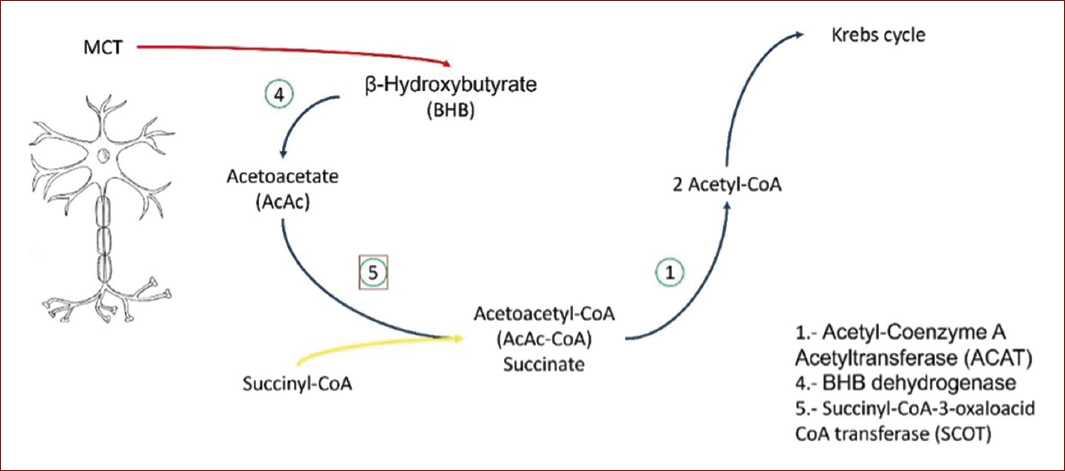
Figure 2 Ketolysis. Acetoacetate is activated into acetoacetyl-CoA by succinyl-CoA-acetoacetate-CoA transferase. CoA transferase is derived from succinyl-CoA to form acetoacetyl-CoA. When added CoA, thiolase cleaves acetoacetyl-CoA to form two acetyl-CoA and thus be oxidized in the citric acid cycle11.
MCT1 localization is in the apical and basolateral membrane, MCT2 is found in the kidney, liver, skeletal muscle, heart, brain, spleen, and testis, and their subcellular localization is in the apical and basolateral membranes, MCT3 is found in the pancreas and retinal pigment epithelium, and their subcellular localization is only in the basolateral membrane. MCT4 is found in the kidney, skeletal muscle, and intestine, and their subcellular localization is only in the basolateral membrane15.
Regarding its presence in the nervous system, we can find that MCT2 is the one that is most expressed in neurons, while MCT4 is in astrocytes16. Although there is a presence of MCT1 in astrocytes, microvascular endothelial cells, and oligodendrocytes16. Even the participation of MCT in astrocytes is related to memory through the lactate transport by MCT116. Regarding its presence in the nervous system, we can find that MCT2 is the one that is most expressed in neurons, while MCT4 is in astrocytes16.
One of the greatest difficulties in the study of ketone bodies is how to raise them without having to fast. Faced with this situation researchers sought to develop a ketone body precursor or analog capable of raising ketone levels within minutes17, they could be a key element for the future therapies.
When ketosis is induced through a ketogenic diet, the production of ketone bodies increases, and βOHB reach plasma levels between 4 and 5 mM. In these ranges, it can have a neuroprotective effect, in some studies, it has been observed that it has such an effect on diseases such as Alzheimer's, Parkinson's, and amyotrophic lateral sclerosis18,19. However, a diet ketogenic can induce changes in the gut microbiome too. Recently was described that microbiota could be modified due to the lack of consumption of grains, vitamins, minerals, and iron19. Another observation that has been made in pediatric epileptic patients, is that consuming a ketogenic diet as a treatment has a beneficial effect on the microbiota, by increasing the Bacteroidetes by 24%, even in obese patients who undergo a ketogenic diet to lose weight, it has been observed that their microbiota has positive changes19.
However, there are limitations for some patients who want to use a ketogenic diet. Especially, patients who live with liver failure, pancreatitis, congenital disorders of fat metabolism, deficiency of carnitine palmitoyl transferase, carnitine translocase, porphyria, and pyruvate kinase will not be candidates for a ketogenic diet20. An additional limitation of the implementation of a ketogenic diet is that during the adaptation period, they could have side effects such as fatigue, headache, dizziness, nausea, vomiting, constipation, and low tolerance to exercise, even this symptomatology has been called the keto flu20.
Diet attachment is complicated, due to the difficult period of adaptation and limitation of food products. In this sense, a synthetic ketone monoester (R)-3-hydroxybutyrate and (R)-1, 3-butanediol has been proven to increase the levels of ketone bodies in plasma, as you can see in table 1 in comparison with other methods21,22, with the advantage that the ester can elevate the ketones level in a short period21, for example, it was observed that a single dose of 573 mg/kg of this monoester induces an increase in of ketone bodies around 3 mM after 10 min, reaching a maximum concentration of 6 mM after 20 min21.
Table 1 Ketone bodies levels. Quantification of the ketone bodies in different nutritional events
| Ketone bodies | Basal levels (mM) | Fasting or diet levels (mM) | Ester (mM) |
|---|---|---|---|
| β-Hydroxybutirate | 0.02-0.05 | 4-5 | > 6-8 |
| Acetoacetate | 0.05 | 1-3 | > 6-8 |
| Acetone | 0.02-0.05 | 0.5 | - |
Although they are not the only mechanisms by which we can increase the levels of ketone bodies. They can be described as two exogenous methods to perform this action, one of them ketogenic salts and the other method is with the use of esters or monoesters, the difference between them can be due to the levels increase much more with the use of the monoester than with salts23.
The ester is administrated orally, when it reaches the small intestine, the monoester will be subjected to hydrolysis by carboxylesterases and esterases located in this part of the intestine, this will give rise to D-β-hydroxybutyrate and R-1-3-butanediol, these two compounds plus acetoacetate will be metabolized by alcohol and aldehyde dehydrogenase in the liver24. Some effect observed with the use of esters, especially in rats, is that their application has a direct effect on glucose levels causing a decrease of almost 50%, like insulin levels which is a positive effect on insulin sensitivity2.
One of the advantages of applying a monoester, apart from increasing the levels of ketone bodies in a very short time, is that it will not generate chronic changes in pH25. In mice studies, it was observed that the administration of a monoester increases the levels of acetyl-CoA, some intermediates of the tricarboxylic acid (TCA) cycle25.
Both the diet and the monoester are effective; however, a relevant difference is that the diet takes time to induce ketosis22.
Implications of ketone bodies in nervous tissue
In several investigations where ketone bodies have been used, it has been observed that they have a neuroprotective effect on nervous tissue26. However, the growing evidence is related solely to the treatment of neurodegenerative diseases.
In the nervous system, ketone bodies will also come from astrocytes, which have the particularity of being the largest glucose sequestrant and being responsible for metabolic regulation with the capacity to synthesize ketone bodies like hepatocytes27. The local production of ketonic bodies is not the only protective source of ketone bodies; in vivo experiments in rodents show that the increase in circulant levels has a neuroprotective effect, neurogenesis is also potentiated, together with a decrease in cerebral edema as well as a reduction in infarct zones, especially in those models of hypoxia27.
In the case of epilepsy, it was shown that ketone bodies increase glutamate, levels altering and producing an increase in inhibitory neurotransmitters, especially g-aminobutyric acid28. However, this excitability suffered by neurons is also affected by ketone bodies, especially acetoacetate and β-hydroxybutyrate, which influence potassium channels dependent on ATP (KATP channels) with the presence of these ketone bodies they can open, producing depolarization of the cell29.
Similarly, the levels of ROS are reduced when ketone bodies are oxidized. It is thought that this is due to a decrease in the reduced form of coenzyme Q and because they can increase the proportion of NADPH/NADP+. By these mechanisms, the ketone bodies intervene as antioxidant agents29. This effect of altering the mitochondria is reflected in some models of neuropathies in peripheral nerves, it is believed that due to the accumulation of ROS and producing lipoperoxidation, affecting mitochondrial functions, it cause axonal degeneration, but when they exposed to a ketonic environment, some genes expressions of complexes I and IV, NADH dehydrogenase, and cytochrome C oxidase, respectively, of the respiratory chain reducing the generation of ROS and improving mitochondrial functions30.
Another mechanism where ketone bodies are involved is in the reduction of oxidative stress damage related to the epigenetic effect of β-hydroxybutyrate. This ketone body regulates histone deacetylase (HDAC) 1, 3, and 428,29, by inhibiting HDAC, it increases the transcription of genes that are related to oxidative stress, especially FOXO3A, SOD2, and metallothionein 2, and protect against free radicals28,29.
In the case of Alzheimer's, which is linked to mitochondrial dysfunction, hypometabolism, inflammation, oxidative stress, calcium concentrations, autophagy deregulation, hypoperfusion, arteriosclerosis, changes in the blood-brain barrier, and an increase in β-amyloid (Aβ) deposits forming amyloid platelets which in high concentrations produces neurotoxicity resulting in loss of neurons and its counterpart having low levels promotes neurogenesis31.
When the body undergoes ketosis, there is an improvement in the production of ATP, restoring the functions of the mitochondria, as mentioned before, ketone bodies decrease ROS, inflammation, and decrease Ab levels31,32.
In the case of Parkinson's disease, it is mentioned that during this disease, there is an accumulation of α-synuclein, the death of dopaminergic neurons, especially in the black matter of the midbrain and it has also been possible to verify that in the mitochondria, the complex I of the respiratory chain is affected33,34. Other pathological mechanisms include Lewy bodies and neurites' presence and the characteristic symptoms related to motor dysfunctions35. Some models in vivo have observed that ketone bodies protect against the neurotoxin 6-hydroxydopamine (6-OHDA), and the metabolic change induces the expression of genes with antioxidant properties36.
In addition, an anti-inflammatory effect was seen after ketone bodies administration by inhibiting the NLRP3 inflammasome. In spinal cord injuries, it has been found that the neurons express NLRP3 and when this inhibited a rapid recovery ensues37. This inflammatory complex is formed by the NLRP3 protein, the union of apoptosis-associated speck-like protein (ASC) and procaspase-1. It activates the IL-1β and IL-1838,39. β-hydroxybutyrate inhibits said the formation of the inflammasome, which, is being increased and present in the nervous system in avoiding the potassium cells release and prevents oligomerization with ASC39. Evidence suggests that the effect of ketone bodies is specific against this inflammasome since studies on NLRC4, found these effects are not replicable39.
On the other hand, evidence suggests potential benefits for migraine and psychiatric disorders. Studies with humans or animals reported that being exposed to a ketogenic diet or some supplement can help patients suffering from schizophrenia, anxiety, and even depression27.
Among some types of acute damage where the interaction of ketone bodies has been studied, we can mention spinal cord damage and brain trauma. As previously mentioned, ketone bodies produce an anti-inflammatory and antioxidant effect. In studies of spinal cord injury in murine models, it has been observed that both NLRP3 and some proinflammatory cytokines, such as IL-1β, IL-6, and TNF- α decrease their expression40, these mechanisms are involved in both recovery and pain from these conditions25,41. In brain trauma, the anti-inflammatory and antioxidant effect also protect from ischemia and reperfusion42.
Another possible cause of the protection provided by ketone bodies in this type of injury is that, due to their presence, the concentrations of succinate increase, this being an intermediary of the citric acid cycle as a source to be able to stabilize HIF-1α, eliminating the activity of the prolyl hydroxylase enzyme, which stabilizes HIF-1α and consequently, protects the organ from the ischemia41,43.
Finally, ketone bodies could inhibit tumor growth studies on glioblastoma, suggest they promote apoptosis of tumor cells44. More specifically the interaction of acetoacetate as a regulator of the mitochondrial uncoupling protein 2 (UCP2) which, by increasing its expression, stops the production of ATP and therefore the intermediates of citric acid accumulate, producing glycolytic inhibition44. In the same way, it has been reported that the combination of ketones/radiation increases survival in murine models, in another treatment, the combination of ketones with chemotherapy demonstrates an effect on the blood vessels of the tumors reducing their density, likewise intracranial hemorrhages are decreased and in combination with bevacizumab, it is observed an antitumor effect in murine models45,46. Knowing that glioblastoma requires the glycolysis pathway for tumor growth, subjecting patients to ketogenic diets produces an inhibitory effect on glycolysis and glutaminolysis, in that way, the glioblastoma cells cannot proliferate due to the deficiency of both metabolic routes47.











 nueva página del texto (beta)
nueva página del texto (beta)


