1. Introduction
According to the World Health Organization (WHO) there are 1,710 million of people suffering musculoskeletal pathologies, where most of them have mobility and dexterity limitations, low social interaction, reduction in functional capacities and deterioration of the mental state, becoming the main causes of disability and early retirement, affecting the cost and access to health care (Cieza et al. 2020; Hartvigsen et al., 2018). Musculoskeletal diseases include more than 150 disorders that can affect joints, bones, muscles, tendons, spine; additionally, organs such as skin, blood vessels, kidney, heart, lungs and central and peripheral nervous system can also be compromised (World Health Organization, 2021b). Among these conditions are rheumatic diseases caused by the accumulation of toxic substances in the body, injuries and infections, generating inflammation, degeneration or alteration of tissues, pain, stiffness and limitation of movement (Briggs et al., 2016). Other pathologies such as osteoarthritis, osteoporosis and fibromyalgia can be found, affecting population of all ages and generating different degrees of disability, significantly affecting people's quality of life (Briggs et al., 2016; WHO, 2021c).
There is a deficit in the provision of services focused on the treatment of musculoskeletal disorders, due to the lack of equipment to perform the appropriate therapeutic treatments. Currently, in low-income countries more than 50% of people do not receive adequate treatment or do not have access to services they require, since it is not considered a priority factor, causing serious consequences that over time make this type of disorders increase (Hartvigsen et al., 2018; WHO, 2021d; 2021c). Evidence has been found on the implementation of electromagnetic fields (EMF) as non-invasive treatment for musculoskeletal conditions, where they reduce the reaction of the immune system, obtaining improvement in inflammation, tissue degeneration, pain, muscle tension and reduction in pharmacological treatments (Abdulla et al., 2019; Hu et al., 2020). Additionally, EMF have antiedematous, vasodilator and angiogenic effects within their therapeutic utilities (Hu et al., 2020; WHO, 2021a). EMF are made up of electric and magnetic waves that travel simultaneously and propagate at the speed of light (Rozo-Clavijo et al., 2017). The higher its frequency, the greater the amount of energy carried by the wave. EMF are classified into two large groups: ionizing radiation (with the ability to break the bonds between molecules) and non-ionizing radiation (Alonso-Fustel et al., 2011). The WHO subdivides non-ionizing EMFs into magnetic fields (MF), static electromagnetic fields (SMF), extremely low frequency magnetic fields (ELF-MF) up to 300 Hz, intermediate frequency fields (IF) with frequencies from 300 Hz to 10 MHzand radio frequency fields (RF) with frequencies from 10 MHz to 300 GHz. I has been evidenced that EMFs do not cause harmful effects either patient, the therapist or any outside person (Mattsson & Simkó, 2019).
Over time and through different studies and experiments it has been shown that EMF are present in the functioning of different systems of living organisms, starting from the activity of cellular systems and the exchange of ions to more robust systems (Bachl et al., 2008). In accordance with technological advances and the need to find alternative treatments for pain, inflammation and tissue regeneration, different experiments, tests and interventions have been developed, where exogenous magnetic and EMF have been used, varying signal shapes, magnetic flux densities, frequencies, among other parameters that have significant impact on biological processes, generating advances to improve therapeutic treatments. Paolucci et al., in their review article focused on the use of EMF in the management of musculoskeletal pain, showing different experiments implemented in people with osteoarthritis, back and neck pain, tendonitis, fibromyalgia and myofascial pain (Paolucci et al., 2020). It is highlighted that in these studies the most used magnetic field was the pulsed electromagnetic field (PEMF) with frequencies between 2 and 100 Hz and a field intensity from 5 µT to 180 mT, evidencing that PEMF relief pain in patients (Paolucci et al., 2020); however, there is a need for standardized protocols that give clear guidelines on the ways to use EMF in structured therapies (Mattsson & Simkó 2019; Paolucci et al., 2020). Accordingly, the aim of this article was to carry out a review of studies that have applied EMF and developed magnetic devices to treat pathologies of the musculoskeletal system.
2. Materials and methods
2.1. Search strategy
Science Direct, Scielo, IEEE, PreQuest, Pubmed, among other databases were searched from 2016 to 2020 for studies involving the use of EMF for the treatment of musculoskeletal diseases, pain management and osteoarthritis. Additionally, this search was performed to report the most recent scientific and technological advances in the devices developed to apply EMF. Keywords such as electromagnetic fields, health, osteoarthritis treatment, musculoskeletal pain, bone and muscle treatment with electromagnetic fields, magnetotherapy technologies, magnetotherapy equipment, electromagnetic field non-invasive treatment, electromagnetic field therapy, electromagnetic field signals and pulsed electromagnetic field were used to look for the information. All keywords were implemented using Boolean Operators (AND and OR).
2.2. Data abstraction and quality assessment
A total of 100 articles were found during search; however, 36 articles were selected, where data was recorded by a bibliographic matrix considering variables such as type and name of article, year, pathology treated, therapy used, frequency range - magnetic flux density and description of tests (intensity of the sessions). Data were obtained by tabulation to organize the information and identify the relevant information about application of EMF. A categorization was implemented to assess pathologies treated with EMF, grouping studies focused on bone, muscular, nervous or pain approach and some additional contributions. Additionally, information about the application of EMF to biomaterials used to restore musculoskeletal disorders was collected and analyzed. Here, the main molecules and proteins synthetized by cells under magnetic stimulations were highlighted.
3. Results
A total of 50% of studies focused on the treatment of pathologies in the joint system, cell stimulation and its influence on bone regeneration, 20% evidenced pathologies of the musculoskeletal system, 11% showed pathologies of the nervous system and pain treatment and 19% focused on application of EMF to biomaterials, devices and signals used for this therapeutic purpose. Most of the studies that carried out trials of the application of EMF in the management of any pathology were developed in accordance with Declaration of Helsinki (Casalechi et al., 2020; Elshiwi et al., 2019; Mohajerani et al., 2019; Multanen et al., 2018) or endorsed by institution's ethics committee. Some studies used measurement scales to know the level of improvement in both pain, inflammation or disability of patients, such as visual analog scale (VAS) (Hattapoğlu et al., 2019; Kopacz et al., 2020), Western Ontario and McMaster Universities Osteoarthritis Index ( WOMAC) (Bagnato et al., 2016; Chen et al., 2019), Fibromyalgia Impact Questionnaire (FIQR) (Paolucci et al., 2016), Generalized Pain Index (WPI) (El Zohiery et al., 2021), Numerical Pain Rating Scale (NRS) (Abdulla et al., 2019; El Zohiery et al., 2021), Health Assessment Questionnaire (HAQ) (Paolucci et al., 2016) and American Spinal Injury Association (ASIA, Tarlov Scale (Ross et al., 2017). It is important to highlight that there were several modalities of EMF therapy approved by the Food and Drug Administration, where one of them was the PEMF, which is one of the most used therapies in the studies analyzed (Hu et al., 2020; Iwasa & Reddi 2018; Pesqueira et al., 2018; Ross et al., 2017; Vicenti et al., 2018). Magnetotherapy is another therapy which may be implemented with three different approaches: using an external magnetic field directly in the injured area, activation and/or magnetic stimulation in the implementation of tissue engineering and implantation of biomaterials that respond magnetically (Pesqueira et al., 2018).
3.1. Treatments focused on joint system pathologies
It was possible to identify that there are two types of fields used to treat bone pathologies PEMF and MF. The former is mainly focused on pathologies such as osteoarthritis (Hu et al., 2020), osteoporosis (Wang et al., 2019), osteonecrosis, infected pseudoarthrosis (Qiu et al., 2020), cervical herniation disc (Hattapoğlu et al., 2019), temporomandibular disorders (Kopacz et al., 2020) and fractures (Mohajerani et al., 2019). In these studies, the range of frequencies used are between 3 and 100 Hz, and the PEMF density varied from 0.1 to 30 mT. Regarding the MF, studies have been used this type of stimulation to treat osteoarthritis and cartilage lesions (Hattapoğlu et al., 2019), low back pain due to discopathy (Taradaj et al., 2018) and rheumatoid arthritis (Zwolińska et al., 2016). Here, the range of frequencies used to apply the MF have been created between 20 and 50 Hz whit a field density from 49.2 μT to 10 mT. In Table 1 is possible to observe in detail the type of field applied to treat pathologies affecting joint systems, emphasizing in pathology type, therapy applied, frequencies and field intensity and stimulation scheme performed.
Table 1 Summary of magnetic stimulation focused on joint system pathologies.
| Pathology | Therapy | Frequency range; magnetic density |
Stimulation scheme |
Main outcome | Reference |
|---|---|---|---|---|---|
| F, OA, OP, ON and TD |
PEMF |
< 100 Hz; 0.1 mT - 30 mT |
--- |
PEMF are a stand-alone or adjunctive treatment for treating musculoskeletal disorders. |
(Hu et al., 2020) |
| OA, SIS, LM and FM |
PEMF, ELF-MF, PRFE and EMTT |
OA: 50 Hz; 100 µT - 10 mT SIS: 50 Hz; 20 mT LM: 4000 Hz; 5 -15 G FM: 0.1 - 64 Hz; 40 µT |
OA: 1 hour/5 per week/2-weeks. SIS: 20 min/2 per week/4-weeks. LM: 20min/once a day/3-weeks FM: 8 min twice a day/12-weeks. |
ELF-MF are used to relief musculoskeletal pain. EMF is a well- tolerated and effective method, which can be integrated with rehabilitation to treat chronic and acute pain in musculoskeletal diseases. |
(Paolucci et al., 2020) |
| IN |
PEMF |
15 Hz; 1 G (1mT) |
--- |
PEMF may treat infected nonunion as an adjuvant therapy by controlling infection, inducing bone consolidation. |
(Qiu et al., 2020) |
| TMDs |
LT-EMF- CT |
Red light 640 nm and infrared light 830 nm - pulses in the range 180 - 195 Hz, frequency from 12.5 to 29 Hz. Groups from 2.8 to 7.6 Hz. Series from 0.08 to 0.3 Hz. |
15 treatments, 10 minutes, 3 times/week |
LT-EMF treatment demonstrate an analgesic effect in terms of the overall discomfort during temporomandibular disorders. |
(Kopacz et al., 2020) |
| OP |
PEMF |
- 72 Hz; 2.85 mT - 8 Hz; 3.82 mT |
- 10 h/day - 12 weeks. - 40 min/day - 24 weeks |
PEMF stimulate osteoblastogenesis and suppress osteoclastogenesis and influence the activity of bone marrow mesenchymal stem cells and osteocytes. |
(Wang et al., 2019) |
| MF |
PEMF |
40 Hz; 1 mT |
6 h immediately post-surgery. 3 h daily for the next 6 days. 1.5 h daily for the next 6 days |
PEMF therapy postoperatively leads to increased bone density, increased formation of new bone and decreased pain. |
(Mohajerani et al., 2019) |
| NSI, OA, CI and MSI |
MT |
NSI: 2 a 10 Hz OA: 20-30 Hz, CI: 30 Hz MSI: 10-20 Hz. |
From 15 min to 1 h. 20-30 sessions. |
MT has an analgesic effect in older adults diagnosed with osteoarthritis. |
(Mori, 2019) |
| L-DP |
MF |
50 Hz; 10 mT, 50 Hz; 5 mT, 195 Hz; 49.2 μT |
20 min, 5 times a week, for 3 weeks |
MF reduces pain symptoms and leads to an improvement of functional ability in patients with L-DP. |
(Taradaj et al., 2018) |
| KJD |
PEMF |
- 0.05 mT - 1.5 mT - 75 Hz; 1.5 mT |
- 6 h/day for 90 days - 4 h/day for 60 days |
PEMF could be used as an adjunct after an arthroscopic knee procedure to control the joint post-operative inflammatory state. |
(Vicenti et al., 2018) |
| OA |
PEMF |
--- |
Exposure time from 30 min to 12 h. Exposure duration from 3 to 6 weeks |
PEMF could alleviate pain and improve physical function for patients with knee and hand OA, but not for patients with cervical OA. |
(Wu et al., 2018) |
| N, LM, F, OP |
PEMF |
- 15 Hz; 1.19 mT - 1.5 Hz; 0.68 mT - 15 Hz; 1.19 mT |
- 8 h/day for 6 months - 8 h/day - 2 h/day for 90 days - 6 - 12 months |
PEMF therapy has been shown to be effective in clinical settings as an adjunct to lumbar and cervical intervertebral fusion and for long bone nonunions. |
(Waldorff et al., 2017) |
| OA |
PEMF |
27.12 MHz |
12 h/day for 1 month |
PEMF therapy is effective for pain management in knee OA patients. |
(Bagnato et al., 2016) |
| RA |
PEMF MF, MT and SMF |
- PEMF: 12 Hz; 2mT - MF: 5-50 Hz; 10 mT - MT: 180 mT - MF: 5 - 23 Hz; 3 - 7.5 mT - SMF: 72 - 190 mT |
- PEMF: 15 to 30 min - 1-2 times/day - MF: Once a day |
PEMF therapy is effective for pain management in knee OA patients. MF are effective in promoting the treatment of inflammatory diseases and musculoskeletal, nervous system muscle, digestive and urogenital pathologies. |
(Zwolińska et al., 2016) |
| OP |
PEMF |
16, 18, 20, 22 Hz; 30, 32, 34, 36 G |
50 min/day, 6 sessions/week, 25 times |
PEMF induce changes in bone metabolism, especially on the regulation of Wnt/β- Catenin and the RANKL/OPG signaling pathways that could play a role in explaining the effects on bone tissue. |
(Catalano et al., 2018) |
| F, CI, NSI and MSI |
MF |
- 0.6 T - 1.5 T |
- 10 or 60 min |
MF can be utilized for the transplantation of various types of cells and the treatment for various kinds of musculoskeletal and neural tissue disorders. |
(Kamei et al., 2018) |
| RA, WH and KJD |
SMF, MF |
- RA: 50 to 180 mT - 2 mT - KJD: 0.3 mT |
--- |
SMF has been shown to affect osteogenic, chondrogenic and adipogenic cells, which in turn offers new therapeutic opportunities. |
(Marycz et al., 2018) |
| MSI |
AC-EMF |
50 Hz; 180 mT |
15 min |
Physiological role of the EMF enhanced blood circulation might help eliminate the metabolic waste products and endogenous pain producing substances inducing muscle stiffness and pain. |
(Okano et al., 2017) |
| F, OA, OP, CI | EMF, ELF- MF and PEMF |
- F and OP: from 15 to 75 Hz; from 0.1 to 5 mT - OA and CI: from 5 to 150 Hz; from 0.1 to 3 mT |
- Three times a day (45 min every 8 h) for 21 days - 30 min/day - 2 h/day - 12 h/day - 8 h/day |
Values of EMF frequencies, times of stimulation, as well as the microenvironmental niche may affect EMFs’ impact on stem cell proliferation, differentiation, and migration to result in the desired therapeutic outcome. |
(Maziarz et al., 2016) |
| Abbreviations: fractures (F), osteoarthritis (OA), osteoporosis (OP), osteonecrosis (ON), tendon disorders (TD), lumbar myalgia (LM), fibromyalgia (FM), shoulder impingement syndrome (SIS), infected nonunion (IN), nonunion (N), temporomandibular disorders (TMDs), mandibular fractures (MF), nervous system injuries (NSI), cartilage injuries (CI), muscular system injuries (MSI), lumbar discopathy (L-DP), knee joint diseases (KJD), rheumatoid arthritis (RA), wound healing (WH), pulsed electromagnetic fields (PEMF), extremely low-frequency magnetic field (ELF-MF), pulsed radiofrequency electromagnetic field (PRFE), electromagnetic transduction therapy (EMTT), light therapy with electromagnetic field and cryotherapy (LT-EMF-CT), electromagnetic field (EMF), cryotherapy (CT), magnetic fields (MF), alternating current electromagnetic field (AC-EMF), static magnetic fields (SMF) and magnetotherapy (MT). | |||||
Studies in Table 1 evidenced the potential of magnetic stimulation as a safe and non-invasive treatment with good tolerance by patients (Hu et al., 2020), reducing significatively pain level (Hattapoğlu et al., 2019; Taradaj et al., 2018; Wu et al., 2018), providing an analgesic effect with a good emotional state and energy in patients (Mori, 2019). Additionally, it has been elucidated that magnetic stimulation improves the functional capacity in the acceleration and consolidation of fractures (Qiu et al., 2020) and bone mineral density (Mohajerani et al., 2019). The role of magnetic stimulation has been also extrapolated at cellular level; specially, in bone regeneration, musculoskeletal and neural tissue disorders (Marycz et al., 2018), cell transplantation (Kamei et al., 2018), bone formation at the tissue, cellular and subcellular level, favoring the process of osteogenesis (Bachl et al., 2008), cell proliferation, extracellular matrix production, chondrocyte apoptosis and negative regulation of inflammatory cytokine (Vicenti et al., 2018) and reduction of pain and infection (Catalano et al., 2018; Maziarz et al., 2016; Okano et al., 2017; Qiu et al., 2020).
3.2. Treatments focused on musculoskeletal pathologies
Studies focused on treatments and/or trials to heal musculoskeletal pathologies are mainly directed to low back pain diseases (Abdulla et al., 2019; Elshiwi et al., 2019; Nayback-Beebe et al., 2017), fibromyalgia (El Zohiery et al., 2021; Multanen et al., 2018; Paolucci et al., 2016), rotator cuff tendinopathy (Klüter et al. 2018), lesions affecting the musculoskeletal system and soft tissues (Pasek et al., 2016), tendon tissue regeneration (Pesqueira et al., 2018). Magnetic stimulation has been focused on the implementation of PEMF between 30 and 50 Hz and magnetic flux densities from 14 µT to 12 mT and MF between 3 Hz and 3 KHz and magnetic flux densities from 0.25 µT to 80 mT. Other kind of magnetic stimulations have been applied such as EMF, PEMFs, TENS, ELF-MF, among other (Table 2). Treatments implemented do not produce heat and do not interfere with nerve or muscle functions. On the contrary, magnetic treatments reduce pain level and disability and increase the range of motion (Elshiwi et al., 2019; Nayback-Beebe et al., 2017). Magnetic stimulation also influences in the increase of blood and lymphatic flow, promoting tissue regeneration (Okano et al., 2017; Ross et al., 2017). MF influence in the expression of tendon genes and inflammatory cytokines which are important factors in tendon regeneration (Pesqueira et al., 2018).
Table 2 Summary of magnetic stimulation focused on musculoskeletal pathologies.
| Pathology | Therapy | Frequency range; magnetic density |
Stimulation scheme |
Main outcome | Reference |
|---|---|---|---|---|---|
| CDH |
TENS and PEMF |
- TENS: 100 Hz with current duration 40 ms - PEMF: 50 Hz; 0.6 mT |
20 min for 5 days a week for 3 weeks |
PEMF therapy in CDH can be used safely in routine treatment in addition to conventional physical therapy modalities. |
(Hattapoğlu et al., 2019) |
| CLBP |
PLFMF |
30 Hz and a pulse duration of 30 ms; 14 µT |
3 sessions/week -20 min session for 6 weeks |
PLFMF is known to be safe, non-invasive, low cost, easy to administer and has no known side effects in the management of patients with CLBP. |
(Abdulla et al., 2019) |
| FM |
PEMF |
- 33.3 Hz; 3-12 μT - 33.3 Hz; 30-150 μT |
8 min/day for 12 weeks |
Low-energy PEMF therapy was not efficient in reducing pain and stiffness in women with FM. |
(Multanen et al., 2018) |
| RCT |
EMTT |
3 Hz; 80 mT |
20 min twice a week during 8 sessions |
EMTT combined with extracorporeal shock wave therapy improves pain and function of patients with RCT. |
(Klüter et al., 2018) |
| RCT and TI |
PEMF, MF and PLFMF |
- PEMF: 75 Hz - PEMF: 5 Hz; 4 μT - MF: 7.8 Hz up to 50 Hz; 0.25 μT up to 0.4 mT - MF: 30 Hz; 1.5 mT -PLFMF: 2 Hz; 350 mT |
- 5-9 hr daily over 4 weeks - 90 min - 30 min vs. 7 days of continuous stimulation 60 min - 8 h |
MT could be an important adjuvant in tendon regeneration, potentially enabling to substitute anti- inflammatory drugs. |
(Pesqueira et al., 2018) |
| CLBP |
PEMF |
--- |
30 min 3 times/week for 4 weeks |
PEMF therapy demonstrated efficacy in studies examining muscle recovery and function in injured athletes, pain control, and treatment of musculoskeletal pain and dysfunction. |
(Nayback-Beebe et al., 2017) |
| FM | PLFMF | 80 Hz; 100 μT | 12 sessions, 3 times/week for 4 weeks |
ELF-MF therapy can be recommended as part of a multimodal approach to reducing pain in FM subjects for short periods and to intensifying the results of drug therapy or physiotherapy. |
(Paolucci et al., 2016) |
| Abbreviations: fibromyalgia (FM), cervical disc herniation (CDH), chronic low back pain (CLBP), rotator cuff tendinopathy (RCT), tendon injuries (TI), electromagnetic field (EMF), transcutaneous electrical nerve stimulation (TENS), pulsed electromagnetic field (PEMF), pulsed low-frequency magnetic field (PLFMF) and electromagnetic transduction therapy (EMTT). | |||||
3.3. Treatments focused on nervous system pathologies
Studies in this category are focused on the treatment of side effects or sequelae that affect functions of the musculoskeletal system obtained with any disease or injury such as strokes (Casalechi et al., 2020), multiple sclerosis (Hochsprung et al., 2021), spinal cord injury (Ross et al., 2017) and chronic pain (Arabloo et al., 2017; Camacho et al., 2019). MF and SMF are mainly used to treat these conditions, implementing frequencies between 5 and 100 Hz with a magnetic flux density of 5 mT, while PEMFs are applied using frequencies from 800 to 900 kHz (Table 3). Magnetic stimulation has been implemented as a promising non-pharmacological treatment for strokes, improving patient mobility (Casalechi et al., 2020). Regarding the spinal cord injuries, magnetic stimulation has decreased inflammatory markers and an increased proliferation and differentiation of endogenous mesenchymal stem cells; additionally, a functional improvement of the sciatic nerve has been obtained (Ross et al., 2017).
Table 3 Summary of magnetic stimulation focused on nervous system pathologies.
| Pathology | Therapy | Frequency range; magnetic density |
Stimulation scheme | Main outcome | Reference |
|---|---|---|---|---|---|
| ST |
SMF |
0 J, 10 J, 30 J, and 50 J |
4 sessions (one dose each week) over 4 weeks |
Photobiomodulation therapy combined with SMF presented positive effects on the functional mobility in ST survivors. |
(Casalechi et al., 2020) |
| CNP |
TMS |
1 - 20 Hz |
--- |
TMS stimulation may be used in the treatment of neuropathic pain in patients with drug- resistant chronic pain. |
(Camacho et al., 2019) |
| MS |
PEMF |
800−900 kHz; 30V |
5 days for 3 weeks - 20 minutes each |
PEMF treatment may be effective in reducing pain in patients with MS, although further research is necessary to confirm its effectiveness. |
(Hochsprung et al., 2021) |
| SCI, MS | PEMF | SCI: 2 Hz; 0.3 mT or 50 Hz, 0.4 mT or 25 Hz, 10 mT MS: 4-5 Hz; 7.5 pT |
6 days or 43 days 30 min/day starting the 7th week after injury |
Mechanisms of action for EMF on SCI include increased blood flow, and changes in ionic currents associated with the electric fields that are induced by EMF, which in turn interact at the plasma membrane and stimulate signal transduction processes and ion transport. |
(Ross et al., 2017) |
| Abbreviations: strokes (ST), chronic neuropathic pain (CNP), multiple sclerosis (MS), spinal cord injury (SCI), neurological disorders (ND), static magnetic field (SMF), transcranial magnetic stimulation (TMS). | |||||
3.4. Technologies associated with electromagnetic stimulation
Devices used in different clinical trials for generating the magnetic stimulation are mostly registered and commercialized (Table 4). The magnetic devices were used to stimulate several systems such as muscular, nervous and bone.
Table 4 Devices used to generate magnetic stimulation.
| Category | Device | References |
|---|---|---|
| ASA magnetic field device (Automatic PMT Quattro pro) |
(Elshiwi et al., 2019). | |
| Muscular | The BEMER 3000 (BEMER Int. AG) |
(Abdulla et al., 2019; Multanen et al., 2018). |
| Duolith SD1 shock wave device (Storz Medical AG) | (Klüter et al., 2018). | |
| The Biomodulator (Senergy Medical Group) | (Nayback-Beebe et al., 2017). | |
| Physicalm (Biotronic Advance Develops) | (Hochsprung et al., 2021). | |
| Nervous | PBMT/sMF device (Multi Radiance Medical) | (Casalechi et al., 2020). |
| Magna Bloc | (Arabloo et al., 2017). | |
| Helmholtz coils | (Ross et al., 2017). | |
| OrthoPulse (IMD) |
(Qiu et al., 2020). | |
| Cyborg Mag generator (Cosmogamma) and Viofor JPS device (Med & Life) |
(Taradaj et al., 2018). | |
| Physio-Stim, Spinal-Stim and Cervical-Stim (Orthofix, Inc.), CMF SpinaLogic and CMF OL1000 (DJO, LLC) and EBI Bone Healing System (Zimmer Biomet, Inc.) |
(Waldorff et al., 2017). | |
| ActiPatch (Bioelectronics Corporation) | (Bagnato et al., 2016). | |
| Viofor JPS (Med & Life) | (Kopacz et al., 2020). | |
| Bone | Dual-channel Chattanooga Intelect Advanced Monochromatic Combo electrotherapy and ASA EASY Quattro PRO (Arcugnano) |
(Hattapoğlu et al., 2019). |
| Portable PEMF device with coil and power supply source generated. |
(Mohajerani et al., 2019). | |
| Biosalus (HSD Srl) | (Catalano et al., 2018). | |
| Neodymium magnets and ferrite magnets | (Marycz et al., 2018). | |
| I-One (Igea) | (Vicenti et al., 2018) | |
| Soken MS (Toride) | (Okano et al., 2017). | |
| Orthopulse II (OSSATEC) and The Biomet EBI Bone Healing System (EBI, LLC) |
(Hu et al., 2020). |
3.5. The role of magnetic stimulation on biomaterials to restore musculoskeletal tissues
Magnetic stimulation has been also implemented to stimulate both cells and biomaterials, which are used to restore tissues that compose the musculoskeletal system. For instance, stromal vascular fraction cells encapsulated into polyethylene glycol (PEG)-based hydrogels have been stimulated with SMF (50 mT) to improve bone regeneration. Results evidenced an increase in the metabolic activity, especially in alkaline phosphatase levels, osteogenic markers (Runx2, Collagen I, Osterix), endothelial, pericytic and perivascular genes and an enrichment in the CD31+ cells population (Filippi et al., 2019). The study performed by Li et al., demonstrated that pre-osteoblasts (MC3T3-E1) cultured into polypyrrole (PPy)/Fe3O4/polylactic acid-glycolic acid (PLGA) magnetic-conductive bifunctional fibrous scaffolds evidenced a good biocompatibility and highest cell viabilities when cells were stimulated with MF (0.072 T) (Li et al., 2019). Studies focused on restoring osteochondral defects have been developed, in which Hydroxyapatite-Collagen type-I (HAC) and PLGAPEG-PLGA thermogels have been stimulated with EMF (15 Hz 1 mT). Here, bone marrow mesenchymal stem cells encapsulated into thermogels promoted proliferation and chondrogenic differentiation of cells partly by activating the PI3K/AKT/mTOR and Wnt1/LRP6/β-catenin pathways (Yan et al., 2021). Similarly, cartilage defects have been treated with PEMF (100 mT), where the magnetic stimulation was applied to gelatin - β-cyclodextrin - Fe3O4 hydrogels implanted into articular cartilage defects of rabbits. Results evidenced that PEMF promoted chondrogenic differentiation and significantly enhanced the expression of cartilage-specific gene markers, such as COL2, Aggrecan, and COL1 (Huang et al., 2020).
Results evidenced that PEMF not only effectively reduced the inflammatory reaction of macrophage cells but also contributed to the incremental proportion of M2 macrophages at the injury site (Wang et al., 2020). Similarly, PEMF (5 Hz; 4 mT) applied to human tendon cells encapsulated into polycaprolactone magnetic nanoparticles have a modulatory effect on the inflammatory profile cells favoring anti-inflammatory cues, which is also supported by the anti-inflammatory/repair markers expressed in macrophages. Here, authors conclude that PEMF may can contribute for inflammation resolution acting on both resident cell populations and inflammatory cells, and thus significantly contribute to tendon regenerative strategies (Vinhas et al., 2020). Magnetic stimulation has been also applied to restore injured muscles; for example, the study developed by Chang et al., demonstrated that MT (1 Hz; 5.500 Gauss) stimulate gelatin - Fe3O4 hydrogels that are implanted into muscle rats. Results elucidated that MT stimulate axial muscle stretch, being superior to massage-like compression in maintaining muscle mass and structure in the animal model, and suggests pathways for combatting muscle disuse atrophy (Chang et al., 2021).
4. Discussion
There is evidence about the use of EMF that contribute to the improvement of diseases of the musculoskeletal system (Abdulla et al., 2019; Bachl et al., 2008; Hu et al., 2020; Kamei et al., 2018; Paolucci et al., 2020).
There are some studies in which results are not entirely conclusive on the effectiveness of the treatment, either the lack of an established protocol or results of the real treatment versus the combination with other methods or treatments (Camacho et al., 2019; Marycz et al., 2018; Qiu et al., 2020). However, it has been found that recent studies have results where the improvement of patients is perceptible either in pain management, inflammation (Abdulla et al., 2019; Arabloo et al., 2017; Chen et al., 2019; Hattapoğlu et al., 2019; Multanen et al., 2018; El Zohiery et al., 2021) or in a reduction of recovery time in the case of fractures (Mohajerani et al., 2019). Most of the studies were carried out to treat bone pathologies; for instance, magnetic stimulation was applied to treat fractures, evidencing faster repair bones (Hu et al., 2020; Kamei et al., 2018; Maziarz et al., 2016; Waldorff et al., 2017). Other studies evidenced that EMF, PEMF, among others had a positive effect over joint diseases, improving patient's symptoms and providing an analgesic effect (Bagnato et al., 2016; Klüter et al., 2018; Marycz et al., 2018; Pesqueira et al., 2018; Vicenti et al., 2018; Wu et al., 2018). Finally, there were applications focused on the regeneration of bone or cartilage tissues by stimulating proliferation, differentiation and synthesis of extracellular matrix proteins (Kamei et al., 2018; Marycz et al., 2018; Maziarz et al., 2016; Pesqueira et al., 2018; Wang et al., 2019), where mesenchymal stem cells are magnetically stimulated, so that the differentiation process is successful, observing an improvement in the functional capacity of patients (Marycz et al., 2018; Maziarz et al., 2016). In treatments proven in muscle and nerve pathologies, transplantation techniques and stimulation of bone marrow stem cells are found in a minimally invasive way (Abdulla et al., 2019; Hattapoğlu et al., 2019; Hochsprung et al., 2021; Ross et al., 2017; El Zohiery et al., 2021). It was evidenced that SMF regenerate peripheral nerves, relieve pain, improve blood flow, microcirculation and tissue inflammation, additionally a bactericidal effect is detected in the case of infections (Casalechi et al., 2020; Marycz et al., 2018; Pasek et al., 2016; Zwolińska et al., 2016).
Studies included in this review showed that Poland, Italy and China are countries with most research on the application of MF and EMF treatments. Figure 1 shows the report of countries in which studies were carried out, evidencing those with the highest and lowest contributions. Regarding the technologies used to generate and apply a magnetic stimulation, it was found that 54%, 25% and 21% were PEMF, MF and EMF, respectively. The most used signals generated by these devices were sine, pulse, triangular and sawtooth waves, with the possibility of varying field intensity, frequency and useful cycle (Bachl et al., 2008; Hu et al., 2020; Qiu et al., 2020; Waldorff et al., 2017). Among the applications of different types of signals, the sine wave was found to be applied to nerves and muscles, pulse wave in bone diseases and triangular wave in cartilage, tendon and similar dysfunctions (Krawczyk et al., 2017).
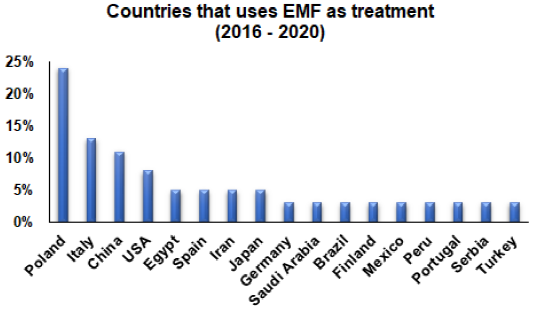
Figure 1 Countries where magnetic stimulation has been used as a non-invasive therapy to treat musculoskeletal diseases.
Most of the studies are focused on the treatment of pain, inflammation and tissue growth or regeneration (Abdulla et al., 2019; Arabloo et al., 2017; Hattapoğlu et al., 2019; Multanen et al., 2018; El Zohiery et al., 2021); nevertheless, pathologies with the greatest application of treatments are osteoarthritis (Bagnato et al., 2016; Hu et al., 2020; Maziarz et al., 2016; Mori, 2019; Paolucci et al., 2020; Wu et al., 2018), low back pain (Abdulla et al., 2019; Elshiwi et al., 2019; Nayback-Beebe et al., 2017), fibromyalgia (Multanen et al., 2018; Paolucci et al., 2016; El Zohiery et al., 2021) and fractures (Hu et al., 2020; Kamei et al., 2018; Maziarz et al., 2016; Waldorff et al., 2017). The most used procedure is the PEMF, generally used in combination with other types of therapies, finding positive results and improvements in the symptoms of musculoskeletal diseases. Here, it was possible to observe that different kind of magnetic stimulations were implemented to treat musculoskeletal diseases, where PEMF was the most used, followed by MF, PLFMF and SMF, while TMS, EMTT, TENS, ELF-MF and MT were less implemented to treat either, cartilage, bone, nervous and muscle pathologies. Several frequency ranges and magnetic densities have been used to stimulate different tissues affected by musculoskeletal pathologies; for instance, frequencies between 1.5 Hz to 27.12 MHz and magnetic densities from 0.1 mT to 1.5 T have been implemented to stimulate joint system diseases. Regarding the musculoskeletal pathologies, frequencies between 3 Hz to 3 kHz and magnetic fluxes from 3 μT to 350 mT have been applied. Finally, frequencies varying from 1 Hz to 900 kHz and magnetic densities from 7.5 pT to 10 mT have been used to treat nervous system pathologies. Here, different kind of devices have been used to generate and apply the magnetic stimulation, where most of them have therapeutic and rehabilitative approaches with available information on their technical characteristics and applications (Abdulla et al., 2019; Hochsprung et al., 2021; Waldorff et al., 2017). The development of biomedical devices focused on tissue regeneration; specially, those as a non-invasive therapy approach , are novel and viable tools to transform the health sector and improve not only the quality of patient care, but also in providing quality therapy with promising results (Caicedo & Smida, 2016).
5. Conclusions
Overall, magnetic stimulation is a promising non-invasive therapy that can be used to treat different kind of musculoskeletal pathologies. In fact, magnetic stimulation may be applied to improve either the whole musculoskeletal system or the cell dynamics of a specific cell type. Here, magnetic stimulation presents several advantages, elucidating its versatility to be implemented at clinical level or in basic science. However, there are some limitations to highlight regarding the stimulation scheme, as several frequencies, magnetic fluxes, stimulation times have been used, which means that there is no standardized protocol to know which of stimulation schemes are the most appropriate to be able to treat a specific pathology. Even though studies described in this review showed positive results in trat musculoskeletal diseases, there is a need to carry out a standardization of the magnetic stimulation parameters so that they are implemented in a regulated way at the clinical level.
Conflict of interest
The authors have no conflict of interest to declare.
Financing
The authors received no specific funding for this work.











 nueva página del texto (beta)
nueva página del texto (beta)


