1. Introduction
Artisanal or small-scale mining is an industry that has been developed in Colombia since colonial times, and it is an oral tradition that has passed from our ancestors to the current days, for more than 400 years Guiza (2013).Thanks to the great mineral wealth that the country possesses, the extraction of metals has taken on great importance in economic and social development, leading international companies to invest and formalize all types of non-legal mining, especially mining, with the Colombian government. Gold mining. The recovery of gold has become such an important axis that the national government projects it as a post-pandemic economic recovery, thanks to its commercial value that reaches 2,000 dollars per ounce El Tiempo (2020). At Marmato Caldas, (Colombia) one of the extractive projects is underway that will expand the mine's production capacity, increasing the recovery from 25,000 ounces of gold to 150,000 ounces per year Portafolio (2020).
The process of extracting gold from minerals usually leads to the use of recovery methods that are aggressive to the environment, additionally they are not profitable. In artisanal mining it has been shown that, due to the lack of technology to the mineral benefit process as well as the low technical and technological training of the miners, the recoveries of the processes do not exceed 60% Pantoja Timarán and Pantoja Barrios (2016). Cyanide and mercury have generally been the most common chemical agents for the extraction of gold and the most polluting for the environment, creating effects on the health and life of the inhabitants of the areas surrounding the mines Echeverry et al., (2016), Rojas-Reyes et al. (2017), Rubiano et al. (2020).
To improve the efficiency of gold extraction in refractory minerals, it is necessary to use a pretreatment to modify the chemical structure of the mineral, and to increase the recovery of the metal. The use of some chemical processes has been studied such as: use of sulfuric acid, bromides, bioleaching, pressure leaching, ultrafine grinding, among others; These methods can be convenient, although they are sometimes difficult to recycle, generating substances that are toxic to the environment Torres and Lapidus (2020), Snyders et al. (2018), Wang et al. (2019).
Additionally, since it is small-scale mining, the producer presents serious economic limitations to technologies that, although from a metallurgical point of view are efficient, have considerable application costs. An alternative to these techniques is the use of microwaves that through a heat source transforms the characteristics of the mineral, increasing the percentages of gold extraction in refractory minerals. Gaviria et al. (2006), Daza et al. (2011), Lovás et al. (2011), Zhang et al. (2019), McGill et al. (2015), Xu (2015), Sun et al. (2012).
With the results of this work, it is expected to demonstrate the efficiency of microwaves as a pretreatment for the extraction of gold from refractory minerals and its application in small-scale artisanal mining. The other improvement in the proposed process consists of the use of thiourea as an alternative leaching agent to those of traditional use such as cyanide and mercury that are usually used in Colombia.
2. Materials and methods
In the study of gold extraction, a refractory pyrite gold mineral was used, which we will call gold mineral, from Marmato - Caldas (Colombia). This material was mechanically prepared and a sample of 1000 g was selected through conventional quartering processes to determine a suitable particle size, which was -60 + 100 ASTM mesh.
Characterization analyses were essential to determine its main compounds. The qualitative study was carried out by X-ray diffraction in the Analytical X'pert-Pro equipment with cobalt anode, Bragg-Brentano mode) and the results were analyzed using the Panalytical HighScore Plus © software (Vers. 3.0.32011). By means of digestion with aqua regia, the quantitative composition was determined in the Agilent 4210 MP-AES EEA atomic emission spectrophotometer, using the MP Expert software (Vers. 1.6.1.10384). A thermogravimetric analysis was also carried out for which a heating ramp of 20 °C/min was carried out until reaching a temperature of 1000 °C. The SDT Q600 V20.9 Build 20 equipment, manufactured by TA Instruments THERMAL ANALYSIS was used for these tests.
Gold dissolution was made with thiourea Panreac at analytical grade and 30% concentrated hydrogen peroxide; distilled and deionized water (Boeco Dest-4) was used for all solutions. The leaching processes were carried out at ambient temperature in Tunja, (Colombia) (17 ºC) with constant mechanical agitation of 300 rpm. Aliquots of the different leaching were taken to evaluate the dissolution of the metal of interest by atomic emission spectroscopy. The reported experimental values, both numerically and graphically, correspond to the arithmetic means of the results obtained in triplicate at the specific operating conditions.
The pretreatment tests were carried out in a GE JES14G1 home microwave oven with approximate cavity measurements of 26.7 x 27 x 18.8 cm, power of 700W and a frequency of 2.45GHz. The simulations for the location of the maximum temperature and the quantity of mass for the tests were carried out in the COMSOL Multiphysics software; this program is used for simulations in coupled or multiphysics phenomena. It was also necessary to use ceramic crucibles with a capacity of 24 ml and resistance to maximum temperatures of 1200 ºC and clay to irradiate the material in the microwave oven. An optical pyrometer - Extech 42570 Dual Laser Infrared was used to measure the temperatures inside the microwave.
The first adjustment made to the homemade microwave oven was the replacement of the turntable and the plastic rail with an aluminum support, which had the rough edges and sharp corners filed down. This procedure was necessary to avoid electric discharges that can be produced by field enhancement and electron emission when the induced electric potential exceeds the coulomb potential Sun et al. (2012).
3. Results and discussion
3.1. Characterization of minerals
Some previous studies that have been carried out with the Marmato gold ore Torres and Lapidus, (2020), Córdoba (2019) and the diffractogram, Figure 1, show that the study ore has high pyrite and quartz contents, and it is confirmed that it is refractory in nature. Given the characteristics of the diffraction technique, it is not possible to find the form in which the gold is associated; in addition, the results were complemented by chemical digestion and quantification by atomic emission spectrophotometry.
Quantitative characterization confirms that the refractory ore contains gold, Table 1. As can be seen in the results, iron is the predominant metal in the ore.
Table 1 Quantitative composition of the study minerals by EEA.
| Gold ore | |
|---|---|
| Metal | Kg/t |
| Au | 0.029 |
| Fe | 182.35 |
| Cu | 5.245 |
The analysis of the thermograms shows the loss of mass as a function of time, showing the temperature at which, the process occurs, Figure 2. The mineral has different behaviors due to its characteristics as a sulfide.
At 400 °C a reaction begins between sulfur and oxygen forming FeO, which is completed above 600 °C. It is incomplete due to the rate of heating and the absence of agitation within the TGA, (Eq. 1).
3.2. Analysis of thermal pretreatment with microwave waves
3.2.1. Analysis of variables
Determining the amount of mineral sample and maximum temperature to achieve the best microwave effect depends largely on its dielectric constant, a measure of the material's ability to retard microwave energy representing the amount of input microwave energy that is loses in the material as it dissipates as heat Lovás et al. (2011). These variables were simulated in the COMSOL Multiphysics software Littmarck and Saeidi (2014), under the previously mentioned home microwave conditions.
In the software, the essential parts for the proper and improved operation of the microwave reactor were simulated; The two-aperture waveguide and the cavity dimensions were represented with the identical characteristics of the test microwave oven simulation, Figure 3. Both the apertures and the width of the waveguide work with frequencies between 1.76 GHz and 3.53 GHz. At the microwave output frequency through the waveguide openings, the power delivered is 700 W. Gold ore with the same characteristics was used in the test to generate a point of comparison in each graph.

Figure 3 Finite element mesh for simulation in the cavity structure, the waveguide of two microwave feed. openings, the rotating support and the sample, in tetrahedral elements, for the microwave reactor.
The wave fronts or electric field distribution for each feed opening have a different phase and angle, providing totally different field distributions within the cavity, Figure 4(a), 4(b). In the mineral, there is a more intense distribution around the surface, since it is located at one of its maximum points in the distribution of the electric field, Figure 4(c). Which contributes to higher temperatures, and likewise a greater absorption of the power evidenced in the resistive loss, increasing the efficiency three times more when absorbing the energy and in the increase of the temperature in the mineral inside the microwave reactor, Figure 4(d).
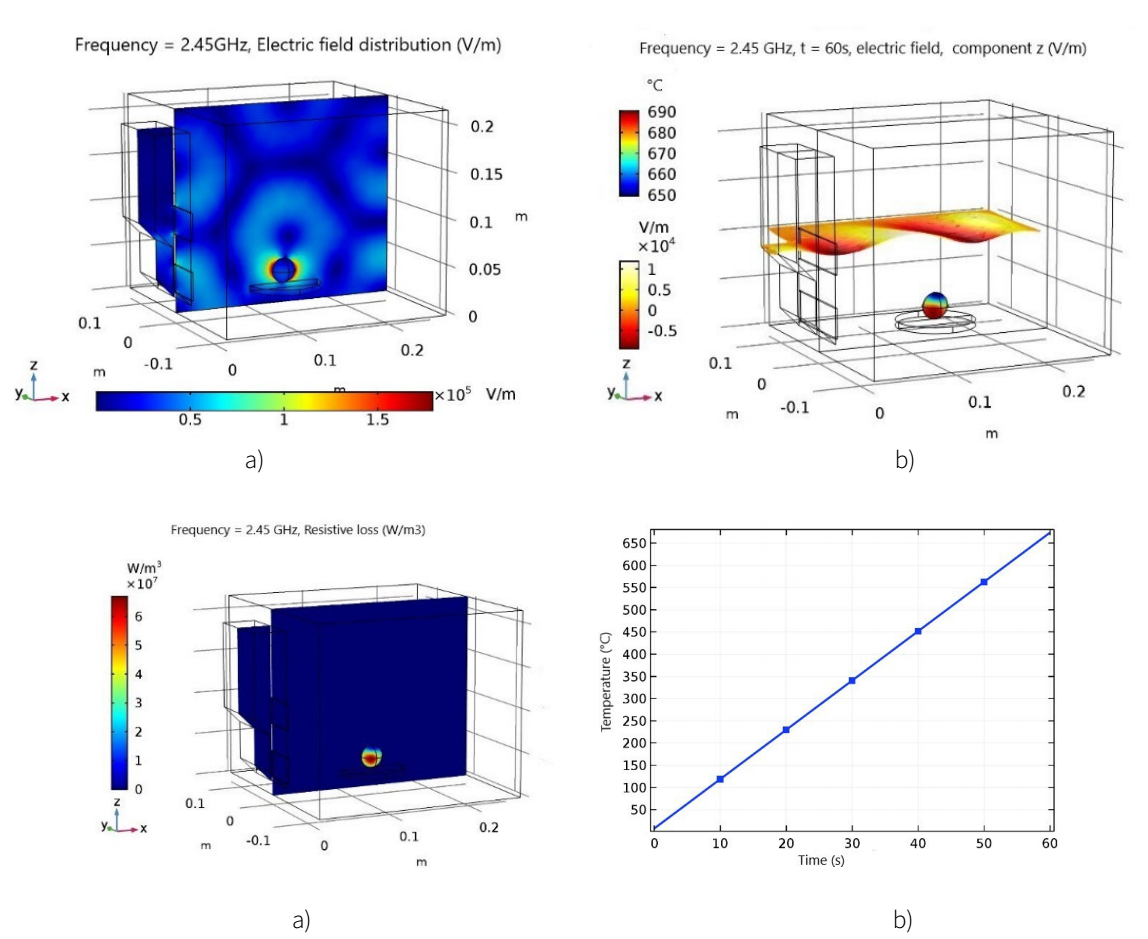
Figure 4 Simulation of variables in COMSOL Multiphysics software, under the conditions of a home microwave oven. a) Distribution of the electric field within the cavity V / m, in the microwave reactor. b) Electric field and temperature distribution for 60s of exposure to microwave waves, in the microwave reactor. c) Average temperature of the sample volume during the first 60 seconds of exposure, in the microwave reactor. d) Distribution of the dissipated microwave power, W / m3, in the microwave reactor
For the simulation of the gold mineral, the constants of its properties were adjusted as its dielectric constant ὲ = 7.6 - j 2.2 and the mass was increased every 10 g by plotting the temperature with respect to the mass, Figure 5(a), and the internal energy absorbed by the mineral with regarding mass, Figure 5(b). In this analysis it can be observed that its maximum point is reached at temperatures higher than 600 ºC with 70 grams, coinciding with the mass of the highest point for internal energy.

Figure 5 Simulation of variables for the gold mineral microwave effect a) Maximum temperature reached by varying the mass. b) Internal energy absorbed by varying the mass.)
From these analyses of variables, it can be deduced that the behavior of minerals depends on their dielectric properties. The complex dielectric permittivity describes the behavior of the material in the microwave field, which makes it possible to evaluate the suitability of heating. According to the data obtained, it is agreed that minerals of sulfide origin such as gold ore obtain higher temperatures with less material Haque (1999).
3.2.2. Microwave heat pretreatment
According to the previously performed analyzes, the pretreatment is carried out with the mineral samples, Table 2. In the simulations with the software, the location of the maximum temperature reached is recorded in the volumetric center of the sample, so the microwave heating is divergent.
Table 2 Data from the microwave effect pretreatment process for each mineral. The tests were carried out with an ambient temperature of 21.4 ºC.
| Gold Ore | |
|---|---|
| Temperature (ºC) | Time (min) |
| 367 | 1.20 |
| 501 | 1.37 |
| 637 | 4 |
The 70 grams of gold mineral were taken and left inside the microwave oven, there the expulsion of a large quantity of gases and an intense blue flame was observed, Figure 6. This phenomenon occurs due to the increase in temperature in a short period of time caused by the interaction of the microwaves with the dipoles producing the oxidation of the sulfur contained in the mineral, this in accordance with the results found by TGA Uslu et al. (2003). Increasing the temperature initiates a radial internal heating with a greater intensity of radiation in the sample.

Figure 6 Microwave effect on gold ore. a) External heating, expulsion of gases. b) Radial internal heating.
When the gold ore is exposed to microwave heat treatment, the sample is transformed into a porous condition caused by the expulsion of the gases resulting from the reaction, Figure 7. This is evidence of oxidation in the presence of air due to the effect of temperature in a mineral of the sulfide species, which, as shown in the thermogram of the characterization, coincides in that at temperatures higher than 630 ºC in the mineral the sulfide is transformed to oxide, expelling all the sulfur contained in the mineral in the form of SOx.

Figure 7 Microwave effect on gold ore at different temperatures. a) Effect at 367 ºC. b) Effect at 501 ºC. c) Effect at 637 ºC.
The increase in temperature in a short period indicates that the sample is highly efficient in microwaves. This phenomenon occurs due to the dipoles of its molecular structure that interact in such a way that it generates an increase in temperature due to the absorption of electromagnetic energy from microwaves Lovás et al. (2011).
It is important to select the right amount of sample for microwave thermal pretreatment. If the amount is very small the material will behave reflectively, preventing the electromagnetic fields of the microwaves from interacting with the dipoles of the mineral making it take more time for the heating of the sample. When the sample is very large, it risks losing a lot of electromagnetic energy. These analyses are based on the microwave input frequency affecting the energy penetration depth Eymery & Ylli (2000). According to the data obtained, it is agreed that the minerals of origin of sulphides such as gold ore are rapidly warming, which is presumably due to their dielectric properties Lovás et al. (2011), Baláž et al. (2000).
After the microwave thermal process, the pretreated mineral was subjected to X-ray diffraction analysis, Figure 8. The diffractogram showed oxidation in the presence of air due to the effect of temperature, where most of the sulfides presented above, Figure 1, were transformed into iron oxides due to the effect of pretreatment. This analysis shows that if a transformation of the species occurs in the gold ore to 637 °C.
3.3. Leaching processes
The leaching process was performed with the material of the microwave thermal pretreatment, and to evaluate its effectiveness was compared with a material without pretreatment. The process is described in Figure 9.

Figure 9 Flow chart for gold extraction from refractory gold ore. The ore is previously heat treated in a microwave oven at 637 °C, with a residence time of 4 minutes. For gold extraction in pretreated and non-pretreated ore, 0.1 M thiourea was used.
For the analysis of gold extraction with thiourea, the thermodynamic conditions of the mineral are previously studied. In the diagram of predominant areas for gold in the presence of thiourea Torres and Lapidus (2020), gold forms an ionic complex with thiourea, indicating that the metal is soluble in potentials greater than 0.58 V. For the use of thiourea, it is advisable to work with a pH close to 2, where it offers better stability with oxidizing agents such as hydrogen peroxide. Some studies have indicated that when used at pH greater than 4, ferric ions can precipitate into the hydroxide compound and thiourea could be oxidized into formamidine disulfide in an unstable manner de la Cruz et al. (2012), Tremblay et al. (1996), Lapidus (1993), Jiménez and Parra (2018).
The characterization showed that the gold ore is refractory and contains iron in greater quantity, Table 1; this information coincides with some previous studies that have been carried out on this mineral Torres and Lapidus (2020). As can be seen in the results of the leaching process, Figure 10, a good part of the gold is encapsulated in iron and quartz oxides, which makes it a limitation for its extraction. Uslu et al. (2003), Eymery & Ylli (2000), Baláž et al. (2000).

Figure 10 Gold leaching process with 0.1 M thiourea with 1ml dosed of H2O2 every hour at pH 1.5, mechanical stirring at 300 rpm, and Tunja room temperature 18°C.
Subsequently, a mineral leaching process was performed with thermal pretreatment and a substantial improvement in gold extraction was found, Figure 10. It can be inferred how the reaction of sulfur with oxygen generates a change in the structure of the ore allowing metal values to be reached by the leaching reagent. The trend of the leaching process indicates that with enough time it is possible to achieve the extraction of most of the gold in the sample.
Although TGA studies show a weight loss due to oxidation reactions of the sulphide phase, it is important to complement these results with scanning electron microscopy analysis so that there is a correlation with surface morphology (texture) in order to establish whether pores or other physical modifications are generated. In a macroscopic way it was possible to observe these changes in Figure 8, where the morphology of the mineral changes at different temperatures with the creation of a type of cracks in the mineral.
It is important to note that, as a relatively simple infrastructure, its implementation in small-scale mining processes would be a cost-effective option for these producers Zhang et al. (2019). Additionally, the results shown that the treatment is effective for the purpose of improving the extraction of gold, with an alternative agent such as thiourea which is environmentally friendly for its recycling properties Asamoah (2020), Guo et al. (2020), Wang et al. (2019), Xie et al. (2021).
Finally, further studies from the point of view of chemical engineering are required to analyze other factors such as the effect of mineral agitation, residence times, particle size, among others.
4. Conclusions
The microwave effect is an effective pretreatment in refractory minerals for the extraction of gold. With a permanence of four minutes of the gold mineral in the microwave, it is possible to reach a temperature of 640ºC in which the transformation of the chemical composition to oxides occurs, making the leaching of gold with thiourea more feasible, managing to extract the 93.10% gold in 180 minutes.
The effectiveness of microwave heat treatment is demonstrated by the results of this study, making it a more cost-effective and environmentally friendly option for gold production from refractory ores in small-scale artisanal mining. In Colombia, one of the main problems for the extractive industry are the high costs of robust equipment and large quantities of inputs, and with the use of this process these expenses can be avoided, in addition to reducing the consumption of energy and even water, as it is a thermal treatment.
The applicability of the treatment is based on the fact that even in difficult access conditions, where fuels, furnaces or robust equipment cannot be used, it would be sufficient to obtain electrical energy from other sources (solar energy) and apply microwave treatment to the ore on a small scale.
Further analyses from a chemical engineering point of view are open to complement the results of this first approach to the problem in small-scale metallurgical mining projects.











 nueva página del texto (beta)
nueva página del texto (beta)





