1. Introduction
For ethanol fermentation to yield maximum results, it is crucial to optimize the operating conditions of the process (Asaithambi et al., 2021; Ciesielski & Grzywacz, 2021; Chen et al., 2021; Estevão et al., 2021; Gao et al., 2022; Li et al., 2021; Rodman & Gerogiorgis, 2020; Shen et al., 2021; Urtubia et al., 2021). Among the key parameters, fermentation temperature plays a significant role in achieving high productivity. As ethanol production involves an exothermic reaction, it impacts the metabolism of the microorganism employed. While tropical regions naturally favor heat, controlling the fermentation temperature within the optimal range of 25-35 °C becomes challenging and economically unviable due to the substantial energy required to prevent heat-induced inactivation of yeast cells (Banat et al., 1992). Therefore, there is a quest for robust microorganisms that can efficiently ferment substrates in hot environments while tolerating high ethanol concentrations. The cost of controlling fermentation heat increases due to cooling requirements (Liu et al., 2019). It is worth noting that industrial ethanol fermentation relies on sugars derived from starchy materials, sugarcane juice, molasses (Lopes et al., 2016; McAloon et al., 2000; Samaniego-Sánchez et al., 2020), and specific substrates for distilled alcoholic beverages (Solís-García et al., 2017). Determining feasible products based on available raw materials and established technologies is essential in industrial zones (Zhao et al., 2020; González-Herrera et al., 2016). In ethanol production, sugarcane addresses the raw material availability concern, but temperature remains a critical factor to be studied (Rivera et al., 2017). Modeling has been employed to elucidate cell growth in relation to temperature (Abunde et al., 2019; Nor-Khaizura et al., 2019; Pereira et al., 2020). However, the outcome of simulation and optimization tools heavily relies on the quality of the mathematical model (Carrillo-Ahumada et al., 2020; Castillo-Santos et al., 2017; Darvishi et al., 2020; Díaz &Tost, 2018; Goelzer et al., 2009; Hebing et al., 2020; Jorayev et al., 2022; Meng et al., 2021; Müller et al., 2020; Rodríguez-Mariano et al., 2015; Salmi et al., 2021; Torralba-Morales et al., 2020; Vignesh & Chandraraj, 2021; Wu et al., 2015). Typically, fermentations rely on ideal laboratory conditions (e.g., synthetic media, stirring devices, heating modes), and it is preferable to consider industrial processes (real fermentation media, steady state, etc.) (de Andres-Toro et al., 1997). Modifying processes introduces new situations, necessitating extensive experimentation to generate data for constructing novel models. Consequently, developing a phenomenological process model becomes challenging due to limited understanding of the physicochemical phenomena and associated kinetic and transport mechanisms (Cheema et al., 2002). Moreover, the nonlinear dynamics of the process further complicate modeling (Feil et al., 2004). Given these challenges, hybrid approaches have emerged, such as genetic programming (GP), an evolutionary artificial intelligence technique for developing mathematical models based on input-output data, in contrast to conventional regression and neural network modeling techniques (Babu & Karthik, 2007). The study and modeling of ethanol fermentation processes have proven effective in improving product quality, enhancing process control, and reducing costs (Fan et al., 2015). genetic programming (GP) has successfully been utilized to model the glucose to gluconic acid bioprocess, resulting in increased overall productivity and improved interaction between dissolved oxygen and fungal mycelia (Babu & Karthik, 2007). In this research, GP was employed to develop a mathematical model that describes the impact of temperature on microorganism growth and ethanol yield. The approach followed the work of Madár et al. (2005), which focused on identifying the structure and parameters of a mathematical model using experimental data. Abonyi (2005) developed a MATLAB toolbox for this purpose, which was utilized in this research without modification. The algorithm selected for parameter and structure identification does not require a pre-defined experimental design and produces satisfactory results with limited data for correlation, as demonstrated in the work of Ramírez-Hernández et al. (2017). This methodology has found applications in various domains such as algorithms, biotechnology, computing, process control, data mining, and modeling (Banzhaf et al., 1998; Dorgo et al., 2021; Kumar et al., 2014). Germec et al. (2020) and Esfahanian et al. (2016) conducted parameter identification based on the Gompertz equation. However, this research focuses on establishing correlations using available data specific to the alcoholic fermentation process, rather than relying on a pre-defined mathematical structure.
1.1. Model identification for the fermentation process
Mathematical models play a crucial role in various scientific disciplines as they enable the description and prediction of system behavior under different conditions by utilizing variables. In this research, the symbolic optimization algorithm known as genetic programming (GP) was employed, following the approach proposed by Madár et al. (2005). This method facilitates the identification of both the model's structure and its parameters using experimental data. The key considerations in this approach include the following:
The target vector
Table 1 Parameters of GP.
| Parameter | Value |
|---|---|
| Population size | 100 |
| Maximum number of tree levels | 10 |
| Symbols | +,*,/,-,^ |
| Type of selection and selection mode | Tournament selection |
| Generation gap | 0.8 |
| Probability of crossover | 0.7 |
| Probability of mutation | 0.3 |
| Mode of tree-recombination | 1.0 |
In this research, the variables
The structure of the article is the following: Section 2 shows the experimental and computational methodologies; The results and discussion are shown in Section 3. Finally, some remarks and conclusions are exposed in Section 4.
1.2. Review of multi-objective optimization design procedures
A multi-objective optimization statement without loss of generality is defined as follows:
subject to: g(θ) ≤ 0,
h(θ) ≤ 0 and ≤ θ
i
≤ θ
i
Since there is no single solution that is optimal for all objectives, a set of solutions called the Pareto set is defined. Each solution in the Pareto set represents an objective vector on the Pareto front. All solutions on the Pareto front are considered a set of Pareto-optimal and non-dominated solutions.
A design procedure employing multi-objective optimization techniques typically consists of three fundamental steps: 1) stating the multi-objective problem (MOP), 2) conducting the multi-objective optimization (MOO) process, and 3) performing the multi-criteria decision making (MCDM) stage (Meza et al., 2017).
• MOP statement
At this stage, the designer must make decisions regarding the design concept to address the problem, how to evaluate the performance of design alternatives, and which solutions are relevant, practical, or feasible. In the case of ethanol fermentation, the design concept refers to the operating conditions, while the design alternative pertains to specific time and temperature settings. Performance measurement requires the existence of a parametric model that establishes a correlation between the decision variables (which lead to specific design alternatives) and their performance
• MOO process
During this stage, the multi-objective optimization algorithm is implemented for the multi-objective problem (MOP). The algorithm can be ad-hoc or selected from a suitable pool of algorithms available. An algorithm is considered suitable for the problem at hand if it possesses desirable characteristics such as convergence, diversity, and relevance.
• Decision-making stage
Finally, with the approximate Pareto front, the designer will evaluate the trade-offs between conflicting design objectives and consider the design alternatives. The goal is to select a solution that strikes a preferable balance in performance for the specific problem. Procedures and visualization tools play a crucial role in assisting designers, particularly when dealing with four or more design objectives.
2. Methodology
2.1. Microorganism and culture conditions
Saccharomyces cerevisiae CSI-1 (abbreviated as CSI) yeast was used as the microorganism in this study. A vial of CSI stock cultures, stored at -10 °C, was thawed and used to refresh the cells in tubes containing 10 mL of culture medium prepared with 20 g/L of glucose and 5 g/L of yeast extract (YE). The medium, sterilized for 20 minutes at 121°C prior to inoculation, was then incubated at 37°C for 24 h in a Shel Lab Sl incubator. Subcultures were performed monthly.
2.2. Inoculum preparation and fermentation medium
The refreshed culture served as the seed to prepare the inoculum using 200 mL of growth medium composed of 30 g/L of glucose and 5 g/L of YE. The inoculum was cultivated in a Shel Lab Sl incubator at a temperature of 37°C for 24 h. The pre-culture was subsequently centrifuged at room temperature using a high-speed centrifuge (Kubota model CR21G) at a speed of 6000×g for 5 min to harvest the cells. The cell pellet was then resuspended in 100 mL of sterilized water and used as the inoculum. The fermentation medium consisted of 5 g/L of YE and 100 g/L of molasses (70 g/L sucrose and 30 g/L glucose). The molasses contained a low concentration of fructose and were not monitored. The media was autoclaved for 20 min at 121°C.
2.3. Batch fermentation
The harvested cells from the inoculum were transferred to the fermenter to initiate the fermentation process. Ethanol fermentations were conducted in a 1 L fermenter with a working volume of 800 mL. The experiment was initiated at temperatures of 30°C, 33°C, 35°C, and 37°C with an agitation speed set at 100 rpm. The optical density, temperature, and pH were monitored and recorded throughout the fermentation process. Samples were withdrawn every 3 h, except at 12 h and 15 h, as these periods corresponded to the logarithmic phase where the monitored parameters were predictable, stable, and reproducible.
2.4. Analysis
2.4.1. Cell growth analysis
The growth of CSI yeast was monitored by measuring the optical density (OD) of cells at a wavelength of 575 nm using a UV-Vis Spectrophotometer and correlated to dry cell weight.
2.4.2. Colony forming units
Colony forming units (CFU/mL) were determined using the decimal serial dilutions method (100 - 900 μL) of 101-107 or 108 in 1.5 mL tubes. An aliquot of 100 μL was spread on a PDA plate medium.
2.4.3. Ethanol, glucose, and sucrose determination
Fermentable sugars (glucose and sucrose) and ethanol were analyzed using an enzymatic method with a model BF-5D (Oji scientific instruments Co., Ltd. Japan) analyzer.
2.4.4. Computational methodology
This section presents the computational methodology used in this research work. Firstly, the experimental dynamics of the fermentation process were observed using the original experimental data. Subsequently, based on the experimental data and utilizing GP (Banat et al., 1992), a nonlinear mathematical model was identified to represent the variable responses: dry cell weight (DCW), residual glucose (RG), residual sucrose (RS), ethanol (E), and colony forming units/mL (CFU/mL) as functions of time and temperature. Then, to validate the obtained model, correlation metrics between the model and experimental data were performed. Finally, multi-objective optimization was employed to determine if there is a certain correspondence between the previously obtained data and the results provided by optimization.
3. Results and discussion
3.1. Experimental dynamics of the fermentation process
The experimental dynamics of the fermentation process are presented using the experimental characteristics DCW, RG, RS, E, and CFU/mL (see "Tables 2-6" and "Figures 1-2").
Table 2 Experimental data for DCW (g/L).
| c | Temperature (°C) | Mean | Std. dev. | |||
|---|---|---|---|---|---|---|
| 30 | 33 | 35 | 37 | |||
| 0 | 1.95 | 2.2 | 2.25 | 2.28 | 2.17 | 0.15 |
| 3 | 2.2 | 2.51 | 2.46 | 3.03 | 2.55 | 0.35 |
| 6 | 2.6 | 2.82 | 3.48 | 5.64 | 3.64 | 1.39 |
| 12 | 6.03 | 6.36 | 6.93 | 8.25 | 6.89 | 0.98 |
| 18 | 10.1 | 9.1 | 11.1 | 8.55 | 9.71 | 1.13 |
| 21 | 10.5 | 9.3 | 11.1 | 8.65 | 9.89 | 1.11 |
| 24 | 9.75 | 9.55 | 10.08 | 8.7 | 9.52 | 0.59 |
Table 3 Experimental data for RG (g/L).
| Time (h) | Temperature (°C) | Mean | Std. dev. | |||
|---|---|---|---|---|---|---|
| 30 | 33 | 35 | 37 | |||
| 0 | 28.0 | 27.3 | 27.0 | 28.0 | 27.58 | 0.51 |
| 3 | 22.0 | 21.5 | 18.0 | 21.0 | 20.63 | 1.80 |
| 6 | 16.0 | 14.0 | 10.0 | 13.8 | 13.45 | 2.51 |
| 12 | 6.0 | 1.1 | 1.3 | 1.6 | 2.50 | 2.34 |
| 18 | 2.0 | 0.8 | 0.5 | 0.08 | 0.85 | 0.82 |
| 21 | 1.6 | 0.09 | 0.2 | 0.2 | 0.52 | 0.72 |
| 24 | 0.2 | 0.0 | 0.1 | 0.0 | 0.08 | 0.10 |
Table 4 Experimental data for RS (g/L).
| Time (h) | Temperature (°C) | Mean | Std. dev. | |||
|---|---|---|---|---|---|---|
| 30 | 33 | 35 | 37 | |||
| 0 | 70.0 | 70.0 | 72.0 | 72.0 | 71.0 | 1.15 |
| 3 | 67.5 | 67.0 | 68.0 | 67.0 | 67.38 | 0.48 |
| 6 | 65.0 | 64.0 | 64.0 | 65.0 | 64.5 | 0.58 |
| 12 | 65.0 | 61.3 | 64.0 | 62.4 | 63.18 | 1.65 |
| 18 | 5.6 | 5.3 | 5.7 | 5.2 | 5.45 | 0.24 |
| 21 | 5.0 | 4.0 | 5.0 | 4.5 | 4.63 | 0.48 |
| 24 | 4.7 | 4.6 | 4.3 | 2.1 | 3.93 | 1.23 |
Table 5 Experimental data for E (g/L).
| Time (h) | Temperature (°C) | Mean | Std. dev. | |||
|---|---|---|---|---|---|---|
| 30 | 33 | 35 | 37 | |||
| 0 | 0.4 | 0.6 | 0.0 | 0.4 | 0.35 | 0.25 |
| 3 | 3.0 | 2.9 | 0.8 | 1.7 | 2.10 | 1.05 |
| 6 | 4.2 | 4.8 | 4.9 | 6.0 | 4.98 | 0.75 |
| 12 | 20.0 | 18.0 | 24.0 | 26.0 | 22.0 | 3.65 |
| 18 | 37.4 | 40.1 | 42.4 | 38.9 | 39.70 | 2.11 |
| 21 | 41.4 | 43.2 | 45.3 | 44.4 | 43.58 | 1.69 |
| 24 | 44.8 | 48.0 | 49.5 | 49.6 | 47.98 | 2.24 |
Table 6 Experimental data for CFU/mL (Log10).
| Time (h) | Temperature (°C) | Mean | Std. dev. | |||
|---|---|---|---|---|---|---|
| 30 | 33 | 35 | 37 | |||
| 0 | 6.54 | 6.82 | 6.70 | 6.48 | 6.64 | 0.15 |
| 3 | 6.88 | 7.98 | 7.54 | 7.96 | 7.59 | 0.51 |
| 6 | 7.15 | 8.32 | 8.88 | 8.64 | 8.25 | 0.77 |
| 12 | 8.20 | 8.85 | 9.08 | 8.95 | 8.77 | 0.39 |
| 18 | 8.93 | 8.95 | 9.12 | 8.66 | 8.92 | 0.19 |
| 21 | 8.90 | 9.0 | 9.01 | 8.68 | 8.90 | 0.15 |
| 24 | 8.91 | 8.98 | 9.04 | 8.52 | 8.86 | 0.23 |
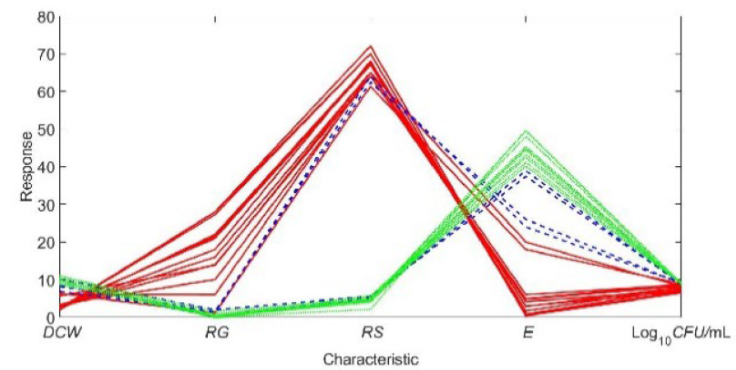
Figure 1 Coupling of the characteristics of the fermentation process. Green lines: E > 40 g/L; blue lines: 20 g/L < E ≤ 40 g/L: red lines: 0 g/L < E ≤ 20 g/L (experimental characteristics).
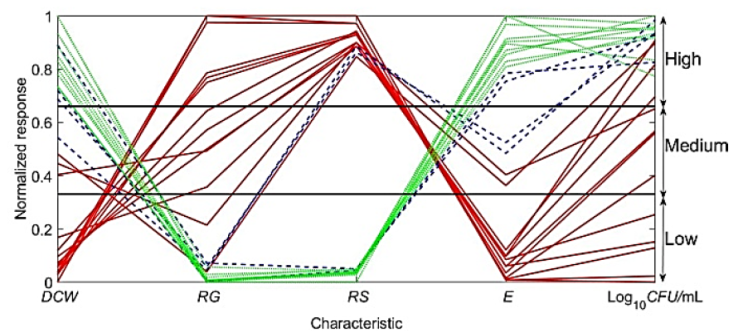
Figure 2 Coupling of the characteristics of the fermentation process. Green lines: E > 40 g/L; blue lines: 20 g/L < E ≤ 40 g/L: red lines: 0 g/L < E ≤ 20 g/L (normalized responses).
"Table 2" displays the dynamics of DCW as a function of time and temperature. The parameters that are within a limited range are DCW and CFU/mL, which show a strong correlation. It is preferable to have low values of DCW and CFU/mL to achieve high specific productivity. However, DCW or its equivalent CFU/mL correlates with the volumetric productivity, represented by ethanol concentration (E) ("Figure 1"). Ethanol (E) can be considered as a reference for analyzing the coupling of the other parameters. This is the optimal way to track the fermentation kinetics, considering that E is the final product of the substrate metabolism. Three intervals can be identified for E ("Figure 2"): a)
For the second interval (b)
For (a)
"Table 4" displays the dynamics of RS as a function of time and temperature. The decrease in RS varies with time, with the most significant changes occurring at
"Table 5" presents the dynamics of ethanol (E) as a function of time and temperature. The increase in E is directly proportional to time, with the most notable changes occurring at
"Table 6" displays CFU/mL as a function of time and temperature. The increase in CFU/mL is directly proportional to time, but there is a stationary phase observed at
The characteristics that are in a limited range are DCW and CFU/mL, and their influence on the other characteristics is observed in the production of ethanol ("Figure 1"). Characteristic E (ethanol) can be considered as a reference to analyze the coupling of the other characteristics, for which there are three intervals ("Figure 2"): a) E > 40 g/L, b)
3.2. Model identification of the fermentation process
The experimental data were used in the GP MATLAB™ toolbox to obtain the mathematical model that describes the behavior of the fermentation process. The parameters of GP are described in "Table 1." The mathematical models are:
where, the parameter models are: DCW is dry cell weight, CFU is colony forming units, RG is residual glucose, RS is residual sucrose, E is ethanol concentration, t is time and T is temperature considering the following restrictions: if DCW < 0 then DCW = 0; if

Figure 3 Validation of the mathematical model vs. experimental data: a) DCW (g/L); b) RS (g/L); c) log10 CFU/mL; d) RG (g/L); e) E (g/L); experiments were performed by triplicate.
The fitness is higher than
3.3. Statement of optimization problem
In this research work, it is considered that the ethanol fermentation process presents the following characteristics: (DCW) g/L, (RG) g/L, (RS) g/L, (E) g/L and (Log10
CFU/mL). These characteristics are considered as dependent variables of the process. The independent variables of the process are time (t) and temperature (T). The relationship between the dependent and independent variables is shown in "Eqs 8-12". Some bibliographic references that consider a methodology like this work is Ramírez-Hernandez et al. (2017) where they consider: a) set of data obtained experimentally from the dependent variables of independent, b) identification of the structure and parameters of the model and finally c) optimization in numerical simulation of the best operating conditions. For point c) optimization in numerical simulation of the best operating conditions in this research work, the following is considered. The multiobjective optimization problem can be posed by finding the values of X
1 and X
2 with
where θ is time (X
1) and temperature (X
2) subject to the decision variables are
3.4. Optimization
"Figures 4a-4b" present the operating conditions and corresponding characteristics obtained from the model and experimental data, showing a correspondence between them. In contrast, "Figures 4c-4d" display the set and Pareto front of operating conditions obtained through the optimization algorithm, along with the characteristics obtained through simulation. Additionally, the maximum values obtained from both experiments and simulation are also indicated.
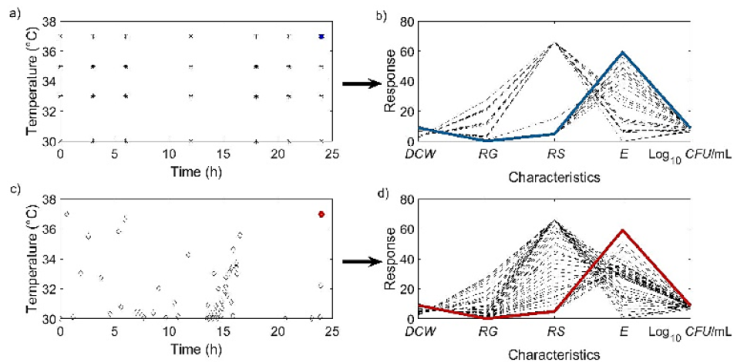
Figure 4 Set and Pareto front of the optimization process: a) Independent variables (t, T) proposed experimentally, b) dependent variables (DCW, RG, RG, E and Log10 CFU/mL) obtained with the mathematical model; c) independent variables (t, T) proposed by means of optimization, d) dependent variables (DCW, RG, RG, E and Log10 CFU/mL) obtained with the mathematical model and c). Blue lines indicate the maximum of E.
It is observed that there is a highly aggregated set in the region corresponding to a time interval of 10 to 15 h, but the maximum value is found with the operating conditions that meet
These results precisely reveal the desired conditions for an exothermic process like ethanol fermentation. Operating ethanol fermentation above 37°C is crucial for tropical countries due to its significant economic advantages. The advantage lies in the fact that if fermentation is conducted at temperatures higher than 37°C, it becomes easier to control using heat exchangers, especially considering that the cooling water temperature in tropical countries typically ranges from 30-35°C. Moreover, operating at temperatures above 37°C is also an economic advantage for processes with a duration of less than 24 h, as it reduces energy consumption required for cooling equipment. Operating at 37°C is a characteristic of the selected microorganism, which can produce ethanol even at temperatures as high as 45°C. However, operating at such elevated temperatures is not advisable as it negatively affects the viability of the microorganism. Maintaining viability is crucial to reuse the microorganism for multiple cycles of operation.
4. Conclusions
The values calculated with the mathematical model align well with the experimental data. Thus, the models can be effectively applied to predict the kinetics of the fermentation process, including cell growth and ethanol production from sugarcane molasses across a range of temperatures, demonstrating the accuracy of the proposed model. Finally, the optimization of ethanol fermentation characteristics highlights the identification of feasible operating conditions.











 nueva página del texto (beta)
nueva página del texto (beta)



