Introduction
Sulfa drugs were the first drug largely employed and systematically used as preventive and chemotherapeutic agents against various diseases [1]. The sulfa drugs are well known for their biological activity. They are active against a broad range of gram positive and gram-negative bacteria and function antagonists to bacterial folate synthesis [2]. The synthesis of transition metal complexes with Schiff base ligands are studied due to strong ability to form complexes, sensitivity, selectivity and synthetic flexibility towards metal atoms [3,4]. Schiff bases and their metal complexes exhibit biological activities as antibiotics, antiviral and antitumour agents because of their specific structure [5,6]. The complexes showed enhanced antimicrobial activity as compared to uncomplexed Schiff base [7-11]. In light of these, we become interested and in continuation of our research work [12,13] the complexes synthesized from 4-((2-hydroxynaphthalen-1-ylmethylene)amino)-N-(pyridin-2-yl) benzenesulfonamide(HL1) [14] in the present paper we report the synthesis, characterization and antimicrobial activity of Mn(II) complexes of Schiff bases 4-((3-ethoxy-2-hydroxybenzylidene) amino)-N-(pyridin-2-yl)benzenesulfonamide(HL2) and 4-((3-ethoxy-2-hydroxybenzylidene)amino)-N-(pyrimidin-2- yl)benzenesulfonamide(HL3).
Experimental
Materials and Methods
The Manganese(II) acetate tetrahydrate - Mn(CH3COO)2•4H2O and solvents were purchased from Merck and used as such as. The ligands HL2 and HL3 were used in this study were synthesized by the same authors in PG and Research Department of Chemistry of Seethalakshmi Ramaswami College, Tiruchirappalli, India; details of the procedure described in the journal [12-14].
The conductance of the complexes in N,N dimethylformamide (DMF) (10-3 M) solutions were measured at 298 K in a Elico conductivity bridge and dip type conductivity cell with cell constant 1.0 cm-1. The IR spectra of ligands and their complexes were recorded in KBr pellets in the 4000-400 cm-1 range on Perkin Elmer IR RXI Spectrometer and Shimadzu IR Affinity 1. The electronic spectra were recorded using Perkin Elmer Lambda 35 spectrometer in 190-1100 nm range using dimethyl sulfoxide (DMSO) as solvent. Magnetic susceptibility measurement was performed on powder samples at 298K on electromagnet/Gouy method-PICO make using mercury tetrathio cyanatocobaltate (II) as calibrant. Simultaneous TG and DTA patterns of the complexes were recorded on Perkin Elmer STA 6000, Diamond TG/DTA with heating rate 40.00 °C to 740.00 °C at 10.00 °C/min. EI mass spectra were recorded on GC-Mass Spectrometer - Jeol GCMS GC-Mate II make. The photoactive potentiality of the ligand and the complexes were investigated by fluorescence measurements using Perkin Elmer LS 45 Spectrofluorometer. Antimicrobial activity studies were carried out by Disc Diffusion Technique.
Synthesis of Schiff base (HL2/HL3)
To a hot stirred ethanolic solution of sulfapyridine/sulphapyrimidine (1.245 g /1.2514, 0.005 mol) an ethanolic solution of 3ethoxysalicylaldehyde (0.834 g, 0.005 mol) was added. The reaction mixture was refluxed for 3h. The coloured solid mass formed during refluxing was cooled, filtered, washed thoroughly with ethanol and dried in a desiccator. The compound was purified by recrystallization from ethanol. The purity of the ligand was checked by melting point, TLC and spectral data. HL2- Yield: 1.7260 g (83 %). Colour: Orange; m.p.185 °C; Elemental Analysis (%) calculated for C20H19N3O4S: C 60.45; H 4.79; N 10.58; S 8.06. Found: C 60.01; H 4.12; N 11.48; S 9.02. 1H NMR spectrum: δ 8.96 ppm (s,1H) -CH = N, δ 12.72 ppm (s,1H) -OH, δ 6.87-7.95 ppm (m,11H) aromatic, δ 1.32-1.36 ppm (t,3H) -CH3, 4.04-4.07 ppm (q,2H) -CH2 protons. 13C NMR spectrum: δ 165.35ppm -CH = N, δ 150.77 ppm phenolic, δ 112.09 - 147.37 ppm aromatic, δ 64.15 ppm -CH2, δ 14.68 ppm -CH2 carbon. EI Mass spectrum: C20H19N3O4S: 397.02 (Calcd., 397). HL3- Yield: 1.7260g (70%). Colour: Orange; m.p.170◦ C; Elemental Analysis (%) calculated for C19H18N4O4S: C 57.29; H 4.52; N 14.07; S 8.04. Found: C 57.20; H 4.50; N 14.34; S 8.79. 1H NMR spectrum: δ 8.45 ppm (s,1H) -CH = N, δ 12.62 ppm (s,1H) -OH, δ 5.94-7.59 ppm (m,11H) aromatic, δ 1.33-1.36 ppm (t,3H) -CH3, 4.05-4.01 ppm (q,2H) -CH2 protons. 13C NMR spectrum: δ 165.54-CH = N, δ 158.28 ppm phenolic, δ 64.12 ppm -CH2, δ 14.68 ppm -CH2 carbon. EI Mass spectrum: C19H18N4O4S: 398.49 (Calcd., 398).
Synthesis of Mn (II) complexes
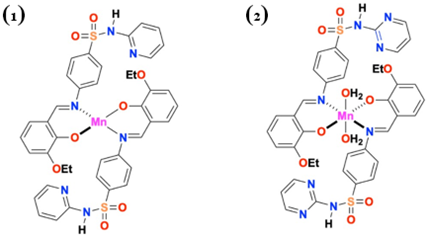
To a hot magnetically stirred ethanolic solution of Schiff bases 4-((3-ethoxy-2-hydroxybenzylidene)amino)-N-(pyridin-2-yl)benzenesulfonamide(HL2) (0.005) / 4-((3-ethoxy-2-hydroxybenzylidene)amino)-N-(pyrimidin-2-yl)benzenesulfonamide(HL3) (0.005) in minimum quantity of dimethyl formamide, an ethanolic solution of the Mn(II) acetate (0.0025 mol) was added separately. The mixtures were refluxed for 6 h on a water bath. The brownish yellow and peat brown precipitates formed during refluxing were cooled in an ice bath and collected by suction filtration, washed thoroughly with ethanol and pet ether, dried in desiccator over CaCl2. The coloured solids obtained were partially soluble in ethylene glycol, ethanol, acetic acid, nitromethane & acetonitrile and soluble in DMF & DMSO. The synthesis of the complexes 1 & 2 is shown in Scheme 1.
Biological assay
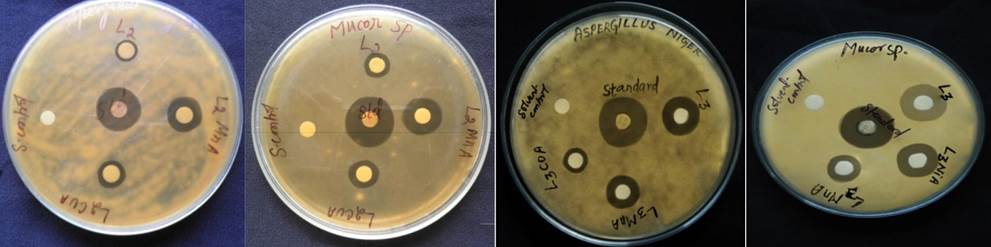
The antimicrobial activity of ligands and their complexes were tested against gram positive bacterial species Pseudomonas aeruginosa (NCIM 2036) and fungal species Aspergillus niger (NCIM 105) and Mucor sp. (NCIM 108) by disc diffusion method. The test was carried out in DMSO solution at a concentration of 100 ppm. The results were compared with standard drugs Ciprofloxacin for bacteria and Nystatin for fungi at the same concentration.
Results and Discussion
The physical characteristics of the Schiff bases and their complex are given in Table 1.
Table 1 Physical characteristics and analytical data of metal complexes.
| S. No. | Metal Complexes | Molecular Formula | Yield % | Elemental Analysis % Found (Calcd) | |||||
|---|---|---|---|---|---|---|---|---|---|
| M. Pt °C | C | H | N | S | Mn | ||||
| 1 | [Mn(L2)2] | MnC40H36N6O8S2 | 64 | 268 | 56.55 (56.68) | 4.18 (4.25) | 9.56 (9.92) | 7.51 (7.60) | 6.33 (6.49) |
| 2 | [Mn(L3)2(H2O)2] | MnC38H38N8O10S2 | 64 | 240 | 51.20 (51.52) | 4.11 (4.29) | 12.08 (12.66) | 7.08 (7.23) | 6.10 (6.21) |
Analytical data
The analytical data obtained for the Schiff bases and their complexes agree very well with the proposed molecular formulae and also indicates the formation of 1:2 metal and ligand ratio in the complexes. The theoretical values are in good agreement with the experimental values of % of elements C, H, N, S and the metal (Table 1). The colour of the complexes are attributed due to d-d transition because of unpaired electron in the d orbital. The sharp and high melting point of the complexes propose the formation of chelate.
Conductance
The molar conductance of metal complexes is measured using 10-3 M DMF solutions and are found 12.00 and 13.50 ohm-1 cm2 mol-1 for the complexes 1 and 2 suggesting the non-electrolytic nature [15-18] and indicate that no anions are present outside the coordination sphere.
IR Spectra
A sharp band at 1627 and 1616 cm-1 are assignable to the azomethine [19-22] ν(-CH=N) present in the Schiff bases HL2 and HL3. Evidence of nitrogen bonding of the azomethine ν(-CH=N) group to the central metal atom stems from the shift of the frequency to 1610 and 1598 cm-1 in the complexes 1 and 2. This is further supported by the appearance of the new bands at 445 and 435 cm-1 due to ν(Mn-N) band [23,24] . A broad band centered at 3433 [25] and 3356 cm-1 is characteristic of hydrogen bonded phenolic ν(O-H) stretching vibration of HL2 and HL3[26]. The coordination through phenolic oxygen, after deprotonation is revealed by the disappearance of the band at 3433 and 3356 cm-1 in the complexes 1 and 2 and it is further confirmed by the appearance of the non-ligand bands at 540 and 544 cm-1 due to ν(Mn-O) in the complexes [27]. The bands at 1358 cm-1 & 1327 cm-1 and 1133 cm-1 & 1157 cm-1 are assigned to νas(SO2) and νs(SO2) remains almost at the same position in the complexes suggesting that the sulfonamide oxygen of HL2 and HL3 is not taking part in coordination [28,29]. The ν(N-H) mode of the sulphonamide group of HL2 and HL3 is observed at 2924 and 2927 cm-1, remain unperturbed in the spectra of its complexes suggests that the sulphonamide nitrogen is not taking part in coordination. The band due to ν(-N=) of pyridine and pyrimidine ring appearing at 1589 and 1581 cm-1 did not show any appreciable change on complexation prove that ring nitrogen not involved in coordination.
Electronic spectra and magnetic susceptibility measurements
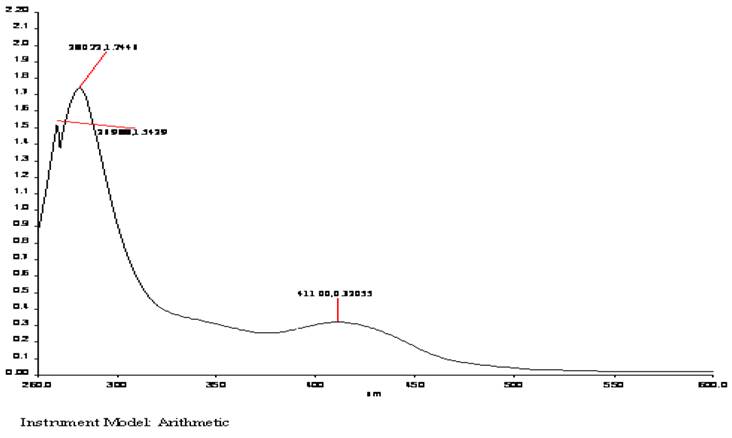
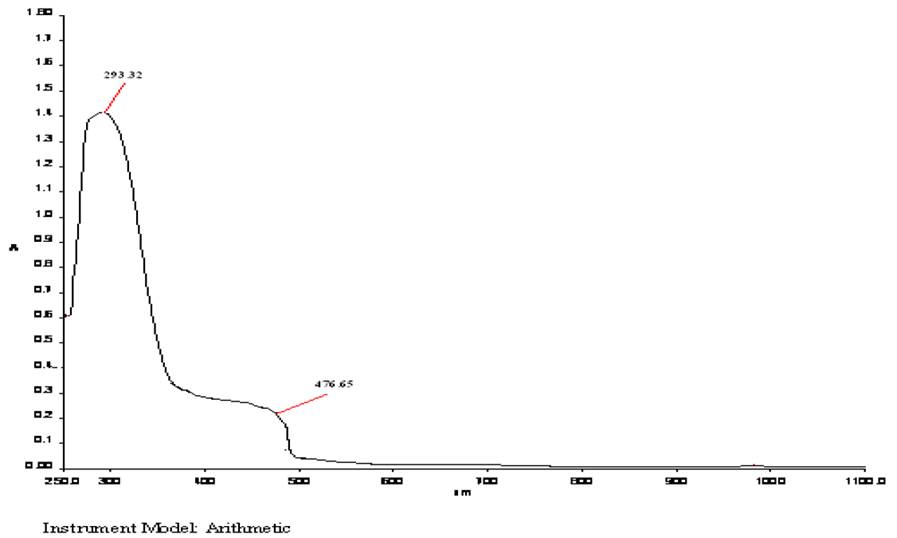
The electronic spectrum of complex 1 (Fig. 1) shows band at 411 nm (24330 cm-1) due to 4A1 ➝4T1 transition [30], exhibits paramagnestism with magnetic moment value of 5.61 B.M. suggesting tetrahedral geometry. The complex 2 (Fig. 2) exhibit weak band at 476 nm (21008 cm-1) which is assigned to 6A1g ➝4T2g(G) transition [31,32]. This observation reveals that the stereochemistry of complex 2 is consistent with the six-coordinate octahedral geometry. It exhibits magnetic moment value of 5.94 B.M. [33] suggesting octahedral geometry.
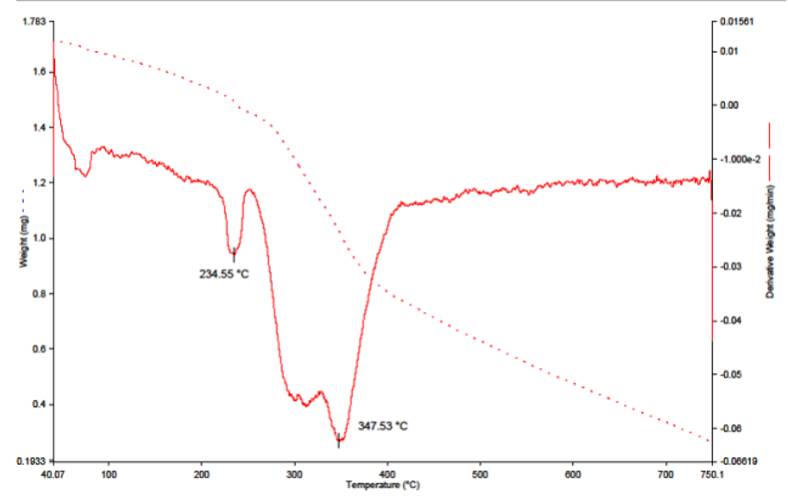
Thermal analysis
The thermogram of complex 1 (Fig. 3) has no endothermic peak in the range of 60-250 oC. This excludes the possibility of lattice or coordinated water molecules in this complex [34] and supports the four coordination and tetrahedral geometry around Mn (II) ion.
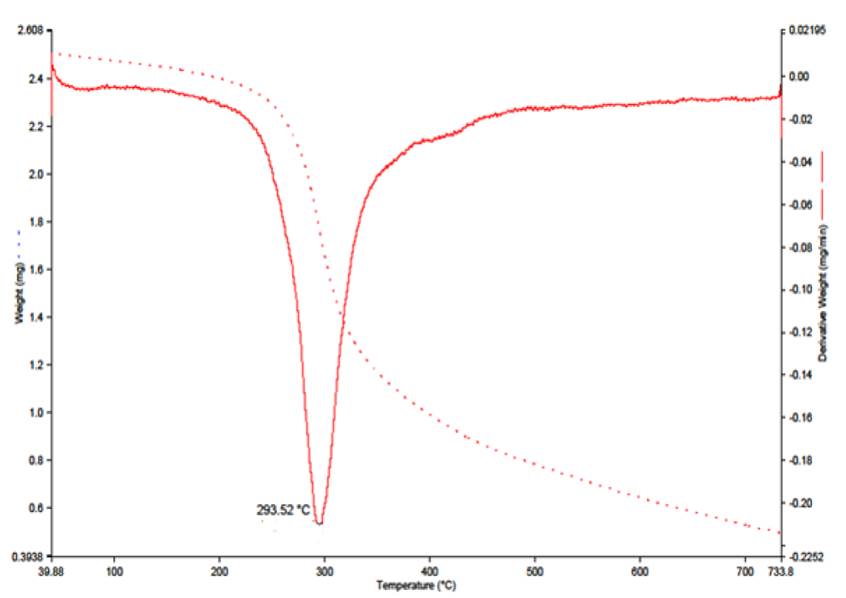
The thermogram of complex 2 (Fig. 4) shows weight loss of 4.0 % (Calcd., 4.03 %) in the temperature 234 °C attributed to the loss of two coordinated water molecules. This thermogram supports the octahedral geometry for complex 2.
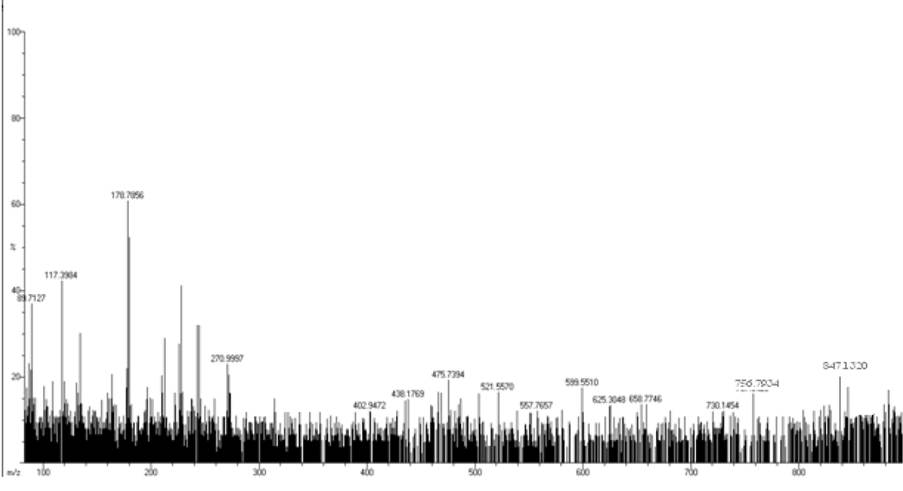
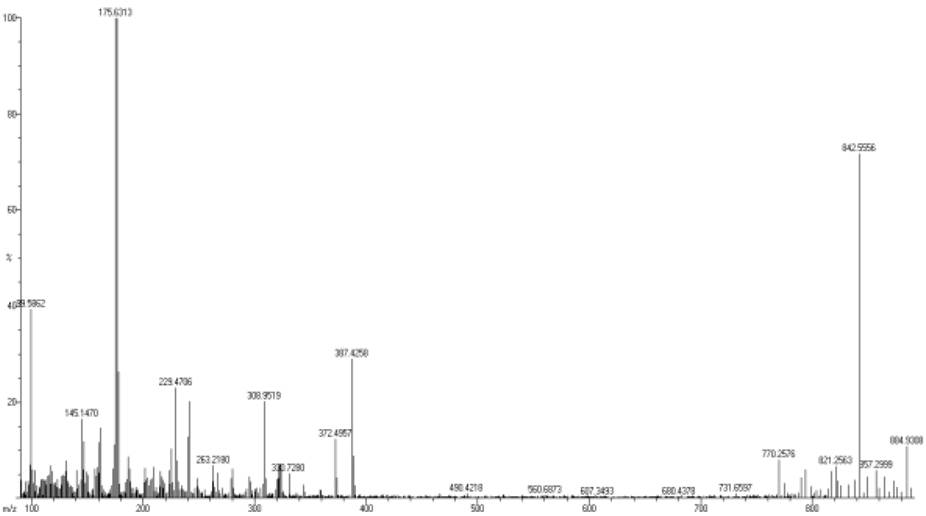
EI Mass Spectra
The mass spectral data of all the complexes (Fig. 5 and 6) are consistent with the formulations: MnC40H36N6O8S2: 847.13 (Calcd., 846.94) and MnC38H38N8O10S2: 884.93 (Calcd., 884.94).
Based on the analytical data, molar conductance value, newly formed band around 400 and 500 cm-1 in IR spectra illustrate non-electrolytic, 1:2 metal to ligand ratio and coordinate covalent nature of bond respectively. The absorption band at 24330 and 21008 cm-1, magnetic moment value and the thermogram of complexes prove the presence of LMCT transitions and tetrahedral arrangement for complex 1 and octahedral environment for complex 2. The above data confirmed that the Schiff bases act as bidentate monobasic ligand and coordination is proposed through azomethine nitrogen and oxygen of phenoxide ion and the following structures have been proposed for the newly synthesized complexes 1 and 2.
Antimicrobial Assessment
Both the Schiff bases and the complexes produced different diameter of inhibition zones was detected in millimeter against the tested microorganisms. The zone of inhibition of the complexes against the growth of bacteria and fungi are shown in Fig. 7 and 8. The comparative study of HL 2, HL 3 and its metal complexes indicates that the Schiff bases have been found to be biologically active and its metal complexes possess enhanced antimicrobial activities. The complex 1 bio potency has been much pronounced compared to the free Schiff base HL2[35-38]. The enhanced activity of the complexes can be explained on the basis of Overtone’s concept [39] and Tweedy’s Chelation theory [40]. The Overtone’s concept of cell permeability states that the cell membrane surrounding the microbial cell favours the passage of lipid soluble particles causing lipid solubility to be a crucial factor in determining antimicrobial activity and chelation tends to reduce the polarity of the metal ion due to the overlap of the ligand orbital and partial sharing of positive charge with the donor groups which results in rise in the lipophilicity of the metal complex [41]. So, the metal complex can easily penetrate into the lipid membranes and block the metal binding sites of enzymes of the microorganisms and block the synthesis of proteins, which limit further growth of the organism [42, 43].
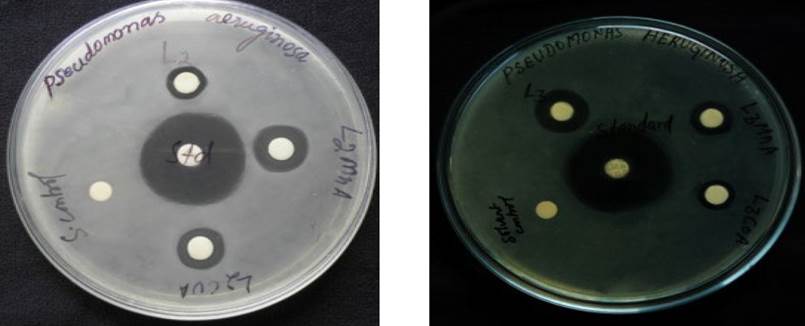
Fig. 7 In vitro antibacterial screening of Schiff bases and their metal complex against Pseudomonas aeruginosa.
Conclusion
Based on the above physical and chemical studies it is concluded that the Schiff bases act as bidentate monobasic ligand and coordination is proposed through azomethine nitrogen and oxygen of phenoxide ion. Based on the spectral data tetrahedral structure has been assigned to complex 1 and octahedral structure has been assigned to the complex 2. The ligand HL 2 and complexes show significant activity. The antimicrobial activity is enhanced for [Mn(L2)2] when compared to Schiff base.











 nueva página del texto (beta)
nueva página del texto (beta)