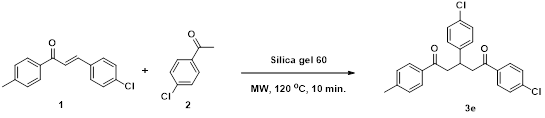Introduction
Enormous significance has been taken in solvent-free synthesis in order to develop conventional methods from the economic and environmentally benign perspective since the method does not employ harmful solvents [1-2]. Michael additions are the effective tool for the generation of new C-C bonds especially, applied for the synthesis of pharmacologically significant β-substituted carbonyl compounds [3-5]. Diketones are the promising precursor for the synthesis of various many heterocyclic and polyfunctional compounds, complex organic molecules and some diverse materials such as OLEDs, DNA binding ligands [3-8]. Several synthetic methods has been explored using a variety of toxic and expensive metal catalysts both acidic and basic such as lanthanides, Bi(NO3)3, Bi(OTf)3, Cu(BF4)2 and others [9-14]. The conjugate addition of aryl ketones to acetohydrazone of aromatic aldehyde with catalyst of 1,2-di-enamine is also an efficient approach [15] to develop the novel organic synthesis by eco-friendly technique accelerate organic synthesis [16-19]. Traditional approaches for the synthesize of 1,5-dicarbonyl compounds typically appeal the use of enolates with aryl/alkyl vinyl ketones through 1,4-addition [20-21]. In many cases, the synthesis of this desired 1,5-dicarbonyl systems can be promoted or catalyzed under strongly basic, Brønsted acid or Lewis acid catalysis conditions [22-26]. The general methods used for the synthesis of 1,3,5-triaryl-1,5-pentanedione in the presence of volatile organic solvents afford only moderate to low yields [27]. However, the use of toxic and expensive catalysts is not economically viable and limits their use in large scale production and also conflicts with aspects of green chemistry. Adsorbent mediated syntheses in solvent-free conditions have fascinated much interest owing to mild conditions, high selectivity and remarkable acceleration [28-29].
It has been planned to synthesize several heterocyclic compounds from simple starting materials during this investigation. α,ω-Diketones are excellent precursors to prepare heterocyclic compounds as they can be reacted with several nucleophiles such as nitrogen, oxygen, sulfur nucleophiles [30-35]. Out of this different α,ω-diketones, the 1,5-diketones are excellent building blocks towards this protocol, as they would lead to stable six membered rings [36-39]. Considering the structural feature of 1,3,5-triarylpentane-1,5-diones, a simple retrosynthetic approach can suggest that a Michael type addition of an acetophenone over a chalcone may lead to the target product. However, a symmetrical 1,3,5-triarylpentane-1,5-diones can be synthesized by a pseudo multicomponent approach involving two molecules of acetophenone and one molecule of arylaldehyde.
Though there are several methods available to get an unsymmetrical 1,3,5-triarylpentane-1,5-diones by the above strategy involving chalcone and aryl methyl ketone, protocols via greener conditions may be more attractive. In last three decades Microwave irradiation has extensively been believed as a clean and useful method for the initiation and acceleration of progressions in organic synthesis and has been consistently used in enormous number of organic syntheses to result higher yield in shorter reaction time under milder conditions [40-41]. In this paper, we desire to report that solvent-free Michael addition for the synthesis of 1,3,5-triarylpentane-1,5-dione derivatives from chalcones and aryl methyl ketones in the presence of silica gel.
Experimental
A CEM Discover microwave synthesizer (Model No: 908010) operating at 180/264 V and 50/60 Hz with microwave power maximum level of 300 W and microwave frequency of 2455 MHz was employed for the microwave-assisted experiments. Nuclear Magnetic Resonance (1H, 13C - NMR) spectra were recorded on 300 MHz spectrometer (Bruker) in CDCl3 using TMS as an internal standard. Chemical shifts are reported in parts per million (δ), coupling constants (J values) are reported in Hertz (Hz) and spin multiplicities are indicated by the following symbols: s (singlet), d (doublet), dd (doublet of doublet), ddd (doublet of doublet of doublet), dt (doublet of triplet), t (triplet), p (pentet), m (multiplet). 13C NMR spectra were routinely run with broadband decoupling. Pre coated silica gel on aluminium plates (Merck) were used for TLC analysis with a mixture of petroleum ether (60 - 80 °C) and ethyl acetate as the eluent. Electrospray ionization (ESI) mass spectra were obtained on an LCQ Fleet mass spectrometer, Thermo Fisher Instruments Limited, US and an Agilent mass spectrometer. HRMS was recorded on Brucker-Daltonics, Micro-TOF-Q II mass spectrometer. Elemental analyses were performed on a Perkin Elmer 2400 Series II Elemental CHNS analyser.
General procedure for the synthesis of 1,3,5-triarylpentan-1,5-diones (3)
A mixture of chalcone 1 (1.0 equiv), aryl methyl ketone 2 (1.0 equiv.) and Silica gel 60 (spherical, 63-200 μm) (3 g) was taken in a 10 mL quartz vial and placed in the microwave oven. The vial was sealed with a pressure cap and subjected to microwave irradiation. The irradiation was programmed between 100 - 120 °C, 120 W, 5 bar, for 10 min. The reaction was monitored by TLC using petroleum ether/ethyl acetate mixture (7:3) as the eluent. After the reaction mixture was cooled to room temperature, ethanol was added, and reaction mixture was separated from the silica gel. The obtained crude was filtered, dried in vacuum, and recrystallized from ethanol to afford 3. Then, the silica gel was carefully washed well with methanol: dichloromethane (1:1) and dried in hot air oven at 130 °C under reduced pressure for 1 h. The recovered silica gel could be recycled.
Characterization of compounds 3
3-(4-Methylphenyl)-1,5-diphenyl-1,5-pentanedione (3a) [42]: Isolated as colorless solid; mp: 120 - 121 oC; IR (KBr): 3069, 3037, 2898, 1678 cm-1; 1H NMR (300 MHz, CDCl3) δ 7.94 (dt, J = 3.5, 1.5 Hz, 3H), 7.53 (dd, J = 10.4, 4.2 Hz, 2H), 7.42 (dd, J = 11.5, 4.3 Hz, 3H), 7.28 - 7.20 (m, 3H), 7.12 (dd, J = 27.9, 8.1 Hz, 3H), 4.03 (p, J = 6.8 Hz, 1H), 3.48 (dd, J = 16.5, 6.9 Hz, 2H), 3.33 (dd, J = 16.6, 6.2 Hz, 2H), 2.28 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 198.6, 140.7, 136.9, 136.1, 132.9, 129.3, 128.5, 128.1, 127.2, 44.9, 36.8, 20.9. ESI-MS m/z calcd for C24H22O2: 342.43 [M]+; Found: 343.59 [M+H]+ . Anal. Calcd for C24H22O2: C, 84.18; H, 6.48 %. Found: C, 84.14; H, 6.52 %.
1,5-Bis(4-methylphenyl)-3-phenyl-1,5-pentanedione (3b) [42]: Isolated as colorless solid; mp: 108 - 110 oC; IR (KBr): 3059, 3028, 2891, 1679 cm-1; 1H NMR (300 MHz, CDCl3) δ 7.88 (d, J = 8.1 Hz, 4H), 7.45 - 7.39 (m,6H), 4.00 (p, J = 6.8 Hz, 1H), 3.45 (dd, J = 16.9, 6.8 Hz, 2H), 3.27 (dd, J = 16.8, 7.2 Hz, 2H), 2.41 (s, 6H). 13C NMR (75 MHz, CDCl3) δC: 198.0, 144.0, 142.4, 134.3, 132.2, 129.3, 128.9, 128.7, 128.2, 44.6, 36.6, 21.6. ESI-MS m/z calcd for C25H24O2: 356.46 [M]+; Found: 357.21 [M+H]+ . Anal. Calcd for: C, 84.24; H, 6.79 %. Found: C, 84.27; H, 6.75 %.
1,3,5-Tri(4-methylphenyl)-1,5-pentanedione (3c) [6]: Isolated as colorless crystal; mp: 87 - 89 oC; IR (KBr): 3058, 3027, 2891, 1679 cm-1; 1H NMR (300 MHz, CDCl3) δH: 7.86 (d, J = 8.1 Hz, 4H), 7.24 (d, J = 8.1 Hz, 4H), 7.17 (d, J = 8.1 Hz, 2H), 7.08 (d, J = 8.1 Hz, 2H), 4.05 (p, J = 6.9 Hz, 1H), 3.45 (dd, J = 16.5, 6.9 Hz, 2H), 3.32 (dd, J = 16.5, 6.9 Hz, 2H), 2.39 (s, 6H), 2.28 (s, 3H). 13C NMR (75 MHz, CDCl3) δC: 198.3, 143.8, 140.9, 136.1, 134.4, 129.3, 129.2, 128.3, 127.3, 45.0, 36.9, 21.6, 21.0. ESI-MS m/z calcd for C26H26O2: 370.49 [M]+; Found: 371.77 [M+H]+ . Anal. Calcd for C26H26O2: C, 84.29; H, 7.07%. Found: C, 84.32; H, 7.11 %.
3-(4-Chlorophenyl)-1,5-di(4-methylphenyl)-1,5-pentanedione (3d) [42]: Isolated as colorless solid; mp: 113 - 114 oC; IR (KBr): 3065, 3031, 2888, 1677 cm-1; 1H NMR (300 MHz, CDCl3) δ 7.70 (d, J = 8.6 Hz, 4H), 7.23 (d, J = 8.6 Hz, 4H), 6.96 (d, J = 8.0 Hz, 2H), 6.90 (d, J = 8.2 Hz, 2H), 3.80 (p, J = 6.9 Hz, 1H), 3.27 (dd, J = 16.6, 7.0 Hz, 2H), 3.10 (dd, J = 16.6, 7.0 Hz, 2H), 2.41 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 198.8, 143.9, 141.9, 136.1, 132.7, 129.6, 128.9, 128.7, 128.2, 44.5, 39.0, 21.1. HRMS (ESI) m/z calcd for C25H23ClO2: 390.9019 [M]+; Found: 390.9021 [M]+. Anal. Calcd for C25H23ClO2: C, 76.81; H, 5.93 %. Found: C, 76.85; H, 5.99 %.
1,5-Bis(4-chlorophenyl)-3-(4-methylphenyl)-1,5-pentanedione (3e) [6]: Isolated as colorless crystal; mp: 72 - 73 oC; IR (KBr): 3077, 3037, 2886, 1679 cm-1; 1H NMR (300 MHZ, CDCl3) δH: 7.88 (d, J = 8.6 Hz, 4H), 7.40 (d, J = 8.6 Hz, 4H), 7.14 (d, J = 8.0 Hz, 2H), 7.07 (d, J = 8.0 Hz, 2H), 3.97 (p, J = 6.9 Hz, 1H), 3.44 (dd, J = 16.6, 7.0 Hz, 2H), 3.27 (dd, J = 16.6, 7.0 Hz, 2H), 2.28 (s, 3H). 13C NMR (75 MHz, CDCl3) δC: 197.4, 140.3, 139.5, 136.4, 135.3, 129.5, 129.3, 128.9, 127.2, 44.9, 36.9, 20.9. HRMS (ESI) m/z calcd for C24H20Cl2O2: 411.3204 [M]+; Found: 411.3207 [M]+. Anal. Calcd for C24H20Cl2O2: C, 70.08; H, 4.90 %. Found: C, 70.12; H, 4.92 %.
3-(4-Bromophenyl)-1,5-bis(4-chlorophenyl)-1,5-pentanedione (3f) [6]: Isolated as colorless solid; mp: 95 - 96 oC; IR (KBr): 3060, 3027, 2894, 1679 cm-1; 1H NMR (300 MHz, CDCl3) δ 7.95 (d, J = 8.5 Hz, 2H), 7.87 (d, J = 8.5 Hz, 2H), 7.67 (d, J = 8.5 Hz, 2H), 7.50 (d, J = 8.5 Hz, 2H), 7.30 (dd, J = 14.7, 8.6 Hz, 4H), 4.08 (p, J = 7.2 Hz 1H), 3.52 (dd, J = 16.9, 6.8 Hz, 2H), 3.34 (dd, J = 17.4, 7.7 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 197.0, 141.9, 139.8, 135.4, 131.9, 129.6, 129.5, 129.0, 128.7, 44.7, 36.5. HRMS (ESI) m/z calcd for: 476.1899 [M]+; Found: 476.1895 [M]+. Anal. Calcd. for C23H17BrCl2O2; C, 58.01; H, 3.60 %. Found: C, 58. 03; H, 3.58 %.
3-(3-Bromophenyl)-1,5-bis(4-chlorophenyl)-1,5-pentanedione (3g) [6]: Isolated as colorless solid; mp: 92 - 93 oC; IR (KBr): 3052, 3031, 2882, 1675 cm-1; 1HNMR (300 MHz, CDCl3) δH: 7.88 (d, J = 8.4 Hz, 4H), 7.42 - 7.12 (m, 8H), 4.06 (p, J = 6.9 Hz, 1H), 3.45 (dd, J = 17.0, 6.8 Hz, 2H), 3.28 (dd, J = 16.9, 6.9 Hz, 2H). 13C NMR (75 MHz, CDCl3) δC: 196.8, 145.9, 139.6, 134.9, 130.4, 130.2, 129.9, 129.5, 128.9, 126.3, 122.7, 44.4, 36.6. HRMS (ESI) m/z calcd for: 476.1899 [M]+; Found: 477.1894 [M]+. Anal. Calcd. for C23H17BrCl2O2: C, 58.01; H, 3.60%. Found: C, 58.04; H, 3.64 %.
1,5-Bis(4-chlorophenyl)-3-[4-(dimethylamino)phenyl]-1,5-pentanedione (3h) [6]: Isolated as pale yellow solid; mp: 82 - 84 oC; IR (KBr): 3061, 3029, 2894, 1679 cm-1; 1H NMR (300 MHz, CDCl3) δH: 7.89 (d, J = 8.4 Hz, 4H), 7.41 (d, J = 8.3 Hz, 4H), 7.11 (d, J = 8.6 Hz, 2H), 6.65 (d, J = 8.6 Hz, 2H), 3.91 (p, J = 7.0 Hz, 1H), 3.42 (dd, J = 16.4, 7.1 Hz, 2H), 3.25 (dd, J = 16.4, 6.9 Hz, 2H), 2.90 (s, 6H). 13C NMR (75 MHz, CDCl3) δC: 197.9, 149.6, 139.5, 135.4, 131.1, 129.7, 129.0, 128.1, 112.9, 45.4, 40.7, 36.6. HRMS (ESI) m/z calcd for C25H23Cl2NO2: 440.3616 [M]+; Found: 440.3617 [M]+. Anal. Calcd for C25H23Cl2NO2: C, 68.19; H, 5.26; N, 3.18 %. Found: C, 68.14; H, 5.30; N, 3.22 %.
3-(2,3-Dichlorophenyl)-1,5-bis(4-fluorophenyl)-1,5-pentanedione (3i): Isolated as colorless solid; mp: 70 - 72 oC; IR (KBr): 3063, 3031, 2896, 1677 cm-1; 1H NMR (300 MHz, CDCl3) δ 8.02 - 7.97 (m, 4H), 7.31 (d, J = 7.8, 1H), 7.23 (d, J = 7.5 Hz, 1H), 7.16 - 7.09 (m, 5H), 4.56 (p, J = 6.9 Hz, 1H), 3.55 - 3.35 (m, 4H). 13C NMR (75 MHz, CDCl3) δ 196.5, 167.6, 164.2, 147.0, 143.1, 133.1, 130.9, 130.7, 128.8, 127.3, 115.9, 115.6, 42.7, 34.8. HRMS (ESI) m/z calcd for C23H16Cl2F2O2: 433.2747 [M]+; Found: 433.2749 [M]+. Anal. Calcd. for C23H16Cl2F2O2: C, 63.76; H, 3.72 %. Found: C, 63.81; H, 3.75 %.
3-(4-Chlorophenyl)-1,5-bis(2-naphthyl)-1,5-pentanedione (3j) [6]: Isolated as colorless solid; mp: 120 - 122 oC; IR (KBr): 3057, 3028, 2889, 1679 cm-1; 1H NMR (300 MHz, CDCl3) δH: 8.50 (s, 2H), 8.01 - 7.83 (m, 8H), 7.63 - 7.51 (m, 4H), 7.30 - 7.23 (m, 4H), 4.19 (p, J = 6.9 Hz, 1H), 3.67 (dd, J = 16.7, 6.7 Hz, 2H), 3.47 (dd, J = 16.7, 7.2 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 198.2, 142.3, 135.6, 134.0, 132.5, 132.3, 129.9, 129.6, 128.9, 128.8, 128.5, 128.4, 127.7, 126.8, 123.8, 44.8, 36.8. HRMS (ESI) m/z calcd for: 462.9661 [M]+; Found: 462.9665 [M]+. Found: %. Anal. Calcd. for C31H23ClO2: C, 80.42; H, 5.01 %. Found: C, 80.38; H, 5.06 %.
3-(4-Chlorophenyl)-1,5-bis(thiophene-2-yl)-1,5-pentanedione (3k) [6]: Isolated as colorless crystal; mp: 110 - 111 oC; IR (KBR) 3298, 3172, 2931, 2852, 1701 cm-1; 1H NMR (300 MHz, CDCl3) δH: 7.76 (dd, J = 3.8, 1.1 Hz, 2H), 7.64 (dd, J = 5.0, 1.1 Hz, 2H), 7.25-7.23 (m, 4H), 7.13 (dd, J = 4.9, 3.8 Hz, 2H), 4.09 (p, J = 6.0 Hz, 1H), 3.43 (dd, J = 16.2, 6.8 Hz, 2H), 3.26 (dd, J = 16.2, 7.4 Hz, 2H). 13C NMR (75 MHz, CDCl3) δC: 190.9, 143.9, 141.6, 133.8, 132.3, 132.1, 128.8, 128.6, 128.1, 45.0, 37.0. HRMS (ESI) m/z calcd for C19H15ClO2S2: 374.9042 [M]+; Found: 374.9047 [M]+. Anal. Calcd for C19H15ClO2S2: C, 60.87; H, 4.03; S, 17.10 %. Found: C, 60.89; H, 4.07; S, 17.12 %.
1,3,5-Tris(thiophen-2-yl)-1,5-pentanedione (3l): Isolated as colorless crystal; mp: 103 - 105 oC; IR (KBR) 3297, 3168, 2934, 2859, 1695 cm-1; 1H NMR (300 MHz, CDCl3) δH: 7.76 (d, J = 3.8 Hz, 2H), 7.62 (d, J = 4.9 Hz, 2H), 7.16 - 7.07 (m, 3H), 6.92 - 6.82 (m, 2H), 4.39 (p, J = 6.9 Hz, 1H), 3.47 (dd, J = 16.3, 6.8 Hz, 2H), 3.34 (dd, J = 16.2, 7.0 Hz, 2H).13C NMR (75 MHz, CDCl3) δC: 190.6, 143.8, 133.6, 132.0, 127.9, 126.5, 124.2, 123.2, 121.9, 45.7, 32.7. ESI-MS m/z calcd for C17H14O2S3: 346.47 [M]+; Found: 347.22 [M+H]+. Anal. Calcd. for C17H14O2S3; C, 58.93; H, 4.07; S, 27.76 %. Found: C, 58.90; H, 4.12; S, 27.72 %.
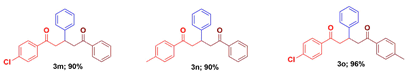
1-(4-Chlorophenyl)-3,5-diphenyl-1,5-pentanedione (3m) [6]: Isolated as colorless solid; mp: 97 - 99 oC; IR (KBr): 3060, 3029, 2894, 1675, 1680 cm-1; 1H NMR (300 MHz, CDCl3) δH: 7.96 (d, J = 7.3 Hz, 2H), 7.90 (d, J = 8.6 Hz, 2H), 7.57 (t, J = 7.3 Hz, 1H), 7.49 - 7.41 (m, 4H), 7.33 - 7.22 (m, 5H), 4.10 (p, J = 6.0 Hz, 1H), 3.50 (dd, J = 16.8, 7.2 Hz, 2H), 3.34 - 3.27 (m, 2H). 13C NMR (75 MHz, CDCl3) δC: 198.4, 197.4, 143.5, 139.4, 136.7, 135.1, 133.1, 129.5, 128.8, 128.6, 128.5, 128.0, 127.4, 126.7, 44.8, 44.7, 37.1. HRMS (ESI) m/z calcd for C23H19ClO2: 362.8488 [M]+; Found: 362.8490 [M]+. Anal. Calcd for C23H19ClO2: C, 76.13; H, 5.28 %. Found: C, 76.18; H, 5.25 %.
1-(4-Methylphenyl)-3,5-diphenyl-1,5-pentanedione (3n) [43]: Isolated as colorless solid; mp: 99 - 101 oC; IR (KBr): 3058, 3027, 2890, 1677, 1679 cm-1; 1H NMR (300 MHz, CDCl3) δ 8.01 (dt, J = 3.5, 1.5 Hz, 3H), 7.59 (dt, J = 10.4, 1.4 Hz, 2H), 7.52 - 7.46 (m, 3H), 7.34 - 7.13 (m, 6H), 4.17 - 4.05 (m, 1H), 3.58 - 3.50 (m, 2H), 3.42 - 3.37 (m, 2H), 2.34 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 198.6, 198.5, 143.8, 140.7, 136.9, 136.1, 132.9, 129.3, 129.2, 128.5, 128.2, 128.1, 127.4, 127.2, 44.8, 37.2, 21.6. ESI-MS m/z calcd for C24H22O2: 342.43 [M]+; Found: 343.22 [M+H]+ . Anal. Calcd for C24H22O2: C, 84.18; H, 6.48 %. Found: C, 84.22; H, 6.43 %.
1-(4-Chlorophenyl)-5-(4-methylphenyl)-3-phenyl-1,5-pentanedione (3o): Isolated as colorless solid; mp: 101 - 102 oC; IR (KBr): 3052, 3031, 2890, 1676, 1681 cm-1; 1H NMR (500 MHz, Chloroform) δ 7.65 (d, J = 7.5 Hz, 2H), 7.60 (d, J = 7.5 Hz, 2H), 7.35 - 7.25 (m, 6H), 7.23 - 7.15 (m, 3H), 3.63 (p, J = 6.8 Hz, 1H), 3.44 (dd, J = 12.4, 8.0 Hz, 1H), 3.37 (dd, J = 12.5, 3.3 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 198.51, 143.7, 143.6, 138.6, 135.8, 129.3, 128.9, 128.8, 128.2, 127.9, 127.5, 127.1, 44.2, 38.7, 20.8. HRMS (ESI) m/z calcd for: 376.8753 [M]+; Found: 376.8756 [M]+. Ana. Calcd. for C24H21ClO2; C, 76.49; H, 5.62 %. Found: C, 76.54; H, 5.68 %.
1-(4-Chlorophenyl)-3-(4-methoxyphenyl)-5-(4-methylphenyl)-1,5-pentanedione (3p): Isolated as colorless crystal; mp: 111 - 113 oC; IR (KBr): 3066, 3029, 2898, 1678, 1681 cm-1; 1H NMR (300 MHz, CDCl3) δ 7.89 (d, J = 6.7 Hz, 2H), 7.84 (d, J = 8.3 Hz, 2H), 7.40 (d, J = 6.7 Hz, 2H), 7.24 (d, J = 8.0 Hz, 2H), 7.17 (d, J = 8.7 Hz, 2H), 6.80 (d, J = 8.7 Hz, 2H), 4.02 - 3.91 (m, 1H), 3.75 (s, 3H), 3.43 (ddd, J = 16.6, 12.0, 6.8 Hz, 2H), 3.25 (ddd, J = 16.2, 14.0, 7.1 Hz, 2H), 2.40 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 198.3, 197.6, 158.4, 143.9, 139.5, 135.7, 135.4, 134.6, 129.6, 129.3, 128.9, 128.4, 128.3, 114.1, 55.2, 45.1, 45.0, 36.6, 21.7. HRMS (ESI) m/z calcd for: 406.9013 [M]+; Found: 406.9017 [M]+. Anal. Calcd. for C25H23ClO3; C, 73.79; H, 5.70 %. Found: C, 73.82; H, 5.68 %.
3-(4-Chlorophenyl)-1-(4-methylphenyl)-5-(2-naphthyl)-1,5-pentanedione (3q): Isolated as colorless solid; mp: 107 - 109 oC; IR (KBr): 3061, 3031, 2882, 1679, 1683 cm-1; IR (KBr) 3288, 3068, 2947, 2852, 1718, 1696, 1654, 1091, 702 cm-1; 1H NMR (300 MHz, CDCl3) δH: 8.48 (s, 1H), 7.97 (t, J = 8.2 Hz, 2H), 7.89 - 7.84 (m, 4H), 7.64 - 7.51 (m, 3H), 7.25 (m, 5H), 4.16 (p, J = 6.9 Hz, 1H), 3.72 - 3.51 (m, 2H), 3.46 - 3.29 (m, 2H), 2.41 (s, 3H). 13C NMR (75 MHz, CDCl3) δC: 198.1, 197.8, 144.0, 142.4, 135.5, 134.2, 134.0, 132.4, 132.1, 129.8, 129.5, 129.3, 128.9, 128.7, 128.4, 128.4, 128.2, 127.7, 126.7, 123.7, 44.7, 44.6, 36.7, 21.6. HRMS (ESI) m/z calcd for: 426.9340 [M]+; Found: 426.9343 [M]+. Anal. Calcd. for C28H23ClO2; C, 78.77; H, 5.43 %. Found: C, 78.01; H, 5.48 %.
1-(4-Chlorophenyl)-3-(4-methylphenyl)-5-(thiophene-2-yl)-1,5-pentanedione (3r) [6]: Isolated as colorless crystal; mp: 90 - 91 oC; IR (KBR) 3288, 3171, 2939, 2849, 1705, 1698 cm-1; 1H NMR (300 MHz, CDCl3) δH: 7.90 (d, J = 6.8 Hz, 2H), 7.76 (dd, J = 3.8, 1.1 Hz, 1H), 7.63 (dd, J = 4.9, 1.1 Hz, 1H), 7.42 (d, J = 8.7 Hz, 2H), 7.18 - 7.08 (m, 5H), 4.04 (p, J = 6.0 Hz, 1H), 3.54 (dd, J = 15.0, 6.0 Hz, 1H), 3.43 (dd, J = 15.0, 6.0 Hz, 1H), 3.33-3.21 (m,2H) 2.30 (s, 3H). 13C NMR (75 MHz, CDCl3) δC: 197.7, 191.8, 144.6, 140.6, 139.8, 136.7, 135.5, 134.1, 132.5, 129.9, 129.7, 129.2, 128.5, 127.6, 46.0, 45.0, 37.5, 21.3. HRMS (ESI) m/z calcd for: 382.9031 [M]+; Found: 382.9036 [M]+. Anal. Calcd. for C22H19ClO2S; C, 69.01; H, 5.00; S, 8.37 %. Found: C, 69.06; H, 5.04; S, 8.40 %.
Results and Discussion
First, to attain suitable reaction conditions for the synthesis of 1,3,5-triarylpentane-1,5-dione. We investigated Michael addition of aryl methyl ketones (1) with 1.0 equivalents of Chalcone 2 under Microwave conditions mediated by various adsorbents such as Activated alumina, Montmorillonite K10, Activated carbon. However, the reactions were not completed even for longer reaction time and also resulted in the lower yields of 3e (12-48 %) simultaneously with the formation of complex mixture which attributed that this lower yield might be due to the more acidic nature of these adsorbents (Table 1. entries 1-4). The lower yield of 3e by using activated carbon (12 %) indicating that activated carbon is much weaker acidic nature (able 1. entry 5). By employing silica gel (Silica gel 60, 63-200 μm) resulted the product 3e in the reasonably excellent yield (95 %) (Table 1. entry 6). Even employing the same reaction condition with other silica gel gave nearly the same results (Table 1. entries 7 and 8). Even the same reaction condition applied with powdered silica gel too led to the as similar result (Table 1. entry 9). Michael addition mediated by sand resulted 3e in the low yield (12 %) with the formation of a huge crude mess which indicating that sand cannot be able to adsorb a large amount of aryl methyl ketones (Table 1. entry 10).
Table1. Optimization of Michael addition mediated by some adsorbentsa.
aChalcone 1 (1.0 mmol), aryl methyl ketone 2 (2.0 mmol) and adsorbent (3 g). bAlumina (activated, acidic, 150 mesh) was used. cMontmorillonite K10 (surface area 220-270 m2/g) was used. dH-Mordenite (5-7 μm) was used was used. eActivated carbon (100 mesh) was used. fSilica gel 60 (spherical, 63-200 μm) was used. gSilica gel 60 (amorphous, 63-210 μm) was used. hSilica gel 60 (45-106 μm, spherical) was used. iSilica gel 60 (crushed, 63-200 μm,) was used. jSand C (40-80 mesh) was used.
Utilizing these optimized conditions, we explore the scope of this strategy employing a library of 1,5-diketones with different aryl substituents at 1,3 and 5 positions. The aryl group bearing electron-releasing/electron-withdrawing groups in the aryl rings of either acetophenone or chalcone does not have any influence on the product yield. Thus, substituent scope of this reaction has been successfully established and their yields are listed in the Table 2.
It is also to be noted that yields are excellent and the reaction mass does not require any column purification technique. Out of the twenty-two compounds synthesized, twelve of them are new and they have been fully characterized by spectral details (See Supporting information).
Furthermore, the recycle experiments for the synthesis of 3e were performed with recovered silica gel. The recovered silica gel was washed well with methanol: dichloromethane (1:1) and dried at 130 °C under reduced pressure for 1 h. Silica gel could be recycled five times without substantial decrease of the yields (Table 3).
Table 3 Recycle experiments for the silica gel mediated reaction.a
aChalcone 1 (1.0 mmol), aryl methyl ketone 2 (2.0 mmol) and Silica gel 60 (spherical, 63-200 μm, 3 g), Microwave, 120 °C, 10 min. b Isolated yields.
Mechanism
A plausible mechanism is shown in Scheme 2 for the formation of 3. The keto form of the aryl methyl ketone 2 is in equilibrium with the enol form 4 which could be formed probably due to the shifting of equilibrium results the enol form 4 by coordination of the weakly acidic hydrogen to the oxygen in 2 from Silica gel. The enol for 4 would attack the Chalcone 1 which is a Michael acceptor to result 3 via the enol form 5. In addition, enol 4 attacks the Chalcone 1 would be accelerated by the coordinating via hydrogen from the Silica gel to the oxygen atom of 1. Accordingly, hydrogen bonding between hydroxy groups which is present on silica gel to substrates would participate in significant role in this particular Michael addition reaction.
Conclusion
In summary, the present work describes a series of symmetrical/unsymmetrical 1,3,5-Triarylpentane-1,5-diketone derivatives have been prudently synthesized from easily available starting materials such as aldehydes and acetophenones using silica gel as an adsorbent using microwave. The entire strategy is realized under mild reaction condition and easy work up by recrystallization with excellent yields. This ultrasonic strategy assures to generate synthetically useful 1,5-diketone precursors which could be utilized for the construction of various heterocyclic systems.











 nueva página del texto (beta)
nueva página del texto (beta)