Introduction
Fishes of the Haemulidae family have an essential ecological role in energy fixation for the reef communities and they also are one of the largest prey of larger species, such as groupers and snappers (Cushing, 1975; Darcy, 1983). Worldwide, a total of 133 species in 18 genera are recognized (Nelson et al., 2016). Haemulon is one of the most representative genera, with 23 species (Froese & Pauly, 2017). Haemulon plumierii and Haemulon flavolineatum are the most abundant species in the Caribbean reef areas (Gaut & Munro, 1983).
Haemulon plumierii (Lacepède, 1801) is distributed from the eastern coast of the United States to the southern coast of Brazil and occupies a wide variety of habitats, such as continental shelf waters, coral and rocky reefs, mangroves, and seagrass beds (Darcy, 1983; Rocha et al., 2008; Sponaugle et al., 2002). In Florida, H. plumierii is mainly caught by hand and hook line (Murie & Parkyn, 2005; Padgett, 1997). It is one of the most representative fishery resources in Brazil (Hoffmann et al., 2017), as well as an important component in artisanal fisheries in several Caribbean countries, including Mexico (Araújo & Martins, 2007; Fernández et al., 2011; Palazón-Fernández, 2007; Potts & Manooch III, 2001; Shinozaki et al., 2013).
The capture of H. plumierii off the northern coast of the Yucatán Peninsula is associated with the scale-fishing of other target species, such as grouper and snapper (Fernández et al., 2011). It is also an important component of recreational fishing, and therefore represents an important species in small-scale and subsistence fishing for the coastal communities (Mexicano-Cíntora et al., 2007; Poot-López et al., 2018; Treinen-Crespo et al., 2012). In this region, fishermen have been previously allowed to catch species considered as unprofitable or of low value due to the considerable decrease in catches of historically relevant fishery resources, like red grouper (DOF, 2006, 2014; Salas et al., 2006).
In the southern Gulf of Mexico, H. plumierii has acquired relevance as a fishing source in the last four decades (Mexicano-Cíntora et al., 2009; López-Rocha et al., 2020). At least from 1976 to 2007, catches have shown fluctuations ranging from 89 to 333 tons (t) (Mexicano-Cíntora et al., 2007, 2009). Additionally, catches remained above 550 t since 2005, reaching almost 1,000 t in the 2009-2014 period (Conapesca, 2018). In consequence, this species may be subject to intense exploitation without considering any fishing regulations since not enough is known about its biology or population dynamics to generate suitable administrative measures.
Information about population dynamics and reproduction in the species is fundamental to outline sustainable management measures, such as closed seasons and minimum catch sizes, that would guarantee its continuity (Domeier et al., 2002; Villacorta & Saint, 1999). Haemulon plumierii is a gonochoric species, without evident sexual dimorphism (Darcy, 1983). In addition, studies related to the reproduction of this species have been carried out in Jamaica (Munro et al., 1973), USA (Murie & Parkyn, 1999), Venezuela (Palazón-Fernández, 2007), and Brasil (Hoffmann et al., 2017; Shinozaki et al., 2013; Silva, 2015; Souza, 2008). However, biological information of this species in the southern Gulf of Mexico is scarce, mainly focused on its growth and mortality, genetic differentiation among populations, and length- weight relationships (Domínguez-Viveros & Avila- Martínez, 1996; Oribe-Pérez et al., 2020; Poot-López et al., 2017; Villegas-Hernández et al., 2014). Only one published study has addressed reproductive traits for H. plumierii on the Campeche Bank (northern coast off the Yucatán Peninsula) (Darcy, 1983), such as those relating to spawning seasonality and sexual maturity; however this reference is more than 30 years old. Other more distinctive details of its reproduction are still missing, such as its oocyte development (whether it is synchronous, group-synchronous, or asynchronous), type of fecundity (whether it is determinate or indeterminate) based on the microscopic characteristics of germ cells, and its oocyte frequency distribution among the reproductive phases present during an annual cycle. The overall aim of this study was to explore the reproductive biology of H. plumierii off the northern coast of the Yucatán Peninsula during an annual cycle to inform future sustainable management measures such as closed seasons and minimum catch sizes, that may guarantee the continuity of the species populations in the area before they reach overexploitation levels, as observed in other species already threatened within the region.
Materials and methods
The study area in the Yucatán Peninsula has a warm semi-dry climate from March to June, with heavy rains from July to October, and strong north winds and lower intensity rains the rest of the year (Mexicano-Cíntora et al., 2007). The average annual sea surface temperature is 25.6 °C, with the highest temperatures usually recorded in May and the lowest recorded in January. The winter season is characterized by the entrance of northern cold fronts (known locally as ‘northerlies’), which bring rainfall and low temperatures. Monthly samplings independent of commercial fishing were carried out on the continental shelf of the central coast of the Campeche Bank off the northern coast of the Yucatán Peninsula (Fig. 1). The study was conducted from September 2015 to November 2016. Fish were collected during daylight hours by hook and line (numbers 8/0, 10/0, and 14/0), using squid tentacles (Dosidicus gigas, d’Orbigny, 1835) as bait.

Figure 1 Location of the study area and the four sampling sites (dots) along the continental shelf off the northern coast of the Yucatán Peninsula.
At the laboratory, specimens were measured to the nearest 0.1 cm to obtain the total length (TL) and to construct the size-frequency distribution of the entire sample; individuals were grouped into class intervals previously defined from the Sturges rule (Scott, 2009). Once total weight (TW) was measured to the nearest 0.01 g, all samples were dissected and eviscerated, and the eviscerated body weight (EW), gonad weight (GW), and liver weight (LW) were obtained to the nearest 0.001 g. The gonadosomatic index (GSI) was calculated for each individual based on EW to avoid possible variations arising from differences in the contents of the digestive tract with the following formula GSI = 100×(GW/EW), where GW and EW represent gonad and eviscerated weights, respectively. The gonads were fixed in Bouin’s solution for further histological processing and fecundity estimations.
Although the sex and reproductive status of specimens were first macroscopically determined, a histological analysis was also performed to provide a more accurate analysis of the reproductive characteristics of H. plumierii. The histological study of the gonads of all individuals was carried out to determine the stages of development of their germ cells. Central portions (transverse sections) of the fixed gonads were dehydrated and embedded in paraffin, sectioned to between 5-7 µm, depending on their state of gonadal development because the early phases required thinner sections to facilitate the identification of their germ cells, and then stained with haematoxylin-eosin (Awruch et al., 2008; Villegas-Hernández et al., 2015). The reproductive phases of the gonads were classified in line with Brown-Peterson et al. (2011) as immature (IMM), developing (DEV), spawning capable (SC), regressing (RGS), and regenerating (RGN). It should be noticed that ovarian wall thickness has been recognized as a key factor in distinguishing between immature and regenerating phases, since a thin ovarian wall is commonly observed in immature specimens whereas a thick ovarian wall (along with atretic oocytes) was observed at specimens in the regenerating phase (Brown-Peterson et al., 2011; Villegas-Hernández et al., 2015). During the analysed annual cycle, the occurrence of spawning of H. plumierii was inferred with both the GSI peaks and the increased frequency of spawning capable specimens according to the reproductive phases (expressed in relative frequency) found throughout the year (Brown-Peterson et al., 2011; De Vlaming et al., 1982).
Once the sexes were confirmed by histology, the sexual proportion was explored annually, monthly, and by size class as the number of females per male (F:M). In all three cases, the chi-square test (χ2) with the Yates correction factor was applied to accept or reject the hypothesis of a female:male ratio equivalent to 1:1 (Zar, 1996).
The length-weight relationship (LWR) was estimated by a power regression model using the curvilinear formula EW = a·TLb, where EW is the eviscerated body weight of the fish, TL is the total length of the fish, a is the intercept, and b the slope (Le Cren, 1951). To determine differences of the LWR between sexes, a covariance analysis (one-way ANCOVA) was applied, where EW was the dependent variable, sex the factor, and TL the co-variable. The condition of each fish was evaluated with the hepatosomatic index (HSI) for each individual as a function of eviscerated weight (EW) as HSI = 100 (LW/ EW), where LW represents liver weight. Additionally, the somatic condition was evaluated by the relative condition factor Kn (Le Cren, 1951), using the formula of Copp (2003): Kn = (EWobs)/(EWexp) × 100, where EWobs is the observed eviscerated weight and EWexp is the expected weight, which was determined by using the ln-transformed LWR relationship for all individuals in the study. Kn differences between sexes were evaluated with a Student’s t-test for independent samples, and a Spearman rank correlation test was applied to explore the relationship between Kn and GSI.
The presence of hydrated (yolked) oocytes was histologically determined to select suitable specimens for the estimates of the fecundity, and discarding those with ovaries that presented post-ovulatory follicles (POFs), since its presence indicates that spawning of mature oocytes has occurred (Hunter & Macewicz, 1985). Fecundity was estimated as batch fecundity (BF defined as the number of eggs spawned per batch) for each specimen in the spawning capable (SC) phase and without POFs using the gravimetric method combined with image analysis (Murua et al., 2003), which facilitates the measurement (diameter in μm), the characterization of oocyte development, and the count of the oocytes in a subsample of a given weight (in our case approximately of 0.05 g) of the central region of the ovary (Gonçalves et al., 2009; Kraus et al., 2000; Witthames et al., 2009) according to Hunter et al. (1985) as BF = WPO ×(Y/Sw), where WPO is the weight of preserved ovary, Y is the number of hydrated (highly yolked) oocytes in a weighted subsample of ovarian tissue, and Sw is the subsample weight. With this aim, once subsamples were taken from the ovary and weighed, their oocytes were separated from connective tissue using a washing process (Lowerre-Barbieri & Barbieri, 1993) and sorted by size through several sieves (from 800 to 200 µm), which eased the subsequent work of counting and measuring oocytes using a computer-aided image analysis system called IMAGEJ V. 1.51, which generates plots of size frequency distributions of the diameters of the oocytes.
For different stages of development of oocytes, comprising primary growth oocytes (PG), cortical alveoli oocytes (CA), early vitellogenic oocytes (Vtg1), secondary vitellogenic oocytes (Vtg2), and tertiary vitellogenic oocytes (Vtg3), the mean diameter (50 oocytes per stage) was estimated from five ‘standard’ 20.0 cm TL females (to avoid any maternal size effect on estimates) whose histological sections were used to calculate the average of major and minor axes using the previously mentioned computer-aided image analysis system. Only those oocytes with a central nucleus were used to make sure that maximum diameter was effectively measured. Moreover, due to their irregular shape in histological transverse sections, the mean diameter of the hydrated oocytes was estimated differently: those largest oocytes previously separated through sieves were separated and then measured making use of the image analysis system.
The selection of the specimens suitable for fecundity estimates was based on those females that showed size frequency distributions with most advanced vitellogenic oocytes (at least Vtg3) plus the hydrated oocytes that were clearly about to outgrow the standing stock of vitellogenic oocytes and/or presented a separate mode of oocytes developed previously to ovulation (Murua et al., 2003). Finally, the relationship between BF and size (TL) was estimated by fitting power functions. The relative batch fecundity (RBF) was also calculated as batch fecundity per gram of eviscerated weight of the fish. The differences in TL and EW between sexes were compared with a Mann Whitney W test for independent samples.
Results
In total, 355 individuals of H. plumierii were captured, with sizes ranging from 7.5 to 34.0 cm TL (mean 22.71 ± 4.44 cm) and from 30.2 to 417.6 g in EW (mean 188.30 ± 82.49 g) (Fig. 2). The histological analysis allowed the identification of 233 males (ranging from 7.5 to 34.0 cm TL) and 122 females (ranging from 7.7 to 29.5 cm TL). Most specimens of both sexes had between 20.0 and 30.0 cm TL (Fig. 2). Regarding the length-weight relationship (LWR), the ANCOVA test showed no significant difference between sexes either in the slope (F = 0.44, p > 0.05) or the intercept (F = 2.64, p > 0.05) of the LWR, thus a single (sex joined) power model was calculated as EW = 0.0383*TL2.6846 (R2 = 0.9069).

Figure 2 Size frequency distribution of H. plumierii caught on the continental shelf off the northern coast of the Yucatán Peninsula. Specimens were separated by sex and sexual maturity.
Annual sex ratio was estimated at 1 female per 2.1 males and differed significantly from 1:1 based on the chi-squared test (χ2 = 37.78, p < 0.01). Although males were more abundant throughout the year of sampling, the sex ratio did not differ significantly (p > 0.05) in March, (χ2 = 0.51), April (χ2 = 0.51), May (χ2 = 2.7), June (χ2 = 1.04), July (χ2 = 2.06), September (χ2 = 2.20), and November (χ2 = 0.00). In regard to size (TL), sex ratio differed significantly (p < 0.05) in the following size classes: 20- 21, 21-22, 27-28 and 28-29 cm, with males more abundant than females in all cases (Fig. 2).
In both sexes, 4 stages of gonadal development were identified: developing, spawning capable, regressing, and regenerating. Females in the developing phase showed primary growth oocytes (PG), cortical alveoli oocytes (CA), early vitellogenic oocytes (Vtg1), and in some cases, secondary vitellogenic oocytes (Vtg2). Besides PG, CA, Vtg1, or Vtg2 oocytes, ovaries in spawning capable phase also showed tertiary vitellogenic oocytes (Vtg3) and a few hydrated oocytes (H). Meanwhile, those females with ovaries in the regressing phase showed PG, CA, Vtg1, Vtg2 oocytes plus some post-ovulatory follicles and atretic oocytes whose presence as well as the disorganized lamellae, spaced oocytes, and residual Vtg3 were all features of the gonadal phases. Finally, female specimens with ovaries in the regenerating phase were observed showing only oogonia and PG as well as a thick ovarian wall (along with atretic oocytes).
Males in the developing phase presented mainly spermatogonia (Sg), spermatocytes (Sc), spermatids (St), and, in some cases, scarce spermatozoa (Sz) in the lumen of the gonad. Males with testicles in spawning capable phase showed high accumulation and concentration of Sz in the lumen. Males in the regressing phase showed some residual Sz, and finally males in the regenerating phase showed proliferation of Sg throughout the testes.
Regarding sexual maturity, 17 immature female specimens, ranging from 7.7 to 15.9 cm TL, showed thin ovarian walls and only with PG within the entire gonad. Similarly, 13 immature males, ranging from 7.5 to 16.4 cm TL, were observed with germinal epithelium and spermatogonia (Sg) evidenced in the testis. The size at 50% maturity (L50) was estimated at 15.22 cm TL for females (Fig. 3A) and 15.15 cm TL for males (Fig. 3B) and no immature individuals were found with TL greater than 17 cm in both sexes (Fig. 3).

Figure 3 The relative frequency logistic curves of mature females (A) and males (B) of H. plumierii as a function of size class.
The monthly variation of the mean GSI showed that the spawning seasonality might be observed during the spring months April-May in females and March-April in males. This was confirmed later based on the histological examination of their gonads, in which similar trends in reproductive phases (expressed in frequency of occurrence) were found throughout the year in both sexes (Fig. 4): the developing phase was observed mainly from November to February followed by spawning capable phase until August when subsequently spawning activity ceased, and the regressing and regenerating phases became more evident for the rest of the year.

Figure 4 Monthly variation of the relative frequency (percent abundance) of development stages and mean (± SD, standard deviation) variation in the gonadosomatic index (GSI) of H. plumierii per sex at the continental shelf off the northern coast of the Yucatán Peninsula. The sample number (n) per month is also given above bars.
The relationship between the relative condition factor (Kn) and the GSI was not significant either in females (Spearman, P = -0.0412, p > 0.5) or males (Spearman, P = -0.0078, p > 0.05), although in April the high values of the GSI coincided with the decline of both fish condition indices Kn and HSI (Fig. 5). The mean values of these two condition indices showed the same monthly variation in both females and males. Apparently higher energetic reserves were observed during the spawning season; this then, decreased as the spawning season advanced and began to recover when spawning finished.

Figure 5 Mean monthly variation of the relative condition factor (Kn ± SD) of H. plumierii per sex at the continental shelf off the northern coast of the Yucatán Peninsula.
The diameters of the oocytes in different phases of development were observed ranging as follows: 126-176.4 μm (CA), 201.6-302.4 μm (Vtg1), 327.6-378 μm (Vtg2), 403.2-529.2 μm (Vtg3), and 554.4- 705.6 μm (H). Oocyte development in H. plumierii was considered asynchronous since oocytes at different stages of development were simultaneously present in the ovary despite the reproductive phase (Fig. 6). These oocyte size-frequency distributions showed a continuous size-frequency development, except for ovaries at the onset of spawning, which, as with all the secondary growth stages, had a separate mode of the most advanced oocytes (Fig. 6b-c). That is to say, just before ovulation, most advanced oocytes did outgrow the standing stock of vitellogenic oocytes and a separate mode of mature hydrated oocytes developed for ovulation, as shown in Figure 6 b-c. This later would be recognized as an asynchronous oocyte recruitment pattern as oocytes at different stages of development were simultaneously present (Fig. 6).
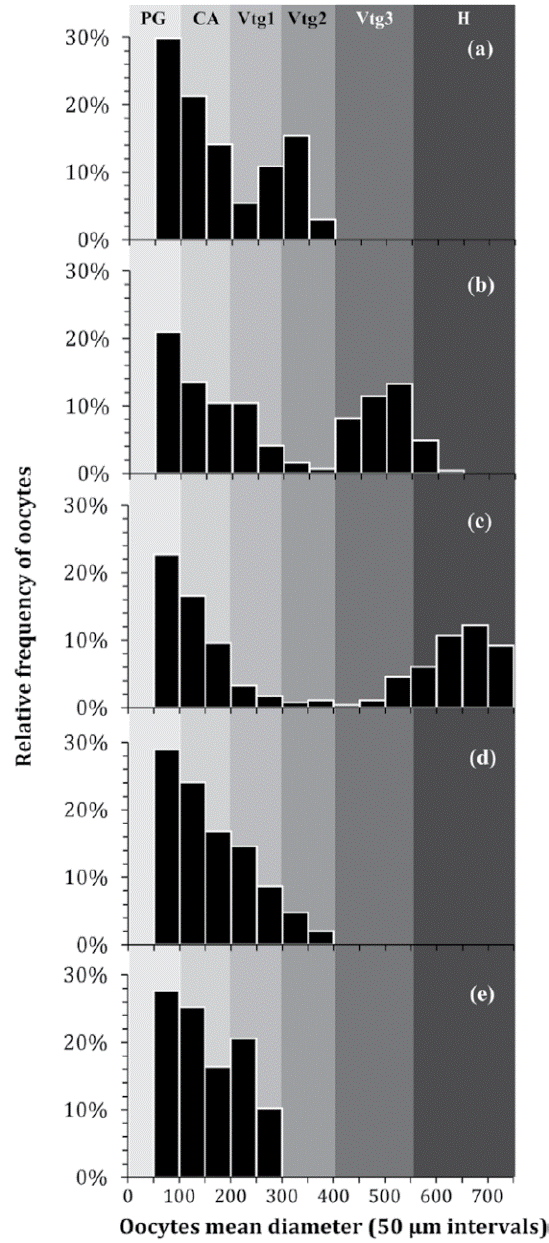
Figure 6 Oocyte size-frequency distributions (percent abundance per 50 μm diameter class) of five ‘standard’ 20.0 cm TL females of H. plumierii through subsequent gonadal development phases: (a) developing, (b) spawning capable, (c) actively spawning, (d) regressing, and (e) regenerating. Each distribution corresponds to an individual fish. The range of oocytes diameters at different stages of development (PG, CA, Vtg1, Vtg2, Vtg3, and H) is also presented based on previous measures.
Fecundity was estimated only from 12 females that showed signs of advanced gonadal development, presence of Vtg2-3 oocytes, which showed a separate mode of hydrated and oocytes in other late stages of maturation. The size range of the females with which fecundity was estimated was 22.5 to 25.5 cm TL (143.0 to 240.7 g EW). The batch fecundity (BF) ranged from 9,363 to 42,371 eggs per batch, with an average (± S.D) of 26,558 ± 10,284. The relationship between BF and TL (Fig. 7A) was fitted to the power regression model BF = 2E-10·TL5.9143 (R2 = 0.4289). On the other hand, the variation of BF was somehow better explained by the weight (Fig. 7B), as observed in the power model that relates the BF to the EW (BF = 0.049·EW2.845, R2 = 0.4945). The overall mean relative batch fecundity (RBF ± S.D) was estimated at 123.3 (± 47.1) eggs per gram of body mass for this species.
Discussion
Several reproductive traits for this important fishing resource are reported in the present study based on histological analysis from gonads of specimens collected in the coastal waters of the northern Yucatán Peninsula. Based on the results of monthly variation of the relative frequencies of the reproductive phases of gonadal maturity and the mean GSI, for both sexes, it is suggested that the reproductive period of H. plumierii in the study area occurred from February to August, with a spawning peak between March to June. This spawning seasonality coincides with that reported from Jamaica (Munro et al., 1973), the Campeche Bank of Mexico (Darcy, 1983), Florida (Murie & Parkyn, 1999), Venezuela (Palazón-Fernández, 2007), and Brazil (Hoffmann et al., 2017; Shinozaki et al., 2013; Silva, 2015; Souza, 2008). Despite the differences in climatic seasonality between hemispheres, northern with winter-spring-dry seasons and southern with autumn and rainfall seasons, this does not seem to influence the spawning peaks of H. plumierii, since their spawnings are probably related to some of the constant environmental characteristics of the tropical regions, such as temperature (Coombs et al., 2006; McMullin et al., 2009; Morgan et al., 2013; Vinagre et al., 2009).
Post-ovulatory follicles (POFs) were poorly observed in regressing and regenerating phases gonads of females, but this could be related to the warm temperatures characteristic of the tropical regions that favor their absorption (Murua & Saborido-Rey, 2003). Also, in spawning capable females, the presence of hydrated oocytes was almost nil, due to the fact that hydration occurs a few hours before ovulation (Brown-Peterson et al., 2011; Wootton & Fletcher, 2009); however, females in this reproductive phase did show at least Vtg3 oocytes and some females (those used for fecundity estimates) even showed a separate mode of oocytes which were about to outgrow the standing stock of vitellogenic oocytes. Since some haemulids spawn at night, it is difficult to observe hydrated oocytes when sampling is done during the day (Gaut & Munro, 1983; Palazón-Fernández, 2007; Trott et al., 2010). This has been recently confirmed in a study on H. plumierii reproduction in Florida, which found that in the spawning season, the number of hydrated oocytes increases considerably during the full moon phase (Stallings et al., 2016). In the present study, only two samplings coincided with the period of the full moon: May (2 days after the full moon) and July (the same day).
In our sample, males were longer (32.0 to 34.0 cm TL) than females (28.0 to 30.0 cm TL), which is a usual characteristic of H. plumierii (Araújo & Martins, 2007; Palazón-Fernández, 2007; Shinozaki et al., 2013). The size at sexual maturity (L50) in this study was similar for females (15.22 cm) and males (15.15 cm). These L50 sizes were greater than reported (≈ 10 cm TL for males and ≈ 14.7 cm TL for females) by Mateo & Appeldoorn (2001) in Puerto Rico and lower than previous data from the Campeche Bank that reported that H. plumierii matures during the third year of life, at < 18 cm TL (Darcy, 1983). The differences are explained by spatial variations because size at sexual maturity is usually closely related to total population abundance over the life of a cohort, having maturing cohorts at an earlier age and smaller size, when population size is low (Köster et al., 2013; Morgan & Colbourne, 1999).
In the present study, sex ratio (annually and monthly) was dominated by males in a 2:1 ratio to females. Previous studies have reported different sex ratios for H. plumierii, for example, a 1:2.27 (F:M) was found in Puerto Rico (Mateo & Appeldoorn, 2001) and a 1:1 (F:M) ratio in Venezuela (Palazón-Fernández, 2007) and Brazil (Araújo & Martins, 2007; Hoffmann et al., 2017; Shinozaki et al., 2013). In fish, the sex ratio can vary within the same species and from one period to another; however, in gonochoric species, the relationship tends to be close to one, but differences in the sex ratio tend to be related to sexual segregations due to food preferences or differences in habitat (Nikolsky, 1963; Wearmouth & Sims, 2008). In our study, the sex ratio of H. plumierii specimens was not significantly different from 1:1 in four months (March- June), which matched the spawning season. The sex ratio of H. plumierii in the study area could also be related to the type of fishing gear used, since it may be more selective for one sex than the other (Arellano-Martínez et al., 2001; Nikolsky, 1963; Oxenford, 1999).
Regarding the condition (Kn and HSI) of H. plumierii, specimens showed a good condition for both sexes throughout most of the year, suggesting that they carry out simultaneous uptake of external energy (i.e., from food) at the time close to reproduction. This biological strategy, known as income breeding, allows the maintenance of constant energy levels in muscle tissue and biomass without compromising the energy reserves necessary for the regulation of metabolic processes, growth, and survival (Alonso-Fernández & Saborido-Rey, 2011; Chong & González, 1995; Rodrigues et al., 2013).
Another important reproductive trait examined in this study was the batch fecundity (BF) estimated at 26,558 ± 10,284 eggs per batch on average, which is higher than 13,977 eggs per batch reported by Shinozaki et al. (2013). However, discrepancies between those BF values could be attributed firstly to our small sample size (12 females that showed signs of advanced gonadal development, presence of Vtg2-3 oocytes, which showed a separate mode of hydrated and oocytes in other late stages of maturation), and secondly to the criteria used in the calculations of Shinozaki et al. (2013), since these authors only included hydrated oocytes, whereas in the present study, BF was estimated from those females that clearly showed a separate mode (at least Vtg3) of advanced oocytes outgrowing the standing stock of vitellogenic oocytes. In addition, we observed oocytes in different stages of development (from primary growth to hydrated) at the time of spawning, together with the presence of massive atresia in the regression phase, indicating an asynchronous development of their oocytes, as has been reported in other Atlantic regions (Darcy, 1983; Palazón-Fernández, 2007).
Accordingly to Murua & Saborido-Rey (2003) the following features suggest multiple batch spawning and indeterminate fecundity of H. plumierii: 1) continuous size-frequency development of oocytes (asynchronously), 2) the presence of recent POFs along with oocytes in the final phases of gonadal development, and 3) massive atresia in post-spawning individuals (i.e., in the regressing phase of gonadal development). Due to the issue mentioned previously referring to the moment in which the spawning and the samplings occurred (Cubillos et al., 2011; McBride et al., 2002; Stallings et al., 2016), the results obtained in our study could hardly be contrasted with research carried out with H. plumierii in other Atlantic regions, such as Venezuela (Palazón-Fernández, 2007) and Brazil (Hoffmann et al., 2017), since they only estimated total fecundity. Besides, in species with indeterminate fecundity and batch spawning, such as H. plumierii, whose eggs are recruited and ovulated from the population of yolked oocytes in several batches throughout the spawning season, it would be imprecise and unreliable to determine total fecundity given the possibility of incorporation of vitellogenic oocytes to the batch of the more mature oocytes with potential for being released (Ganias et al., 2015; Gordo et al., 2008).
Although in most fish, greater fecundity has been associated with size and weight, with a better fit through a power model (Coward & Bromage, 1999; Cubillos et al., 2011); in the present study batch fecundity was weakly associated either with size (TL, R2 = 0.4289) or weight (EW, R2 = 0.4945). This poor adjustment between BF and size/weight could be due to fishes of similar size showing wide variations in fecundity. This lack of adjustment in the BF-TL relationship has also been observed for H. plumierii by Gaut and Munro (1983) in Jamaica. These authors attributed this variation to the kind of fecundity by batches. A similar result was reported for the same species by Hoffmann et al. (2017) in Brazil when relating fecundity to size and weight, who assumed that factors such as the availability of food, variations in temperature, age, and the number of spawning could affect fecundity. Besides, differences between BF-TL and BF-EW have also been reported in other marine and freshwater species, especially in tropical species with indeterminate fecundity (Cubillos et al., 2011; Fitzhugh et al., 2012). Usually fish with indeterminate fecundity exhibit an asynchronous development of (Murua et al., 2003; Murua & Saborido-Rey, 2003). The same was observed in H. plumierii, and thus fecundity estimates could be subject to many variations when there is no clear separate mode of the most advanced oocytes that outgrow the standing stock of vitellogenic oocytes (Korta et al., 2010; Witthames et al., 2009). Notably, in H. plumierii accuracy of the batch fecundity estimates may be related to the time of capture, and it may be increased by sampling ovaries at the onset of spawning just before ovulation since hydration of oocytes is increased considerably during the full moon phase (Stallings et al., 2016). Hence, to improve the accuracy of the fecundity estimates for this species in future studies, sampling of specimens is encouraged to be carried out during the full moon phase and at sunset.
Although this study is not the first contribution of information on the reproduction of H. plumierii, the information presented here from the northern coast of the Yucatán Peninsula can be used to compare the behavior and the reproductive potential of this species with other stocks from different regions and as a frame of reference for future work on the H. plumierii fishery in Yucatán, considering that this natural resource is very important for regional fisheries. This kind of biological information may represent part of the baseline data for future regulations if needed. For example, fisheries stakeholders from the northern Yucatán Peninsula are encouraged to apply some fishing regulations, such as a fishing closure at least from March to June when spawning peaks were observed. Moreover, based on our L50 estimates, 15 cm TL would be the shortest allowed length (minimum catch size) for a caught fish of this species. Although other fishing considerations (i.e., catch quotas, maximum sustainable yield, or catching technical regulations) are still needed to maintain the population size in spite of the harvest of individuals, biologically based management strategies would help to guarantee the continuity of this species in the region.











 nueva página del texto (beta)
nueva página del texto (beta)



