Introduction
Carbon steels, are by far, the main engineering material used in volume, mainly in flat rolled products. Their manufacturing processes have experienced constants innovations aimed to improve mechanicals properties [Herrera, 2009, 3518 ]. Nowadays, there is a growing demand both in automotive and machinery industries for advanced ultra-high strength steels (UHSS). Commercial low-alloy structural steels have been used in many engineering components in quenched and tempered conditions [Herrera, 2009, 3519]. Medium-carbon steels can be heat treated to have a good balance of ductility and strength. The uses of medium carbon-manganese steels include shafts, couplings, crankshafts (AISI 1040), axles, gears (AISI 1050), and forgings. Steels in the 0.40 to 0.60% C range are also used for rails, railway wheels, and rail axles [ASM, 2005, 262]. Currently, the 38MnVS6 steel series are well used to manufacture the automotive parts pointed above by forging process. These steels have sulfur content from 0.050 to 0.065%wt. with the target to make easy its maquinability but with a negative effect on the fatigue resistance which is still an improvement field. Thus, a non-metallic inclusions - mainly MnS - shape modification is required to enhance its fatigue resistance.
Usually, components of machines, vehicles and structures, work under repeated loading that may cause failure by fatigue [Wen-Fung., 1999, 3 ]. That is the reason why the effects on vehicle dynamics and durability of the vehicle parts, must be tested before taking it as mass production material [Pompetzki, 1990, 209]. According to statistics analysis, most of the mechanical failure in machine´s parts is due to fatigue. Moreover, a large percentage of fatigue failure for the components in service is the multi-axial fatigue [Wen-Fung., 1999, 3]. On the other hand, it is widely known that, fatigue and mechanical anisotropy are foremost caused by non-metallic inclusions in steel [Maciejewski, 2015, 171 ]. Therefore and not surprisingly, the demand for extra clean steels is important in order to ensure their intrinsic properties and to produce functional materials [Nisha, 2009, 256 ]. However, it is almost impossible to avoid these foreign particles, which get elongated during the rolling and forging processes, and produce a highly anisotropic microstructure [Nisha, 2009, 527 ]. It is very common to find the sulfur (S) as sulfide inclusions, like manganese sulfide (MnS). The presence of S in controlled amounts, improves the machinability of steel.
However, according to Nisha et al. [2009, 526 ], sulfide inclusions are malleable and become elongated in the rolling direction, leading to a considerable difference between the effective inclusions areas of the longitudinal and transverse rolling directions. Also, sulfides can be found forming clusters which facilitate crack propagation, and as a result, toughness and ductility are considerably lowered [Nisha, 2009, 526 ]. According to their chemical composition, inclusions can be classified as sulfides, oxides, nitrides and silicates, their size vary from 0.1 to 100 µm. However, not only the size of inclusions affects the mechanical properties but also their shape, volume fraction, and distribution [Barbosa, 2009, 23]. Thus, it is important to control these variables in order to mitigate their harmful effects on the mechanical properties [Nisha 2009, 527 ]. Under this basis, rare earth elements cause important effects on the MnS inclusions, particularly its shape. These elements increase the possibilities for wide applications of steels as functional materials [Isshiki, 1996, 885].
It has been reported that in steel, the addition of rare earth not only can purify molten steel and modify inclusions, but also it has a microalloying effect. With the development of new metallurgical techniques, the contents of impurity elements such as sulfur and oxygen can be considerably reduced in steel [Qin, 2007, 485].
The main objective of this research work is to study the effect of adding rare earths on the modification of MnS inclusions, and the consequent influence on the fatigue properties of medium carbon steel. A detailed study of the type, size, shape and distribution of the sulfide inclusions was undertaken to correlate it with the tensile and fatigue properties.
Method
The experimental base alloy, with and without additions of rare earths, were melted in an electric induction furnace. The chemical composition of these steels is shown in Table 1.
Table 1. Chemical composition of experimental steels.
| Element | Steel | |||
|---|---|---|---|---|
| A | B | C (0.01% Mischmetal) |
D (0.01% Mischmetal) |
|
| C | 0.501 | 0.479 | 0.491 | 0.511 |
| Mn | 0.813 | 0.791 | 0.951 | 0.977 |
| S | 0.023 | 0.045 | 0.051 | 0.065 |
| P | 0.015 | 0.014 | 0.021 | 0.018 |
| Si | 0.289 | 0.326 | 0.312 | 0.321 |
| Al | 0.126 | 0.133 | 0.041 | 0.053 |
| Cr | 0.019 | 0.017 | 0.021 | 0.019 |
| Mo | 0.046 | 0.045 | 0.047 | 0.046 |
| V | 0.015 | 0.015 | 0.018 | 0.017 |
| Ti | 0.003 | 0.003 | 0.002 | 0.002 |
In order to evaluate the rare earths addition effect, sulfur was intentionally added in a range from 0.023 wt. % to 0.065 wt. %. The maximum sulfur content reported for conventional medium carbon steel is from 0.040 to 0.050 wt % [John, 2004, 43 ]. The rare earths alloy was added as Mischmetal, whose chemical composition is shown in Table 2.
Table 2. Mischmetal alloy chemical composition.
| Element | Wt.% |
|---|---|
| Cerium | 45 - 51 |
| Lanthanum | 23 - 26 |
| Neodymium | 15 - 19 |
| Praseodymium | 4 - 6 |
Both base composition and Mischmetal treated medium carbon steels were hot rolled in a 50 ton reversible rolling mill. The ingots (5.12 x 5.12 x 5.12 cm) were re-heated at 1000°C, with a holding time of ~2 h for homogenization. Then, the ingots were controlled-rolled from 1000°C to 930°C, with a final rolling thickness of 1.64 ± 0.1 cm and air cooled to room temperature. Steel plates of 12 cm x 20 cm were obtained. For the uniaxial tensile test, the specimens were prepared according to the ASTM A370 standard in an Instron Tensile Testing Machine. The fatigue resistance was conducted under the ASTM E466 standard procedure; nine samples per composition were tested in a R.R. Moore - rotating beam fatigue testing equipment. The size and geometry of the specimens is shown in Figure 1.
This figure also shows the place of the rolled plate where the specimens were extracted and that they were aligned with the rolling direction. The hardness values were measured in the Rockwell “B” scale, using a hardness tester with a steel ball of 1.58 mm of diameter and a load of 100 kg. In order to follow the evolution of the sulfide inclusions, specimens were analyzed in both as-cast and thermomechanically treated conditions, by optical and scanning electron microscopy. Samples for metallography were mechanically prepared by grinding and polishing up to obtain scratches-free surfaces. Etching was undertaken with 2% Nital solution. 40 pictures randomly selected were analyzed by using a PC-based image analyzer.
Results and discussion
Chemical composition:
The chemical composition of the experimental steels (Table 1), is in agreement with the composition specified for a commercial medium carbon steels. The carbon content of the steels “A” and “B” are within the normal range for a medium carbon steel (0.43 - 0.50 wt. %) [10 ]. The amounts of sulfur and manganese are enough to form MnS instead of FeS inclusions. The sulfur content was systematically increased (from 0.023 to 0.065 wt. %) in order to evaluate its effect on the mechanical properties of these steels. The steels “C” and “D” were treated with 0.01 wt.% of mischmetal as inclusions modifier. Thus, the mischmetal effect on the sulfide inclusions could be evaluated in terms of the content, type, size, and distribution of MnS inclusion, and this in turn, could be correlated with the tensile and fatigue resistance.
As cast conditions analysis:
It is well known that rare earths (RE) bind easily with oxygen, sulfur and nitrogen, forming oxides, sulfides, oxy-sulfides and nitrides [Gschneidner, 1998, 295]. This is the main reason why RE are used in the production processes of ferrous metallurgy. The mischmetal alloy is composed by several rare earth elements which purify molten steel and modify inclusions [Gschneidner, 1998, 312]. Thus, it is important to determine its modifier effect on evolution of the sulfide inclusions from the as-cast to the final processing conditions, and to correlate it with the tensile and fatigue properties.
RE modifier effect on as cast inclusions morphology:
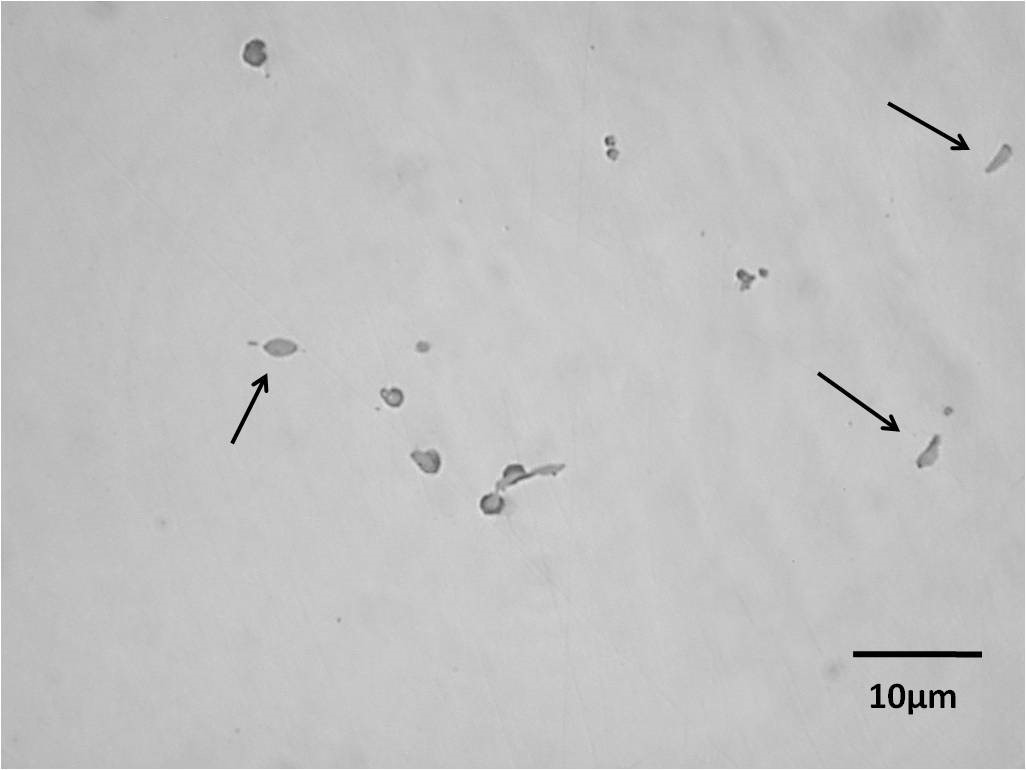
Not surprisingly, three types (globular, elongated and angular geometric) of nonmetallic inclusions were found; their identification was carried out according with the Krauss classification [2005, 169], which is in good agreement with the ASTM E45 standard test method for determining the inclusion content in steels [ASTM, 2002]. Optical micrographs from Figures 2 and 3 , show the as cast inclusions of the untreated RE steels. In the case of the steel “A”,Figure 2 shows a typical morphology of the type II MnS inclusions, which have an elongated shape (indicated by arrows). On the other hand, for the steel “B”, MnS are polygonal shaped, which is classified as type III MnS inclusions, see Figure 3.
The kind of nonmetallic inclusions developed by the steels treated with 0.01 wt.% mischmetal (“C” and “D”), was only of the type I, with a globular shape. The above modifying effect has been widely reported by several researchers in the calcium treatment of steel [Tomita, 1994, 2877, Rungta, 1988, 16, Wilson, 1988, 26, Hetzner, 1988, 43], however, the calcium amount must be controlled and should be proportional to the sulfur content in the steel, due that calcium bit is considered an undesirable element which decreases the mechanical properties when used in excess due to the formation of undesirable compounds [Saleil, 1989, 72]. In this case, the globular shape is attributed to the modifier effect of rare earths elements; particularly by the presence of Cerium [Gschneidner, 1998, 301]; see Figure 4.

Fig. 4 Type I globular inclusions of experimental steel with RE addition. Inclusions characterization by optical microscopy is limited by their resolution, so that scanning electron microscopy (SEM) was also used.
Inclusions morphology analysis by scanning electron microscopy (SEM):
Figure 5 shows an image of the fractured surface of the untreated steel “B”. A ~2μm size, type III MnS inclusion with a polygonal shape can be observed.
As said before, this kind of nonmetallic inclusions is well fitted to the Krauss classification [2005, 169]. Nonmetallic inclusions classified as type III (MnS) have an angular geometric shape (considered as deleterious) with a variety of sizes and arbitrary distribution. Inclusions having a sharp form are harmful for the material because they are stress concentrators, leading to crack initialization [Krauss 2005, 172]. The energy dispersive spectrum (EDS) from the inclusion arrowed in Figure 5 is shown in Figure 6, which is mainly composed of manganese and sulfur (MnS).
The composition of the inclusion shows the peaks of manganese and sulfur, but also, peaks of other elements, due to interaction of the bulk with the electronic beam. We assume that the particles found correspond to manganese sulfides. Type III MnS inclusions are formed by a eutectic reaction, in which a liquid solidifies to two solid phases, L→α+β, where MnS forms as a crystalline phase [Adolfi, 2007, 2]. In addition, sulphide morphologies are determined by the chemical composition variations, both by major (such as chromium and nickel), and minor elements additions, (such as carbon, oxygen, aluminum and titanium); and evidently, the sulphur content. Therefore, these factors can affect the segregation behavior and the sulphide nucleation [Durand-Charre, 2004, 130].
The type II MnS inclusions are generally elongated, with different sizes, and are commonly assumed to be formed as rod-like eutectic particles [Durand-Charre, 2004, 321]. A typical and relatively large MnS particle with a size of about 7μm is shown in Figure 7 on a fractured surface. This type of MnS inclusions are formed in the liquid between solidifying dendrites and are usually found in deoxidized aluminum-killed steels.
An increase on the cooling rate may benefit the formation of type II MnS inclusions [Krauss, 2005, 155]. Adolfi [2007, 1] points out that the formation of type II MnS inclusions are by a monotectic reaction, where MnS forms as a liquid phase, in which a melt gives a solid phase and another liquid phase, L1→α+L2.
Type I MnS inclusions are globular particles and randomly distributed in the matrix of steel. Figure 8 shows inclusions with a globular shape over the fractured surface of the mischmetal treated steel “C”, with an average size of 1.35 μm, which are smaller than type II and III MnS inclusions.
According to the theory, the globular form is attributed to their precipitation as liquid globules rich in sulfur and oxygen during the solidification process. Type I MnS inclusions are formed early in the solidification process, may be constituted as duplex inclusions with MnO [Krauss, 2005, 154]. This could indicate that the oxides have acted as a nucleus, but may simply be due to the joining of the oxides to the sulphur-rich globules [Rungta, 1988, 13]. However, as mentioned before, the cerium has a globular modifier effect which could affect the inclusions shape of the mischmetal treated steels; this is in accordance with the EDS undertaken on this kind of inclusions, see Figure 9, where it can be seen a high sulfur peak and also the Al, O, Ce, La, Nd, Fe and Mn peaks, the presence of this elements can lead to the formation of a more complex compound, according to G.R. SPEICH, et al. [1982, 2243], these inclusions could be RE2O2S or RExSy , depending of the sulphur content.
As has been reported by Maloney et al. [2005, 550 ]Rare earths elemens can modify the shape and the structure of the inclusions, in their research work, Lantanum additions can produced mainly La2O2S type inclusions, improving the fracture toughness of a HY180 steel. The improvement in toughness was attributed to an increase in inclusion spacing.
Hot rolled conditions analysis:
In general, significant changes on the shape and size of the MnS inclusions take place during the hot - rolling processes of the medium carbon steels, where the presence of the inclusions classified as type II and III are unavoidable. During hot rolling, some of the inclusions are broken out, forming a kind of chains aligned with the rolling direction, some of them are elongated and some other just do not suffer any modification [Durand-Charre, 2004, 318]. Of course, any substantial change is governed by the hot rolling process parameters.
Optical microscopy analysis as hot rolled conditions:
Under this basis, after hot rolling, the medium carbon steels were analyzed by optical microscopy to evaluate the mischmetal effect as inclusions modifier. Thus, the untreated mischmetal steels (“A” and “B”), exhibited elongated inclusions as expected, which could be classified as type II and III; see Figures 10(a) and 10(b).
The deformed inclusions exhibit an irregular lengthened form, characteristic of the MnS inclusions. Then; it is believed that the deformation of inclusions during hot rolling is produced by forces operating at the inclusion/matrix interface. In addition, Lou [2001, 96] suggests that the rolling temperature is the most active parameter that affects the inclusion deformation. He argues, that there is a narrow transition temperature region, before which the inclusion behaves as rigid and after which it becomes plastic and easily deformed.
In contrast, steel “C” (0.05%S + 0.01%mischmetal) kept the globular-shaped inclusions (type I) obtained from solidification, Figure 10(c). This effect is attributed to the mischmetal addition and particularly to the effect of cerium, which is a very strong sulphide former [Holappa, 1995, 179]. These inclusions appear small and well distributed; however, some of them were elongated.
On the other hand, the inclusions observed on steel “D” (0.065%S + 0.01%mischmetal) were large, elongated and some of them with an elliptic form; others kept its globular shape but with a considerable size, Figure 10(d). It is easy to infer that the mischmetal loss effectiveness when the sulfur content is higher than 0.05%. Then, there is a relation between sulfur and mischmetal. Also, in medium carbon steels but using calcium as modifier agent, Tomita et al. [1994, 2876] suggested that the optimum ratio of added calcium content to sulphur content (Ca/S) for modification of the inclusions morphology is 3. They also reported that the sulphur content should be as low as possible to avoid the formation of inclusions clusters. However, Blais et al. [1997, 36] suggest a 0.7 Ca/s ratio. In another research work that obtain similar results, Garrison et al. [2005, 309] found that lanthanum additions of 0.015 wt% can improve the fracture toughness of an HY180 steel with low Mn content, but additions of 0.06 wt% leads to an increase the inclusions volume fraction decreasing the fracture toughness, in this case the lanthanum additions modifies form La2O2S inclusions, as part of lanthanum is forming the inclusion, the increase in the lanthanum addition leads to the increases in the inclusions volume fraction. In the present work the manganese and cerium content in the inclusions is predominant due to the higher manganese content in the steel and cerium in mischmetal compared with the Garrison research work, but we agrees in the fact that the proper additions should be directly proportional to the sulphur content in the steel.
Ferrite and pearlite volume fraction estimation:
Rare earth elements play three main roles in steel, these are: a) modifications of the inclusions shape, b) deep steel purifying and c) microalloying; therefore, no microstructural changes are expected [Long-Mei, 2008, 534-535]. The ferrite and pearlite content are presented on Table 3, for the studied steels.
Table 3. Phase quantification and Ferrite grain size of experimental steels.
| Steel | Pearlite (%) | Ferrite (%) | Ferrite grain size (μm) |
|---|---|---|---|
| A (0.023 %S) | 78 | 22 | 8 |
| B (0.045 %S) | 80 | 20 | 9 |
| C (0.050%S+0.01%Mischmetal) | 81 | 19 | 9 |
| D (0.065%S+0.01%Mischmetal) | 80 | 20 | 8 |
Since no significant microstructural variations were observed, the effect of mischmetal on inclusions can be evaluated and correlated to the mechanical properties. Furthermore, the ferrite grain size measurements, Table 3, are quite similar and are in agreement with those obtained by Jahazi, referenced in [Atkinson, 2003, 505] in a 1050°C hot rolled medium carbon steel (0.35%) after a reduction of 60%. However, the presence of elongated inclusions at the ferrite/pearlite borders in the untreated steels must be taken into account, since these inclusions have a deleterious effect on the mechanical properties as shown later.
Measurements of the inclusions:
Table 4 shows the results of the size, volume fraction, and the number of inclusions per unit area.
Table 4. Microstructural characteristics of the different experimental steels.
| Steel | Feret Diameter (μm) |
Shape Factor |
Measured Volume Fraction |
Calculated Volume Fraction* |
Inclusions (No/mm 2 ) |
|---|---|---|---|---|---|
|
A (0.023%S) |
5 | 0.323 | 0.0019 | 0.0012 | 89 |
|
B (0.045%S) |
4 | 0.288 | 0.0032 | 0.0023 | 174 |
|
C (0.05%S+0.01%RE) |
3 | 0.855 | 0.0039 | 0.0026 | 451 |
|
D (0.065%S+0.01%RE) |
4 | 0.721 | 0.0048 | 0.0034 | 612 |
Note: RE = Mischmetal
*f MnS = 5.3(pctS)
On the current research, the volume fraction of inclusions depends directly on the sulfur content; thus, the volume fraction increased as the sulfur content increased. The volume fraction of MnS was measured by automatic image analysis on the light microscope at random 1000X images. Although the volume fraction is reported as MnS, these volume fractions include some oxides associated to those MnS inclusions as highlighted by Speich and Spitzig [Speich, 1982, 2243]. From the same Table 4, it is reported the calculated MnS fraction according to the f MnS = 0.053(pct S) equation used by Speich and Spitzig [1982, 2243 ] to compare the measured and calculated values of manganese inclusions. Similar results are observed for the number of inclusions per mm2, the density of inclusions increases with the sulphur content.
So far, nothing new occurred, but in reference to the inclusions diameter, the smallest size was developed by the “C” steel with an average diameter of 2µm, the relative largest size was produced in steels “A” and “B”, 4µm, and the steel “D” with the higher sulfur content developed an inclusion size of 3.5µm. As a consequence, the inclusion size results indicate that the mischmetal addition of 0.01% with sulfur content up to 0.05% is enough to control the shape and size of the inclusions; over this limit, the mischmetal loss effectiveness. A similar trend was obtained by Long-Mei el at. [2008, 534] with RE addition from 0.0 to 0.039 % in an advanced low alloy steel where the inclusion size was of 2 µm and also, the highest volume fraction developed the smallest inclusion and vice versa, the lowest volume fraction obtained the relatively largest inclusions. On the other hand, Atkinson [2003, 461] estimated that the critical inclusion size for fatigue failure in bearing steels is around 10µm when the inclusion is close to the surface, and increases to about 30 µm if they are about 100 µm below the surface. In this case, the untreated steels have a major axle length of 9 µm which is a determining factor for the fatigue results as show later.
Tensile and hardness results:
The tensile and hardness results are showed in the Table 5, where it can be seen that tensile properties of the steels “A” and “B” are within of the expected ranges, but the “C” and “D” steels, which were mischmetal treated, showed a strength increment on the UTS value due to the shape and size of the MnS inclusions.
Table 5. Tensile and Hardness tests results of experimental steels.
| Steel | UTS (MPa) |
YTS (MPa) |
Elongation % |
Hardness HRB |
|---|---|---|---|---|
| A (0.023 %S) | 752 | 490 | 18.9 | 94 |
| B (0.045 %S) | 741 | 490 | 19.3 | 96 |
| C (0.050%S+0.01%Mischmetal) | 842 | 565 | 19.5 | 97 |
| D (0.065%S+0.01%Mischmetal) | 812 | 470 | 19.1 | 99 |
| Standard | 735 | 334 | 17 |
Note: RE = Mischmetal
As mentioned before, rare earth elements have a strong deoxidization and desulfurization function, which tend to increase the purity of the final steel and reduce the impurity content at the grain boundaries and the tensile strength is evidently improved. Similar results have been reported [Wang, 2007, 492] when adding RE amounts from 0.04 to 0.08% to a medium carbon steel, the tensile strength was increased, but for higher amounts (0.12%) the tensile properties decreased. On the current study, the MnS inclusions formed in the untreated steels were found along the grain boundaries (see Figure 11) and also, the UTS of the steel “D” (0.01%RE) was lowered with a 0.065% of sulfur content which is attributed to the increase in inclusions volume fraction. Again, it is evident that mischmetal additions have a positive effect on the properties of medium carbon steel up to a maximum sulfur amount of 0.05%.
Fatigue test results:
Low sulfur content is not necessarily related to high fatigue strength if also the size of sulphides is not decreased concurrently; on this basis, the mischmetal addition accomplish very well the purpose according to Atkinson [2003, 461]. Figure 12 shows the fatigue for the different steels.
It can be seen that the steel “C” (0.05%S + 0.01%RE) obtained the major fatigue limit with 375.4 MP. This steel developed an improved fatigue limit since the inclusion size was smaller and more globular shaped than the others steels. Zhang et al. [2010, 1123] pointed out that the size of inclusions is the most important factor to determine the fatigue crack initiation behavior. Such observations have also been made by Yang et al. [2004, 964-965] in high strength 42CrMoVNb steel, in which the size of the inclusions is directly related with the fatigue life, decreasing with the increase in size. In this case, both the fatigue strength and the delay of crack initiation tend to decrease with the increase of inclusion size. The above effect is supported by the fact that large inclusions experience larger stresses in the presence of cyclic loads and then are prone to earlier crack nucleation as mentioned by B. Zhang [2010, 1123].
Now, the untreated steels “A” and “B” developed a quite similar behavior, but their fatigue strength was lower than steel “C”, see Figure 12. The inclusions size was almost the same but the difference is the sulfur amount of 0.023 and 0.045% respectively, where the main difference is on the inclusions volume fraction. This is in good agreement with the explained by Zhang et al. [2010, 1123] and Atkinson [2003, 465], it is no enough to reduce the sulfur content and the inclusions volume, but the inclusions shape has a strong effect on the fatigue properties. On the other hand, the Mischmetal treated “D” steel enhance its fatigue strength despite the sulfur content was of 0.065% in comparison with the steels “A” and “B”. Again, it is evidence that the Mischmetal addition of 0.01% is less effective when the sulfur content is over 0.05%, but it is still beneficial as inclusion modifier.
Fatigue fracture analysis:
Figure 13 shows the crack initiation on the fatigue test specimen of the steel “C” (0.01%RE + 0.05%S).
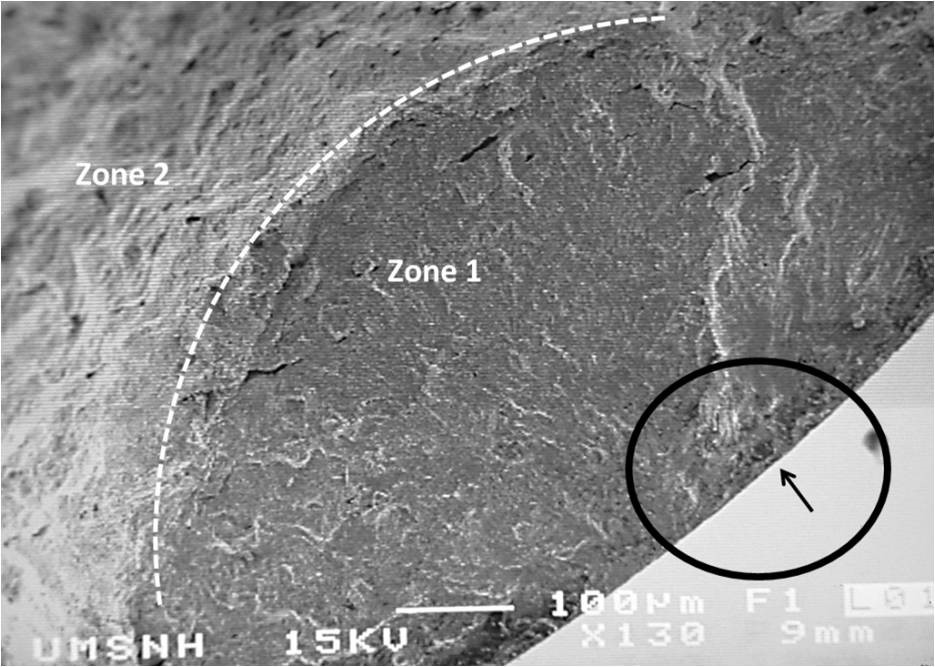
The micrograph exhibits different failure areas produced during the fatigue test, which are described as follow: 1) a relative flat zone produced by friction during the crack propagation across the section. This area shows ring-shaped marks which were generated at the stress point concentration or crack nucleation; also, some beach marks can be seen. They could have been formed in front of the fracture and propagated due to the cyclic load. Also, radial lines were observed, which are more frequently produced on the surface of the material. 2) A rough zone, which is produced when the specimen is broken by reduction of the effective section.
Figure 14 shown a close up of the defect were the crack started, this defect it seems to be surface discontinuity (within the circle on Figure 13).
It is widely known that the cracks can nucleate due to a discontinuity or inclusions on the material surface [2003, 465 ]. It appears that the discontinuity was formed in a hole left by inclusion decohesion, since the test sample was perfect polished in order to avoid any scratch that could act as stress concentration.
The crack initiation process can be explained as follow: just after a few cycles of the applied load, several microstructural changes must have been occurred in critical points of the material; subsequently, sub-microscopy holes form at inclusions since they are stress concentrators. Such holes soon became visible. Finally, once the holes or micro-cracks grew to a critical size, the fracture is produced (see Figure 14).
The evolution of the process can be divided in three stages, nucleation, propagation and rupture, as described by Atkinson et al. [2003, 464].
SEM micrographs of the fractured surface corresponding to the “C” steel are shown in Figure 15, where type I MnS globular inclusions can be observed.
In contrast, type II MnS inclusions were found in the untreated steels shown in Figure 16.
The cerium is easily combined with sulphides to promote its globular shapeand reducing the probability of cracking [Murakami, 1989, 296]. In addition, it is widely accepted that inclusions clusters will produce lower fracture toughness than well distributed inclusions. This is because inclusions play an important role in the void formation and crack nucleation; due to that, the adhesion of inclusions to the matrix is usually weak [Yu, 2009, 4279 ] and it is common to find some cavities between inclusion and matrix due to detachment. The distribution of these cavities determines the relatively easy coalescence and they accumulate stress to initiate the failure.
For the “D” steel (0.01%mm+0.065%S), as said before, the mischmetal addition was less effective when the sulfur content was over 0.05%, letting the formation of type III MnS inclusion, see Figure 17. Also, some holes can be observed which could be formed as a result of the inclusion detaching.
A similar effect was observed in the untreated steel“A” (0.023%S) as can be seen from Figure 18.
Figure 19 shows two micrographs of the untreated “B” steel (0.045%S). It can be observed that the fracture crosses through the inclusions.
When a material is subjected to repetitive loads, it will failure to a tension load lower than the required to produce a fracture under a constant load. The repetitive tension load will produce a progressive deterioration of the material at low loads; this deterioration is reflected through the formation of cracks in the material that eventually could produce the fracture.
Conclusions
1. - The steels treated with mischmetal developed well distributed, globular, small MnS inclusions which are classified as type I.
2. - The type I MnS globular inclusions markedly improve the fatigue strength compared to inclusion with elongated or irregular forms.
3. - The fatigue resistance for medium carbon steel with 0.05% of sulfur was improved with an addition of 0.01% of Mischmetal.
4. - Addition of 0.01% of mischmetal had a strong effect as inclusions shape modifier, from elongated (type III MnS inclusions) to globular shape (type III MnS inclusions) which improves the fatigue and tensile properties.
5. -Addition of 0.01% of mischmetal is less effective as inclusion modifier with sulfur content of 0.065%, but it is still good enough to obtain satisfactory fatigue resistance than an untreated steel with a sulfur content of 0.023%.











 nueva página del texto (beta)
nueva página del texto (beta)