Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.14 no.2 Texcoco feb./mar. 2023 Epub 19-Jun-2023
https://doi.org/10.29312/remexca.v14i2.3054
Articles
Induction of androgenic embryos and regeneration of haploid plants in experimental genotypes of poblano chili through anthers culture
1Programa de Biotecnología-Campo Experimental Bajío-INIFAP. Carretera Celaya-San Miguel de Allende s/n, km 6.5, Celaya, Guanajuato, México. CP. 38110.
2Programa de Hortalizas/Chile-Campo Experimental Bajío-INIFAP. Carretera Celaya-San Miguel de Allende s/n, km 6.5, Celaya, Guanajuato, México. CP. 38110.
Traditional methods to obtain pure or homozygous lines involve several generations of self-fertilization with a significant time requirement. The obtaining of haploid and double haploid plants represents an alternative that reduces the production of lines with identical alleles in all their chromosomes to one generation. The objective of this work was to implement the protocol for obtaining androgenic embryos in populations of mulato and ancho chili of the chili improvement program in the Bajío Experimental Field of the National Institute of Forestry, Agricultural and Livestock Research during 2019 and 2020. Thirty chili genotypes included in the two populations mentioned below were subjected to androgen induction treatment. a) heterogeneous landrace population of mulato and ancho chilies with characteristic of cytoplasmic male sterility; and b) population of interspecific crosses of ancho chili with habanero chili (C. annuum x C. chinense) resistant to geminiviruses (PepGMV) and Phytophthora sp. The treatment consisted of N 6-furfuryladenine (kinetin) (0.01 mg L-1) and 2,4-dichlorophenoxyacetic acid (2,4-D) (0.01 mg L-1) for the induction of somatic embryogenesis and subsequently of kinetin (0.1 mg L-1) for induction of embryo germination. Chromosomal duplication was performed by colchicine (0.5%) prior to adaptation in vivo. The population made up of genotypes derived from interspecific crosses obtained greater efficiency of double haploid regenerated plants, between 4.29% and 14.67%, while the landrace population generated embryos in a smaller proportion, between 1.02% and 5.26%. These results are the first reported to obtain double haploid plants of mulato and ancho chilies.
Keywords: Capsicum annuum; homozygosity; plant regeneration
Los métodos tradicionales para obtener líneas puras u homocigotas involucran varias generaciones de autofecundación con un requerimiento importante de tiempo. La obtención de plantas haploides y doble haploides, representa una alternativa que reduce a una generación, la producción de líneas con alelos idénticos en todos sus cromosomas. El objetivo de este trabajo fue implementar el protocolo de obtención de embriones androgénicos en poblaciones de chile mulato y ancho del programa de mejoramiento de chile en el Campo Experimental Bajío del Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias durante 2019 y 2020. Treinta genotipos de chile comprendidos en dos poblaciones. a) población criolla heterogénea de chiles mulato y ancho con característica de esterilidad masculina citoplasmática; y b) población de cruzas interespecíficas de chile ancho con chile habanero (C. annuum x C. chinense) resistentes a geminivirus (PepGMV) y Phytophthora sp., fueron sometidas a tratamiento de inducción androgénica. El tratamiento consistió en N 6 -furfuriladenina (cinetina) (0.01 mg L-1) y ácido 2,4-diclorofenoxiacético (2,4-D) (0.01 mg L-1) para la inducción de embriogénesis somática y posteriormente de cinetina (0.1 mg L-1) para la inducción de germinación de embriones. La duplicación cromosómica se realizó por medio de colchicina (0.5%) previo a su adaptación in vivo. La población conformada por genotipos derivados de cruzas interespecíficas obtuvo mayor eficiencia de plantas regeneradas doble haploides entre 4.29% y 14.67%, mientras que la población criolla generó embriones en menor proporción entre 1.02% y 5.26%. Estos resultados son los primeros reportados para obtención de plantas doble haploides de chiles mulato y ancho.
Palabras clave: Capsicum annuum; homocigosis; regeneración de plantas
Introduction
Obtaining double haploid (DH) plants from a germ cell is one of the biotechnological tools that is part of crop improvement programs around the world and establishes the possibility of reducing the time to obtain a homozygous plant. Other advantages of using DH include making the most efficient selection of qualitative and quantitative characters, since it facilitates the fixation of beneficial recessive alleles, facilitates the recovery of unique recombinations and eliminates the effects of dominance and segregation within families. In free-pollinated species, DHs can be used as parental lines of a synthetic variety and are useful for estimating additive values or general combining ability if used in crosses with other materials of interest (Snape, 1989).
Chili (Capsicum annuum L.) is a very important vegetable at the economic level due to its commercial value at the international level. In Mexico, chili grows in both tropical and temperate environments and its genetic diversity is the basis of the great variability of chili types, as well as its biological, culinary and industrial value. Androgenesis is the most widely used method for obtaining haploids of C. annuum.
In androgenesis, an immature gametophyte is diverted from its normal development and is induced to sporophytic development (Jha et al., 2021). Obtaining haploid chili plants based on the culture of anthers or microspores (precursors of pollen) is the most reported method for embryogenic induction from male gametophytic cells. The experiences published so far include cultivated species of C. annuum (Comlekcioglu and Ellialtıoğlu, 2018), as well as wild species and interspecific crosses (Munyon et al., 1989). However, it is from the publication of Dumas et al. (1981) that the protocol for obtaining double haploid chili plants has been developed to increase the efficiency of androgenic induction and reconversion to complete plants of different genotypes of chili.
Several studies have demonstrated the high frequency of embryos derived from anthers; nevertheless, few of them were able to develop normal plants (Supena and Custers, 2011). Grozeva et al. (2009) established that the formation of embryos ranged between 0 and 9%, of which they obtained 8 complete plants. For their part, Ercan and Ayar (2011) reported similar results, which range from 0 to 7.69% of embryo formation; nonetheless, they only obtained 12 complete plants. The effect of age of the donor plant of the explant (Kristiansen and Andersen, 1993), growing conditions (Büyükalaca et al., 2004), the growing season (Ata et al., 2019), growth regulators (Nowaczyk et al., 2016) and the genotype of the donor plant affect the efficiency and success of embryogenesis and subsequent development to complete plant; however, the genotype is the main factor that defines the successful embryogenesis of microspores from anther culture (Niklas et al., 2012; Keles et al., 2015).
One of the main objectives of improvement in mulato and ancho chili in the Chili Improvement Program in the Bajío Experimental Field of INIFAP is to obtain lines with resistance to Phytophthora capsici Leo. and to the geminiviruses PepGMV and PHYVV, as well as ‘B’ lines or maintainers of cytoplasmic male sterility. Important advances in the selection of resistant materials were obtained, so it is necessary to obtain homozygous lines of elite genotypes for the generation of commercial hybrids.
Therefore, the objective of this work was to implement and develop a protocol that allows regenerating haploid and double haploid plants in advanced genotypes of mulato and ancho chilies via anther culture, and to characterize the response, efficiency of induction and obtaining plants.
Materials and methods
Plant material
Twenty-seven experimental genotypes of mulato and ancho chilies were used, which were from two main populations: a) heterogeneous landrace population of mulato and ancho chilies with characteristic of cytoplasmic male sterility (CMS); b) population of interspecific crosses (C. annuum x C. chinense) resistant to geminiviruses (PepGMV) and Phytophthora sp.; and c) commercial chili varieties ancho San Luis and ancho 3015, susceptible to geminiviruses (PepGMV) and Phytophthora sp. The donor plants of the experimental explants were planted in the soil in a greenhouse, they were irrigated every three days by drip system and were applied foliar fertilizer at the beginning of flowering and production with Maxi-Grow Excel® 20 ml 25 L-1.
Collection of flower buds
The flower buds were collected from chili plants during the months of autumn and boreal winter of 2019-2020. The collection stage of the flower buds of chili was when they had a size of approximately 6 to 6.8 mm and the petals approximately 1 mm longer than the sepals, that is, five days from the phenological stage of 50% flowering. The flower buds were cut with a disinfected scissors and deposited in 50 ml Falcon® tubes with sterile distilled water.
Disinfestation of flower buds
The water where the flower buds were collected was discarded from the Falcon® tubes and 30 ml of 70% ethanol was added in stirring (100 rpm) for dos minutes. At the end of this time, ethanol was decanted and 30 ml of 10% commercial chlorine (Cloralex®; 6% active chlorine) was added, remaining in stirring for 10 min. Finally, the chlorine solution was decanted, and three washes were carried out with sterilized distilled water, of 1 minute each. After the disinfestation process, the flower buds were kept in an ice bath or in the refrigerator at approximately 4 °C, until the moment of isolation of the anthers.
Culture of anthers in induction medium
The disinfested flower buds were placed on a sterilized Petri dish to extract the anthers with the help of two sterile surgical forceps. The sepals were carefully separated from the petals to maintain the integrity of the anthers, and these were dissected with a scalpel. The anthers were cultured on the convex side in contact with the induction medium, Macroelements: (NH4)2SO4 0.034 g L-1, NH4NO3 1.238 g L-1, CaCl2.2H2O 0.313 g L-1, KH2PO4 0.142 g L-1, Ca(NO3)2.4H2O 0.05 g L-1, Mg SO4.7H2O 0.412 g L-1, KNO3 2.15 g L-1, NaH2PO4.H2O, 0.038 g L-1, KCl 0.007 g L-1; microelements: H3BO3 3.15 mg L-1, CoCl2.6H2O (stock 2.5 mg ml-1) 0.016 mg L-1, CuSO4.5H2O (stock 2.5 mg ml-1) 0.016 mg L-1, MnSO4.H2O 22.13 mg L-1, NaMoO4.2H2O 0.19 mg L-1, KI 0.7 mg L-1, ZnSO4.7H2O 3.625 mg L-1 (Dumas de Vaulx et al., 1981), supplemented with 0.01 mg L-1 of kinetin and 0.01 mg L-1 of 2,4-dichlorophenoxyacetic acid (2,4-D), sucrose 30 g L-1, vitamins: thiamine 2 mg L-1, pyridoxine HCl 1 mg L-1, nicotinic acid 1 mg L-1, calcium pantothenate 1 mg L-1, glycine 2 mg L-1, folic acid 1 mg L-1, biotin 1 mg L-1, myo-inositol 10 g L-1 and agar-agar 8 g L-1. The pH was adjusted to 5.9 prior to sterilization at 121 °C and 15 pounds per square inch for 20 min, the medium was poured into sterilized Petri dishes of 90 ( 15 mm.
The cultured anthers were selected for having an approximate size of 2.5 to 3 mm in length. The Petri dishes were sealed with Micropore® tape and incubated at 32 °C in darkness for eight days. Subsequently, the Petri dishes with the anthers were transferred to a growing chamber at 25 °C with a photoperiod of 16 light hours at a light intensity of 45-70 μmol m-2 s-1 for 4 days in the same induction medium.
Culture of anthers in germination medium
The anthers were transferred to a germination medium with the same macroelements and concentration as the induction medium and microelements in concentrations as follows: H3BO3 1.55 mg L-1, CoCl2.6H2O (stock 2.5 mg ml-1) 0.011 mg L-1, CuSO4.5H2O (stock 2.5 mg ml-1) 0.011 mg L-1, MnSO4.H2O 20.13 mg L-1, NaMoO4.2H2O 0.14 mg L-1, KI 0.33 mg L-1, ZnSO4.7H2O 3.255 mg L-1 (Dumas de Vaulx et al., 1981), added with kinetin 0.1 mg L-1, sucrose 30 g L-1, vitamins (see induction medium) and agar-agar (8 g L-1), the pH was adjusted to 5.9 prior to sterilization. The Petri dishes with the anthers were incubated under the same conditions of photoperiod and temperature mentioned above.
Isolation and development of embryos
Once the embryos germinated and showed their apical and root poles, they were transferred to medium of elongation and hormone-free rooting (Chambonnet, 1988) Macroelements: KNO3 1.9 g L-1, NH4NO3 1.65 g L-1, MgSO4.7H2O 0.37 g L-1, CaCl2.2H2O 0.44 g L-1, KH2PO4 0.17 g L-1, microelements: MnSO4.H2O 0.076 mg L-1, ZnSO4.7H2O 1 mg L-1, H3BO3 1 mg L-1, KI 0.01 mg L-1, CuSO4.5H2O 0.03 mg L-1, AlCl3.6H2O 0.05 mg L-1, NiCl2.6H2O 0.03 mg L-1, supplemented with sucrose 30 g L-1, myo-inositol 10 g L-1, FeSO4.7H2O 18.65 mg L-1, Na2-EDTA 13.9 mg L-1 and agar-agar 8 g L-1. The pH was adjusted to 5.9 prior to sterilization.
In a first stage of elongation, the embryos were transferred to Petri dishes 90 x 25 mm and as their size and space need increased, they were transferred to 170 ml Gerber®-type bottles. The bottles were sealed with Micropore® tape and incubated in the growing chamber at 25 °C with a photoperiod of 16 light hours, at a light intensity of 45-70 μmol m-2 s-1, until the seedlings formed four pairs of true leaves and secondary roots.
Determination of the level of ploidy
Root apices of two roots of 1 to 2 cm were dissected and analyzed by means of ploidy staining with the squash technique (Ahloowalia, 1965). The roots were placed in a solution of acetic acid (45%) and incubated at room temperature for three hours in gentle stirring (100 rpm). The roots were then transferred to a 70% ethanol solution stirred for two hours and refrigerated at 4 °C until analysis.
The roots stored in alcohol were transferred to a 1.5 ml microtube with 600 μl of the inhibitor 8-hydroxyquinoline at 0.002 M for four hours in darkness at room temperature (Valladolid et al., 2004). After the four hours of pretreatment, the inhibitor was removed and 600 μl of Farmer’s solution (absolute ethanol + glacial acetic acid in a 3:1 ratio) was added. The samples were incubated for 24 h at 4 °C. Farmer’s solution was removed with a micropipette and acid hydrolysis was performed with 1 N HCl for 15 min at 60 °C and then enzymatic hydrolysis with 0.2% cellulose for 15 min at 37 °C. The last hydrolysis solution was removed with the micropipette and two washes were carried out adding 600 μl of the 1 mM EDTA solution and 500 μl of 1% acetocarmine dye was added for 30 min. The roots were taken with the help of forceps and placed on a slide. A drop of 45% glacial acetic acid was added, the cover slip was placed, and gentle pressure (Squash) was applied.
Microscope observation
After the squash technique, the sample was observed under the optical microscope, with the objective of magnification of 10X and 40X. Once the cells were located, the 100 X objective was adjusted for the observation of the chromosomes.
Chromosomal duplication
The seedlings were carefully extracted from the culture medium and the remains of the culture medium were removed from the roots with sterilized distilled water. Each seedling was immersed to the base of the stem in a bottle with lid that contained a 0.5% colchicine solution (Sigma®) for 8 h at 25 °C (Arjunappa et al., 2016). After this time, the seedlings were rinsed with sterilized water.
Acclimatization of chili seedlings
After the colchicine treatment, the seedlings were transferred to 250 ml pots with previously sterilized Sunshine®-type substrate. The seedlings were kept in the same chamber at 25 °C with a photoperiod of 16 light hours, at a light intensity of 45-70 μmol m-2 s-1. The substrate was lightly irrigated every third day with a solution of macroelements and microelements (Chambonnet, 1988) at 50%. Once the seedling reached 25 to 30 cm in length, a root sample was taken again to check for chromosomal duplication.
Experimental design and statistical analysis
The experimental design was a completely randomized design, where 27 experimental genotypes of chili were analyzed. The experimental unit was a dish with 25 anthers per Petri dish and 6 repetitions per chili genotype. The statistical analysis consisted of an analysis of variance and comparison of means through the Tukey test (p≤ 0.05), with the statistical package Minitab 17 (Minitab Statistical Software).
Results and discussion
Induction and germination of haploid embryos
The anthers showed response to the induction of embryogenic development between four and six weeks after culture. The first evidence of induction of androgenesis was the appearance, in the pollen ducts of the anther, of an embryo in the globular stage of a light green hue from week four and five of culture, Figure 1A. Then the embryo increased in size and began the differentiation process, the structure that is defined first are the root buds, this begins with the formation of villi at one end of the embryo (Figure 1B). Subsequently, the apical bud that forms the cotyledons differentiated on the opposite side where the root bud originated (Figure 1C). After the separation of the anther (Figure 1D), the embryos germinated showing definition of root, hypocotyl and cotyledons (Figure 1E).
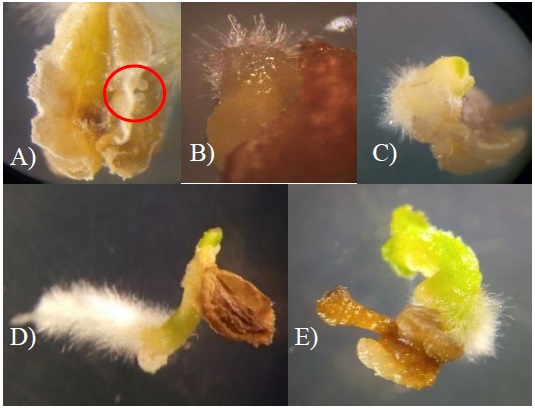
Figure 1 Germination of embryos and development of haploid plants during the culture of anthers of genotype 3328 of ancho chili. A) proembryos in the pollen duct of the anther; B) root bud formation; C) embryo with start of apical bud differentiation; D) embryo independent of the anther; and E) differential stages of embryo germination and seedling elongation.
It was possible to detect the loss of the cotyledon differentiation capacity in some embryos, it is common for proembryos and embryos to stop their development in some part of the process, this was observed in 80% of the proembryos and embryos obtained with the genotypes of chili tested. This can be attributed to the fact that differentiation routes are not genetically determined when dealing with haploid tissue and the induction process (culture medium and thermal shock) does not necessarily culminate in a morphologically complete structure.
Several reports agree on the importance of temperature on cellular mechanisms (Bajaj, 1978; Touraev et al., 1997) as well as the effect on gene expression that causes the change of microspores to embryogenesis (Gonzalez-Melendi et al., 1996; Testillano et al., 2000; Barany et al., 2001), deviating the gametophytic transition from a normal development to a sporophytic one. Thermal shock stimulation has been fundamental, particularly in genotypes with poor response; nevertheless, even in genotypes without response, it will be convenient to consider other factors such as culture medium, regulators, supplements, genotype, growing conditions of the donor plant, stage of development of microspores, among others.
Ten of the 27 chili genotypes used showed no response to induction and germination media. These genotypes showed mostly non-viable anthers and those that initiated germination in induced embryos stopped at some point in their differentiation. For their part, the 17 genotypes that showed induction signals showed variability in the efficiency of response and obtaining of haploid plants from 0.67 to 14.67% with respect to viable anthers (Table 1).
Table 1 Efficiency of regeneration of haploid plants by inducing androgenesis in chili.
| Chili genotypes | Characteristic | Type | No. of viable cultured anthers+ | No. of plants derived from anthers | Efficiency (%)* |
| 3239 | Fruit size Susceptible | mulato | 123 | 1 b† | 0.81 |
| 3328 | CMS maintainers | ancho | 150 | 7 bc | 4.67 |
| 3334 | CMS maintainers | mulato | 136 | 7 bc | 5.15 |
| 3335 | CMS maintainers | mulato | 150 | 0 a | 0 |
| 3336 | CMS maintainers | mulato | 98 | 1 b | 1.02 |
| 3337 | CMS maintainers | mulato | 132 | 3 b | 2.27 |
| 3338 | CMS maintainers | mulato | 133 | 7 bc | 5.26 |
| 3339 | CMS maintainers | mulato | 148 | 0 a | 0 |
| 3340 | CMS maintainers | mulato | 80 | 5 bc | 6.25 |
| 3341 | CMS maintainers | mulato | 124 | 3 b | 2.42 |
| 3342 | CMS maintainers | ancho | 98 | 2 b | 2.04 |
| 3343 | CMS maintainers | mulato | 107 | 0 a | 0 |
| 3344 | CMS maintainers | mulato | 126 | 3 b | 2.38 |
| 3345 | CMS maintainers | mulato | 82 | 0 a | 0 |
| 3346 | CMS maintainers | mulato | 150 | 1 b | 0.67 |
| 3359 | CMS maintainers | ancho | 114 | 2 b | 1.75 |
| 3375 | CMS maintainers | ancho | 115 | 4 b | 3.48 |
| 3378 | CMS maintainers | ancho | 138 | 0 a | 0 |
| 3381 | PepGMV R, BC3 | ancho | 82 | 0 a | 0 |
| 3382 | PepGMV R, BC3 | ancho | 150 | 0 a | 0 |
| 3383 | PepGMV and P. capsici R, BC3 | ancho | 150 | 22 c | 14.67 |
| 3384 | PepGMV R, BC3 | ancho | 147 | 12 c | 8.16 |
| 3385 | PepGMV R, BC3 | ancho | 140 | 6 bc | 4.29 |
| 3386 | Powdery mildew R, BC3 | ancho | 68 | 0 a | 0 |
| 3387 | PepGMV R, BC3 | ancho | 133 | 0 a | 0 |
| Ancho San Luis | Susceptible | ancho | 133 | 9 c | 6.77 |
| Ancho 3015 | Susceptible | ancho | 142 | 2 b | 1.41 |
Susceptible= genotypes susceptible to Phytophthora capsici, PepGMV and Powdery mildew; R= resistant segregating genotypes; BC3= interspecific cross ancho 3015 x habanero BG3821 and backcross with ancho 3015; += the number of viable anthers comes from the population of 150 anthers divided into six repetitions of 25 anthers each; *= number of plants per 100 anthers cultured; †= Means with the same letter in the same column are statistically equal (Tukey, p≤ 0.05).
The above has been uniformly reported by various authors, who establish that one of the main factors for the induction of androgenesis for the purpose of obtaining haploid plants is the genotype, finding that there are chili genotypes that do not respond to the induction of androgenesis (Comlekcioglu and Ellialtıoğlu, 2018; Shimira et al., 2019).
Although this study did not use pretreatment at low temperatures (4 °C), there is no consensus that it provides any relevant advantage; however, thermal shock at 35 °C was mentioned as fundamental for stimulating chili microspore division (Kim et al., 2004; Barany et al., 2005; Vivek et al., 2017; Shimira et al., 2019). According to Barany et al. (2005), the morphogenesis of microspore-derived embryos with the same stimulation of high temperatures revealed changes in cell organization, the sequence in time of structural events related to proliferative and differentiation activity. These changes mainly affect the plastids, vacuolar compartment, cell wall and nucleus. In this work, the anthers were incubated at 32 °C because at 35 °C the anthers dehydrated to the point of their unviability. In this way, the reduction of the induction treatment to 32 °C allowed maintaining the viability of the microspores, the induction of androgenesis and subsequent germination of embryos.
Once proembryo structures were observed, the next stage was embryo germination from root bud elongation from 28 days in germination medium (Figure 2A), as well as thickening and separation of the lateral edges of the anther at approximately 40 days of culture in germination medium (Figure 2B). Subsequently, after 50-60 days of culture, the appearance of foliar primordia was observed (Figure 2C).

Figure 2 Anthers with evidence of androgenesis and embryo germination. A) root bud of an anther belonging to line 3385 at 28 days in germination medium; B) separation of the lateral edges of the anther of line 3384 at 42 days in germination medium; and C) leaf primordia of line 3344 at 56 days in germination medium.
Another differentiation route has been reported with the production of callus derived from the culture of anthers in Murashige and Skoog medium supplemented with zeatin 1 mg L-1, 2, 4-D 0.2 mg L-1 and AgNO3 15 mg L-1 with treatment at 35 °C for 8 days, from 54.02% to 60.92%. However, even when the calli showed green coloration, they did not show differentiation of shoots in the F1 hybrids of sweet chili Bharat and Indra, respectively (Vivek et al., 2017). According to what was obtained in this work, the formation of callus was observed in low proportion and did not constitute a limitation for the androgenic development of microspores.
Determination of the level of ploidy
Chromosomal duplication of chili haploid plants (n= 12) is necessary to restore diploid status (2n= 24) and thus fertility. In this work, no spontaneous double haploids were observed, so it was necessary to establish incubation with colchicine for all plants derived from androgenesis and embryogenesis (Figure 3A). The concentration of colchicine used (0.5%) for 8 h of exposure (Figure 3B), coincides with most reports of double haploids in chili, the method of identification of chromosomal content in metaphase stage (Figure 3C), was efficient and chromosomal duplication was verified days later in the acclimatization stage (Figure 3D). Comlekcioglu (2021) reports that the inclusion of colchicine (0.3%), from the induction medium, increases the rate of spontaneous chromosomal duplication from 33% to 57.6%, which is contemplated within the perspectives to incorporate in future trials. The double haploid plants of chili developed a normal size in the greenhouse, regained their fertility and produced fruits (Figure 3E).
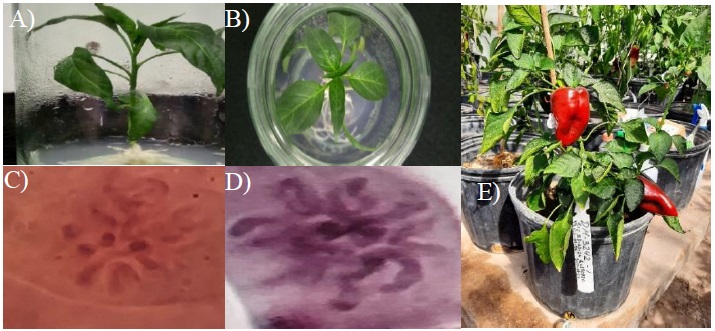
Figure 3 Chromosome duplication and method of staining by the squash method. A) seedling of mulato chili 3342 (n); B) treatment with colchicine (0.5%) for 8 h; C) chromosomes in metaphase before chromosome duplication; D) chromosomes after chromosome duplication; and E) double haploid plant of mulato chili 3242, in greenhouse with fruit formation.
Conclusions
The protocol developed in this research to obtain double haploid plants of poblano chili establishes differential rates of induction of androgenesis and germination of embryos in the populations used. The results suggest that, although the chili genotypes used have different genomic and genetic characteristics, the general bases of induction of androgenic embryos are established, which constitute prospects for improvement.
Acknowledgements
The results are part of the fiscal project: ‘adaptation of technology for the production of double haploid plants for genetic improvement’ No. SIGI: 13232234789.
REFERENCES
Ahloowalia, B. S. 1965. A root tip squash technique for screening chromosome-number in Lolium. Euphytica. 14(1):170-172. https://doi.org/10.1007/BF00038983. [ Links ]
Arjunappa, H. M.; Sateesh, K. P. and Prema, L. D. 2016. Studies on ploidy analysis and chromosome doubling in androgenic plants of chilli pepper (Capsicum annuum L.). Inter. J. Agric. Innov. Res. 4(4):627-633. [ Links ]
Ata, A.; Keles, D.; Taskin, H. and Buyukalaca, S. 2019. Effects of season, genotype, and nutrient medium on pepper anther culture and microspore development. Turk. J. Agric. For. 43(2):123-137. https://doi:10.3906/tar-1802-35. [ Links ]
Bajaj, Y. P. S. 1978. Regeneration of haploid tobacco plants from isolated pollen grown in drop culture. Indian J. Exp. Biol. 16(1):407-409. [ Links ]
Barany, I.; Gonzalez-Melendi, P.; Fadon, B.; Mityko, J.; Risueno, M. C. and Testillano, P. S. 2005. Microspore-derived embryogenesis in pepper (Capsicum annuum L.): subcellular rearrangements through development. Biol. Cell. 97(9):709-722. [ Links ]
Barany, I.; Testillano, P. S.; Mityko, J. and Risueno, M. C. 2001. The switch of the microspore developmental program in Capsicum involves HSP70 expression and leads to the production of haploid plants. Int. J. Dev. Biol. 45(S1):39-40. [ Links ]
Büyükalaca, S.; Comlekcioglu, N.; Abak, K.; Ekbic, E. and Kilic, N. 2004. Effects of silver nitrate and donor plant growing conditions on production of pepper (Capsicum annuum L.) haploid embryos via anther culture. Eur. J. Hort. Sci. 69(5):206-209. [ Links ]
Chambonnet, D. 1988. Production of haploid eggplant plants. Bulletin interne de la Station d’Amelioration des Plantes Maraicheres d’Avignon-Montfavet, France. 1-10 pp. [ Links ]
Comlekcioglu, N. 2021. Effect of colchicine addition to culture medium on induction of androgenesis in pepper (Capsicum annuum L.). Pak. J. Bot. 53(3):1001-1005. http://dx.doi.org/10.30848/PJB2021-3(14). [ Links ]
Comlekcioglu, N. and Ellialtıoğlu, S. S. 2018. Review on the research carried out on in vitro androgenesis of peppers (Capsicum annuum L.) in Turkey. Res. J. Biotech. 13(6):75-84. [ Links ]
Dumas de Vaulx, R.; Chambonnet, D. and Pochard, E. 1981. Culture in vitro d’anthères de piment (Capsicum annuum L.): amélioration des taux d’obtention de plantes chez différents génotypes par des traitements à + 35 °C. Agronomie, EDP Sciences. 1(10):859-864. https://hal.archives-ouvertes.fr/hal-00884205. [ Links ]
Ercan N and Ayar Şensoy, F. 2011. Androgenic responses of different Capsicum annuum L. cultivars, Biyoloji Bilimleri Arastirma Dergise. 4(2):59-61. [ Links ]
Gonzalez, M. P.; Testillano, P. S.; Prestamo, G.; Fadon, B. and Risueno, M. C. 1996. Cellular characterization of key developmental stage for pollen embryogenesis induction. Plant Dev. Biol. 127S-128S. http://hdl.handle.net/10261/252482. [ Links ]
Grozeva, S.; Rodeva, V.; Todorova, V. and Pundeva, R. 2009. Obtaining of pepper plants via anther culture. Genet. Breed. 38(1):25-31. [ Links ]
Jha, K.; Kumar, P. C. and Agarwal, A. 2021. Doubled haploid production in Capsicum annuum L. using anther culture: a review. Plant Archiv. 21(1):168-173. https://doi.org/10.51470/plantarchives.2021.v21.S1.031. [ Links ]
Kim, M.; Kim, J.; Yoon, M.; Choi, D. and Lee, K. 2004. Origin of multicellular pollen and pollen embryos in cultured anthers of pepper (Capsicum annuum). Plant Cell Tissue Organ Cult. 77(1):63-72. [ Links ]
Kristiansen, K. and Andersen, S. B. 1993. Effects of donor plant temperature, photoperiod, and age on another culture response of Capsicum annuum L. Euphytica. 67(1):105-109. https://doi.org/10.1007/BF00022732. [ Links ]
Munyon, I. P.; Hubstenberger, J. F. and Phillips, C. 1989. Origin of plantlets and callus obtained from chile pepper anther cultures. In vitro Cell. Dev. Biol. 25(3):293-296. https://doi.org/10.1007/BF02628469. [ Links ]
Niklas, N. A.; Olszewska, D.; Kisiała, A. and Nowaczyk, P. 2012. Study of individual plant responsiveness in anther cultures of selected pepper (Capsicum spp.) genotypes. Folia Hort. 24(2):141-146. https://doi:10.2478/v10245-012-0017-x. [ Links ]
Nowaczyk, L.; Nowaczyk, P. and Olszewska, D. 2016. Treating donor plants with 2,4-dicholophenoxyacetic acid can increase the effectiveness of induced androgenesis in Capsicum spp, Sci. Hortic. 205(23):1-6. https://doi.org/10.1016/j.scienta.2016.03.044. [ Links ]
Shimira, F.; Keleş, D.; Taşkın, H. and Abak, K. 2019. The assessment of androgenic response of two nematode resistant pepper (C. annuum L.) genotypes. Turkish J. Agric. Food Sci. Technol. 7(12):2103-2110. https://doi.org/10.24925/turjaf.v7i12.2103-2110.2828. [ Links ]
Snape, J. W. 1989. Doubled haploid breeding: theoretical basis and practical applications. In: review of advances in plant biotechnology 1985-1988. 2nd International Symposium on Genetic Manipulation in Crops. Mujeeb-Kazi A. and Sitch, L. A. Eds. (Mexico y Filipinas). International Maize and Wheat Improvement Center (CIMMYT) and International Rice Research Institute (IRRI). 19-30 pp. [ Links ]
Supena, E. D. J. and Custers, J. B. M. 2011. Refinement of shed-microspore culture protocol to increase normal embryos production in hot pepper (Capsicum annuum L.). Sci. Hor. 130(4):769-774. https://doi.org/10.1016/j.scienta.2011.08.037. [ Links ]
Testillano, P. S.; Coronado, M. J.; Segui, J. M.; Domenech, J.; González, M. P.; Raska, I. and Risueno, M. C. 2000. Defined nuclear changes accompany the reprogramming of the microspore to embryogenesis. J Struct Biol. 129(2-3):223-232. https://doi.org/10.1006/jsbi.2000.4249. [ Links ]
Touraev, A.; Vicente, O. and Heberle-Bors, E. 1997. Initiation of microspore embryogenesis by stress. Trends Plant Sci. 2(8):297-302. https://doi.org/10.1016/S1360-1385(97)89951-7. [ Links ]
Valladolid, A.; Blas, R. and Gonzáles, R. 2004. Introducción al recuento de cromosomas somáticos en raíces andinas. In: seminario J. Ed. Raíces Andinas. Contribuciones al conocimiento y la capacitación. Serie: conservación y uso de la biodiversidad de raíces y tubérculos andinos núm. 6. CIP. Agencia Suiza para el desarrollo y la cooperación. Lima, Perú. 96-99 pp. [ Links ]
Vivek, H.; Partap, P. S.; Yadav, R. C. and Baswana, K. S. 2017. In vitro androgenesis in Capsicum (Capsicum annuum L.). Int. J. Curr. Microbiol. App. Sci. 6(5):925-933. https://doi: https://doi.org/10.20546/ijcmas.2017.605.102. [ Links ]
Received: October 01, 2022; Accepted: February 01, 2023











 texto en
texto en 


