Introduction
Motility is an important characteristic linked to the progress of spermatozoa throughout the female reproductive tract1 and to the oocyte penetration. Motility assessment is an essential procedure to evaluate the sperm quality and to approach the potential of male fertility2. In this context, it is widely accepted that mammalian ejaculates constitute a heterogeneous cell pool with the presence of several sperm subpopulations. In order to select the best spermatozoa, many procedures have been developed to reduce debris, nonspermatozoal cells and bacteria contained in the sperm samples or to remove the seminal plasma to avoid the early capacitation process3. This practice could have potential for improving results by artificial insemination (AI) and is essential when other biotechnological procedures, such as in vitro production or intracytoplasmic sperm injection, are implemented4. Percoll, based on polyvinylpyrrolidone-coated silica particles, was one of the most used colloids for sperm separation in numerous species5-7. However, the detection of certain endotoxins exerting a negative effect on the spermatozoa has reduced its use, and new media have been designed to overcome the shortcomings of Percoll. Silane-coated silica colloids are currently the most used solutions, having proved to be more stable and standardized8. These colloids are employed in a variety of procedures, such as double layer centrifugation (DLC) or in a simplification of this technique, called single layer centrifugation (SLC)9.
The volume and concentration of the sperm sample could affect the effectiveness of these procedures10. In this context, DLC has been regarded as impractical as a means of processing whole ejaculates for AI9, and has been indicated for oligospermic ejaculates, although not for complete normospermic samples. SLC is an easier and less time-consuming technique that has been widely used in equine11, but there are few reports of its use in other species. Studies carried out in bulls showed that spermatozoa selected by SLC exhibit an increase in sperm chromatin integrity12 and high mitochondrial membrane potential, although increased superoxide production13. However, motility was only improved when low quality sperm samples were processed14. In reference to the comparison between fractions obtained after colloidal centrifugation, Gosalvez et al15 observed that the spermatozoa isolated in the bottom pellet showed lower sperm DNA fragmentation, although their longevity was lower than in human neat semen. These findings suggest that sperm fractions obtained by different sperm enrichment or separation procedures should be more deeply analyzed, in order to determine their efficiency in viability, concentration and other terms.
The high concentration of sperm (as occurs in ruminant ejaculates) make it difficult to use layer centrifugation techniques for processing whole ejaculates10 and to the best of the present authors’ knowledge, few studies evaluate the effect of colloidal separation in whole fresh, normospermic ram ejaculates. The present study was conducted in ram sperm samples containing different high sperm concentrations, to determine the sperm recovery rate, sperm quality and how different motile sperm subpopulations are distributed in each sperm fraction obtained after separation by SLC.
Material and methods
Animals and semen collection
Four mature Merino breed rams ranging from 3 to 5 yr old were involved in this study. The animals were housed in individual boxes located at Diputacion of Cordoba (Spain) and commercial concentrate (0.5 kg/d), alfalfa hay, water and minerals were supplied. Semen was collected once a week during non-breeding season (March-June) by artificial vagina and using a sheep as a teaser. Ejaculates were maintained at 37 ºC and mass motility (using the scale from 1 to 5; 40 × magnification; Olympus, Tokyo, Japan), sperm concentration (Accurread, IMV technologies, France) and volume were determined. Ejaculates with mass motility ≥4, concentration ≥3000 × 106 spermatozoa/mL and individual motility ≥70 % were used.
All experiments were authorized by the Bioethics Committee of the University of Cordoba (n. 2018PI/29) and they were carried out according to the Spanish Animal Protection Regulation (RD 53/2013), as stipulated by EU Regulation 2010/63.
Experimental design
As shown in Figure 1, semen from four rams was collected, assessed, diluted (1:2) and pooled in a home-made extender (TCFEY) containing Tris (33.19 g/L), fructose (9.55 g/L), 10% clarified egg yolk, citric acid (17.29 g/L), penicillin G (4 g/L) and streptomycin (3 g/L) in bi-distilled water. After dilution, two sperm aliquots were placed into conical tubes for centrifugation (300 g× for 20 min); after the supernatant was removed, TCFEY extender was added to reach a final concentration of 800 × 106 spermatozoa/ml (C800) and 3000 × 106 spermatozoa/ml (C3000) and sperm quality was assessed.
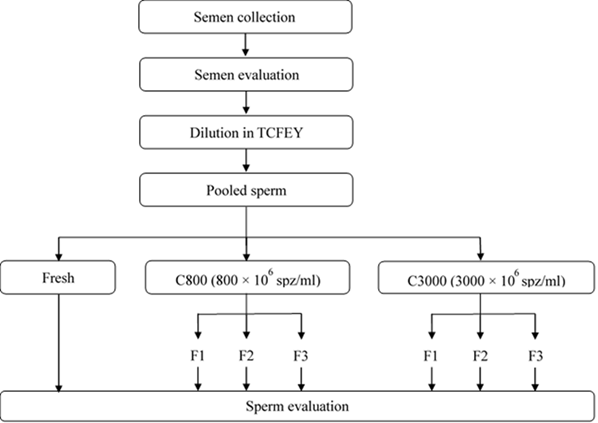
Figure 1 Experimental design scheme used to study the variation of sperm quality and subpopulations at the different fractions obtained after SLC in samples containing 800 and 3000 × 106 spz/ml (C800 and C3000)
SLC based in silane-coated silica (BoviPure and BoviDilute, Nidacon, Sweden) was prepared according to the manufacturer´s instructions. While these solutions were developed for bull sperm, they can be used also for small ruminants. Briefly, one layer of 1.5 ml of 80 % (v/v) BoviPure was deposited into a 15 ml conical tube. Then, sperm samples containing C800 or C3000 were layered on the top (1.5 ml) and centrifuged at 300 g× 20 min. After centrifugation, seminal plasma was discarded (aprox. 1.5 ml), and three different fractions were isolated per sample: the top (F1), the medium (F2) and the bottom (F3) phases, consisting in around 0.5 ml each one using Pasteur pipettes by rounded movements. The experiment was six times replicated using a total of 24 ejaculates.
It was determined the sperm recovery rate, sperm quality (sperm motility, concentration, viability, morphology and membrane functionality) and the sperm subpopulations in each of the different fractions obtained after the colloid centrifugation.
Sperm recovery rate
Sperm samples were diluted 1:200 and concentration was assessed using a Thoma counting chamber. It was determined the sperm recovery rate in each fraction:
Sperm motility assessment
ISAS software v.1.2 (Proiser, Valencia, Spain) was used for sperm motility assessment. Sperm samples were diluted with TCFEY extender to reach a final concentration of 25 × 106 spermatozoa/ml. Samples were maintained at 37 ºC for 10 min and later, a drop (5 µL) was placed on a slide covered with 22 × 22 mm coverslips. Four fields or a minimum of 500 spermatozoa were randomly captured at 25 frames per second. Head sperm area was considered from 10 to 70 µm2. Spermatozoa were categorized as motile when VAP >10 µm/sec, and when they deviated <80 % from a straight line were classified as linearly motile. The following parameters were determined for each analysed sperm sample: total motility (TM; %), progressive motility (PM; %), curvilinear velocity (VCL; µm/sec), straight-line velocity (VSL; µm/sec), average path velocity (VAP; µm/sec), straightness (STR; %), linearity (LIN; %), wobble (WOB; %), amplitude of lateral head displacement (ALH; µm), and beat/cross frequency (BCF; Hz).
Sperm membrane functionality assessment
Hypo-osmotic swelling test (HOST)16was used to determine the functional membrane status of spermatozoa. From each sperm fraction, 10 µL was diluted into 100 µL of hypo-osmotic sodium citrate solution (1.351 g fructose, 0.735 g sodium citrate, and 100 mL bi-distilled water; 100 mOsmol/kg) and warmed at room temperature for 30 min. After incubation, samples were fixed in 2% glutaraldehyde and observed under phase contrast microscopy (× 400 magnification). The sperm membrane was considered intact and functional when the sperm tail exhibited coiling. A total of 200 sperm cells were analyzed and the results were expressed as percentage of positive endosmosis.
Sperm morphology assessment
Hemacolor staining (Merck, Darmstadt, Germany) was used to identify different sperm morphological abnormalities17. A volume of 10 µl of semen was spread on a slide and stained according to the manufacturer’s instructions. The percentage of sperm abnormalities was determined by counting around 200 sperm cells (× 1000 magnification) (Olympus, Tokyo, Japan).
Sperm viability assessment
Sperm viability was assessed by eosin-nigrosin stain18. In brief, a total of 0.67 g Eosin Y (Panreac, Barcelona, Spain) and 0.9 g sodium chloride (Panreac, Barcelona, Spain) were dissolved in bi-distilled water (100 mL) under gentle heating, and then 10 g nigrosin (Panreac, Barcelona, Spain) was added. A 10 µL drop of sperm sample was mixed with a 10 µL drop of stain on a glass slide and the smear was made. For evaluation, 200 sperm cells were analyzed (× 1000 magnification) (Olympus, Tokyo, Japan). The spermatozoa were categorized as live (i.e. membrane was intact) when cells were unstained or as dead (i.e. membrane was altered) when they were pink-stained by eosin. Results were expressed as percentage of live sperm.
Statistical analysis
The SPSS 17.0 package (SPSS, Chicago, IL, USA) was used for statistical analysis. Data are shown as a mean ± SD. Normality was tested using the Shapiro-Wilks test and when data were not normal, they were transformed. Those variables expressed as percentages (LIN, STR and WOB) were arcsine transformed, while others expressed as absolute values (VCL, VSL, VAP, ALH and BCF) were log transformed. Sperm parameters at the different fractions obtained after SLC in samples containing C800 or C3000 were compared with the fresh sperm values in order to determine the recovery rate and the sperm quality. One-way ANOVA was performed to compare the sperm recovery rate and sperm quality. Bonferroni post hoc test was used when significant differences between fractions (P≤0.05) were detected.
In order to identify specific sperm subpopulations based on the kinetic parameters, a total of 20,485 observations from fresh and processed semen were evaluated using clustering procedures. Firstly, a principal components (PC) analysis was carried out on the data to reduce the eight studied variables (VCL, VSL, VAP, LIN, STR, WOB, ALH, and BCF) to the smallest number of linear combinations of the initial variables (called PCs) that save the majority of information of the original variables. It was expected that a few PCs explain a high proportion of the total variance. The VARIMAX rotation method was used and the number of PCs was selected using the Kaiser criterion for selecting those with an eingen value greater than 1. After that, a two-step cluster procedure was used to analyze the sperm-derived indexes obtained after PC. Different subpopulations were then identified and outliers were detected. The type of sperm subpopulation was analyzed in each sperm sample and the Chi square test was used to compare the relative frequencies of subpopulations within each sperm sample (or fraction).
Results
Recovery rate by SLC
After sperm separation by SLC, the percentage of sperm recovery in the fraction containing the theoretically best spermatozoa (F3) showed no significant differences (P>0.05) between C800 and C3000 (36.1 % and 27.5 %, respectively) (Figure 2). In addition, it was observed a significantly higher percentage of isolated spermatozoa in F1 in C3000 samples than in C800 samples, in contrast with those happened in F2. No significant differences were observed between the three obtained fractions after SLC in C800 sperm samples. By contrast, C3000 samples recovered significantly higher percentage of spermatozoa in F1 than in F3.

Asterisk indicate significant differences (P≤0.05) between the same fraction in samples containing different sperm concentrations.
A, B Different uppercase letters indicate significant differences (P≤0.05) between different fractions.
Figure 2 Sperm recovery rate (%) at different fractions obtained after SLC in samples containing 800 and 3000 × 106 spz/ml; (C800 and C3000)
Sperm assessment
Table 1 shows the motility values corresponding to spermatozoa isolated in the different fractions after SLC. No sperm motility differences were observed between the different fractions isolated after SLC both in C800 and C3000.
Table 1 Mean ( ( SD) values of motility and kinematic parameters from different sperm fractions obtained after SLC in samples containing 800 and 3000 × 106 spz/ml (C800 and C3000)
| Item | Fraction | C800 | C3000 | ||
|---|---|---|---|---|---|
| FRESH | SLC | FRESH | SLC | ||
| TM (%) |
F1 | 89.0 ± 2.0 | 86.0 ± 3.5 | ||
| F2 | 88.4 ± 6.8 | 86.5 ± 4.0 | |||
| F3 | 89.5 ± 2.5 | 88.5 ± 6.1 | |||
| Whole | 87.9 ± 3.7 | 89.8 ± 3.06 | |||
| PM (%) |
F1 | 50.3 ± 7.0 | 48.2 ± 4.8 | ||
| F2 | 41.7 ± 9.6 | 56.2 ± 12.2 | |||
| F3 | 37.0 ± 18.4 | 48.4 ± 20.2 | |||
| Whole | 48.8 ± 12.2 | 50.2 ± 9.0 | |||
| VCL (µm/s) |
F1 | 121.4 ± 17.6 | 119 ± 5.3 | ||
| F2 | 127.3 ± 12.8 | 120 ± 9.7 | |||
| F3 | 133 ± 18.6 | 129.9 ± 12.9 | |||
| Whole | 117.9 ± 11.1 | 124.2 ± 4.7 | |||
| VSL (µm/s) |
F1 | 72.2 ± 13.9 | 69.7 ± 7.1 | ||
| F2 | 66.5 ± 12.4 | 81.8 ± 11.8 | |||
| F3 | 69.1 ± 12.3 | 71.7 ± 14.6 | |||
| Whole | 59.9 ± 18.6 | 58.2 ± 18.1 | |||
| VAP (µm/s) |
F1 | 94.9 ± 16.5 | 91.5 ± 7.4 | ||
| F2 | 94.2 ± 15.4 | 100.2 ± 6.9 | |||
| F3 | 96.5 ± 14.5 | 95.1 ± 12.0 | |||
| Whole | 80.23 ± 18.0 | 77.9 ± 17.5 | |||
| LIN (%) |
F1 | 59.4 ± 6.3 | 58.4 ± 3.5 | ||
| F2 | 52.2 ± 9.0 | 68.4 ± 12.3 | |||
| F3 | 52.9 ± 11.2 | 56.7 ± 14.9 | |||
| Whole | 50.0 ± 10.2 | 46.6 ± 12.9 | |||
| STR (%) |
F1 | 76.1 ± 5.5 | 76.1 ± 2.1 | ||
| F2 | 70.5 ± 5.7 | 81.3 ± 8.7 | |||
| F3 | 71.4 ± 6.9 | 74.7 ± 7.8 | |||
| Whole | 72.8 ± 6.3 | 73.7 ± 5.8 | |||
| WOB (%) |
F1 | 77.9 ± 4.6 | 76.8 ± 3.4 | ||
| F2 | 73.7 ± 8.3 | 83.8 ± 7.8 | |||
| F3 | 73.5 ± 10.6 | 74.6 ± 13.3 | |||
| Whole | 66.7 ± 9.4 | 62.5 ± 12.3 | |||
| ALH (µm) |
F1 | 3.4 ± 0.4 | 3.5 ± 0.4 | ||
| F2 | 3.8 ± 0.4 | 3.1 ± 0.5 | |||
| F3 | 3.9 ± 0.9 | 3.9 ± 1.2 | |||
| Whole | 3.78 ± 0.3 | 4.3 ± 0.4 | |||
| BCF (Hz) |
F1 | 8.9 ± 3.2 | 10.3 ± 0.3 | ||
| F2 | 8.9 ± 2.7 | 10.0 ± 0.8 | |||
| F3 | 10.1 ± 4.0 | 10.9 ± 2.0 | |||
| Whole | 11.9 ± 0.9 | 12.9 ± 1.6 | |||
C800= Sample with 800 × 106 spz/ml; C3000= Sample with 3000 × 106 spz/ml; SLC= Single layer centrifugation; F1= top phase, F2= medium phase; F3= bottom phase; TM= total motility; PM= progressive motility; VCL= curvilinear velocity; VSL= straight-line velocity; VAP= average path velocity; STR= straightness; LIN: linearity; WOB= side to side movement of the sperm head; ALH= amplitude of lateral head displacement; BCF= beat/cross frequency.
Table 2 represents sperm viability, morphology and membrane functionality of samples processed. Although F3 showed the higher values in all the studied parameters, no significant differences (P>0.05) were observed in relation with the other fractions or between samples with different sperm concentrations.
Table 2 Mean ( ± SD) values of viability, morphology, membrane functionality from different sperm fractions obtained after SLC in sperm samples
| Parameters | Fraction | C800 | C3000 | |||
|---|---|---|---|---|---|---|
| FRESH | SLC | FRESH | SLC | |||
| Viability (%) | F1 | 81.2 ± | 91.3 ± | |||
| F2 | 92.0 ± | 93.5 ± | ||||
| F3 | 95.4 ± | 94.8 ± | ||||
| Whole | 89.0 ±8.9 | 89.5 | ||||
| Morphology (%) | F1 | 61.0 ± | 83.5 ± | |||
| F2 | 80.7 ± | 90.3 ± | ||||
| F3 | 85.3 ± | 93.5 ± | ||||
| Whole | 87.6 ±5.1 | 90.1 ± | ||||
| Membrane functionality (%) | F1 | 64.5 ± | 75.3 ± | |||
| F2 | 65.5 ± | 78.7 ± | ||||
| F3 | 68.3 ± | 80.7 ± | ||||
| Whole | 65.4 ± 1.7 | 80.6 ± 4.5 | ||||
C800= sample with 800 × 106 spz/ml; C3000= sample with 3000 × 106 spz/ml; SLC= Single layer centrifugation; F1= top phase, F2= medium phase; F3= bottom phase.
Sperm subpopulations
Three sperm subpopulations were identified in a total of 20,485 individual motile spermatozoa analyzed, as shown in Table 3.
Table 3 Mean (± SD) values of kinematic and velocity parameters used for the characterization of three sperm subpopulations identified in fresh ovine semen samples
| Subpop. | N | % | VCL (µm/s) |
VSL (µm/s) |
VAP (µm/s) |
LIN (%) |
STR (%) |
WOB (%) |
ALH (µm) |
BCF (Hz) |
|---|---|---|---|---|---|---|---|---|---|---|
| SP1 | 3906 | 19.1 | 65.5 ± 41.4 |
18.5 ± 13.4 |
38.9 ± 27.4 |
31 ± 2 |
52 ± 2 |
60 ± 1 |
2.9 ± 1.7 |
5.5 ± 2.5 |
| SP2 | 8950 | 43.7 | 121 ± 36.2 |
93.1 ± 31.7 |
106.9 ± 34.4 |
77 ± 1 |
87 ± 1 |
88 ± 8 |
3.1 ± 1.2 |
9.4 ± 2.8 |
| SP3 | 7629 | 37.2 | 154.4 ± 35.2 |
61.4 ± 26.7 |
96.7 ± 26.9 |
40 ± 2 |
65 ± 2 |
63 ± 1 |
5.4 ± 1.4 |
12.9 ± 3.1 |
| Total | 20485 | 100 | 122.8
± 48.6 |
67.1
± 38.7 |
90.1
± 39.6 |
55
± 2 |
72
± 2 |
73
± 2 |
3.9
± 1.8 |
10
± 3.9 |
VC=curvilinear velocity; VSL= straight-line velocity; VAP= average path velocity; STR= straightness; LIN= linearity; WOB= Side to side movement of the sperm head; ALH= Amplitude of lateral head displacement; BCF= Beat/cross frequency.
Three PCs were obtained after the data reduction, which explain the 76.70 % of the variance. PC1 was associated with VCL, VSL, and VAP; PC2 was related with STR and LIN, and PC3 showed association with WOB (Table 4).
Table 4 Principal component analysis of 8 sperm parameters, using Varimax rotation
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Eigenvalue | 2.55 | 2.27 | 1.31 |
| Percentage | 31.91 | 28.35 | 16.44 |
| Cumulative percentage | 31.91 | 60.26 | 76.70 |
| VCL | 0.96 | 0.01 | - 0.06 |
| VSL | 0.70 | 0.64 | 0.24 |
| VAP | 0.92 | 0.16 | 0.29 |
| ALH | 0.46 | -0.18 | - 0.28 |
| BCF | 0.23 | 0.13 | - 0.02 |
| LIN | 0.11 | 0.82 | 0.53 |
| STR | 0.06 | 0.98 | 0.15 |
| WOB | 0.17 | 0.39 | 0.89 |
Loadings exceeding 0.70 are highlighted in bold.
Spermatozoa included in subpopulation 1 (SP1) showed lower velocity (based on VCL, VSL and VAP) and no progressive motility (low values for LIN, STR, WOB, ALH and BCF); a total of 19.1 % of spermatozoa came within this subpopulation. Subpopulation 2 (SP2) showed high velocity (VCL, VSL, VAP and ALH), high progressivity (high LIN, STR and WOB) and good ALH and BCF. This subpopulation could be defined as consisting of rapid and progressive spermatozoa, with 43.7 % of the spermatozoa came within this subpopulation. Subpopulation 3 (SP3) showed high values of VCL, ALH and BCF, while LIN and STR values were low; these were defined as rapid and nonlinear spermatozoa and a total of 37.2% of spermatozoa were included in this subpopulation.
In C800 samples, the SP3 was significantly higher in the F2 and F3 than in F1, while SP1 and SP2 were significantly higher in the F1 than in the others (Figure 3).

a b Different uppercase letters indicate significant differences (P≤0.05) between different fractions for each concentration.
Figure 3 Relative frequency distribution of sperm subpopulations into the different sperm fractions obtained after SLC in samples containing 800 and 3000 × 106 spz/ml; (C800 and C3000)
The distribution of subpopulations varied in C3000 samples. SP2 (rapid and progressive) was significantly higher in F1 than in F3 (or selective fraction) and F2. SLC in this type of samples provoked that the majority of spermatozoa contained into F3 (i.e. fraction containing the theoretically best spermatozoa) were from SP3 (rapid non progressive).
Discussion
The present study evaluates the sperm separation capacity and the characteristics of the isolated sperm fractions after SLC in normospermic ram samples. These samples were prepared with a volume around 1.5 mL and containing 800 or 3000 × 106 spermatozoa/mL, in order to invest if this sperm separation procedure could be efficient on ram fresh ejaculates. In addition, by focusing on eight kinematic parameters, three different sperm subpopulations were found in whole fresh ejaculates and in separated fractions obtained by SLC.
It is known that sperm centrifugation can affect motility and membrane integrity in small ruminant spermatozoa19,20. However, the use of centrifugation combined with colloids could be an interesting option to select the best spermatozoa for improving AI results. The present study suggest that SLC does not offer an adequate separation of spermatozoa based on motility, viability, morphology or membrane functionality when normospermic ram semen is processed. In a recent study carried out on red deer21, it has been demonstrated that the type of colloids affects the efficiency of the layer centrifugation technique, and DLC offers better separation ability if low-quality sperm samples were used22.
In the present study, spermatozoa isolated in F3 after SLC (where are expected the best spermatozoa were found) did not achieve higher sperm motility (total and progressive) than spermatozoa located in the other fractions after centrifugation. As far as sperm morphology is concerned, although the results showed that morphological abnormalities are reduced while sperm crosses the colloid layers, differences were not significant. These results are in line with those obtained in wild ruminants23) and in llama24, observing no morphological sperm differences between fresh and separated spermatozoa in good quality semen samples. In bull sperm, colloidal procedures increased the number of normal morphological spermatozoa in poor-quality samples25. However, when SLC was used with normospermic samples, it was here observed that no advantages were obtained in terms of separating morphologically normal spermatozoa.
The sperm membrane functionality did not vary after application of SLC in C800 or C3000 sperm samples. The present results showed that SLC is not able to separate spermatozoa with injured membrane functionality, morphology and viability, in agree with results reported for equine samples separated by SLC using Androcoll26. In addition, studies of normospermic bull sperm have concluded that there was no effect on samples before and after application of SLC in comparison with untreated samples25.
To the present authors’ knowledge, this is the first study of ram sperm to analyze sperm subpopulations in different sperm fractions obtained by SLC. It has been affirmed that ejaculates have heterogeneous sperm subpopulations, which determine their fertility, i.e. their success after insemination through natural spermatozoa selection27. And fertility can be also promoted by the different procedures for spermatozoa selection, such as centrifugation through colloids. After the evaluation of individual kinematic parameters in ram spermatozoa samples, three different subpopulations were selected with differential characteristics, in keeping with other authors28,29.
As expected in normospermic ejaculates, the subpopulation showing spermatozoa with low and non-progressive motility (SP1) was poorly represented in the sperm sample (19.1 %). By contrast, SP2 (classified as rapid progressive subpopulation) showed a total of 43.7 % of spermatozoa came within this subpopulation, being the higher subpopulation. Finally, SP3 showed high values of VLC and ALH, while LIN and STR values were low, and a total of 37.2 % of spermatozoa were included in this subpopulation, being classified as rapid and nonlinear, in line with other authors28,29. The results obtained after centrifugation and different fractions isolation suggest that SLC does not separate the best motility quality subpopulation, whether in C800 or in C300 sperm samples, not being SP2 the most abundant subpopulation in F3, as expected. It was observed that many spermatozoa belonging to SP2 (considered as the best subpopulation) were retained in the second interface; one possible explanation for this observation is that the high number of spermatozoa included in the sample may impede other optimal spermatozoa from swimming to the bottom of the tubes30. As previously recommended31, SLC it is better to use for processing of large volumes of semen, suggesting that the increment of the sperm concentration was an impediment to the efficient operation of this method. In keeping with others23, the present results showed that sperm selection techniques do not improve sperm quality in non-stressed sperm samples.
Conclusions and implications
In summary, colloid centrifugation is not an effective method for sperm selection when samples containing a high concentration of spermatozoa with good quality (i.e. normospermic sperm samples) are processed. Further studies should be conducted with low quality sperm samples to determine the sperm separation efficiency of colloidal centrifugation in ram sperm, and also to determine the freezability of sperm fractions separated by colloid centrifugation.





![Rendimiento de la planta de frijol caupí [Vigna unguiculata (L.) Walp] y calidad nutricional en los sistemas de cultivo intercalado de frijol caupí y sorgo](/img/es/next.gif)





 texto en
texto en 


