Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias pecuarias
versión On-line ISSN 2448-6698versión impresa ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.13 no.2 Mérida abr./jun. 2022 Epub 20-Jun-2022
https://doi.org/10.22319/rmcp.v13i2.5960
Review
Apis mellifera in Mexico: honey production, melliferous flora and pollination aspects. Review
a Instituto de Ecología, AC. Departamento de Ecología Funcional, Carretera Antigua a Coatepec No. 351, El Haya, 91070, Xalapa, Veracruz. México.
2b Universidad Nacional Autónoma de México. Posgrado Ciencias de la Sostenibilidad, Ciudad de México, México.
The honeybee, Apis mellifera, is a species that, since its introduction to Mexico, has had great social, cultural and economic importance, representing an important source of income for thousands of families who are engaged in beekeeping. However, in the context of the so-called “pollinator crisis”, it is considered that we do not know how this phenomenon affects A. mellifera in Mexico. In review, it is analyzed and discussed the information about A. mellifera in Mexico related to the phenomena that affect its distribution, honey production and its ecology, including interactions with the local flora. In general, it is considered that there is a need for an integration of data on beekeeping at the national level, and that there are few studies on the ecology of A. mellifera in Mexico, from the flora they visit, their efficiency as a pollinator and competition with other native bee species. It is believed that increasing studies on A. mellifera will help to predict changes in honey production as well as understand and address threats to these pollinators, contributing to generate better management practices and establish better pollinator conservation strategies that include the presence of A. mellifera.
Key words Apis mellifera; Beekeeping; Pollination; Honey; Hives
La abeja de la miel, Apis mellifera, es una especie que, desde su introducción a México, ha tenido una gran importancia social, cultural y económica, representando una importante fuente de ingreso para miles de familias que practican la apicultura. Sin embargo, en el contexto de la llamada “crisis de los polinizadores” se considera que se desconoce cómo este fenómeno afecta a A. mellifera en México. Esta revisión analiza y discute la información sobre A. mellifera en nuestro país relacionada a los fenómenos que afectan su distribución, la producción de miel y su ecología, incluyendo las interacciones con la flora local. De manera general se considera que hace falta una integración de datos sobre la apicultura a nivel nacional, y que existen pocos estudios sobre la ecología de A. mellifera en México, desde la flora que visitan, su eficiencia como polinizador y la competencia con otras especies de abejas nativas. El incrementar los estudios sobre A. mellifera ayudará a predecir cambios en la producción de miel, así como comprender y abordar las amenazas a dichos polinizadores, abonando a generar mejores prácticas de manejo y a establecer mejores estrategias de conservación de polinizadores que incluyan la presencia de A. mellifera.
Palabras clave Apis mellifera; Apicultura; Polinización; Miel; Colmenas
Introduction
From the identification of the so-called “pollinator crisis”1,2, which identifies the collapse of different groups of pollinators in various parts of the world, especially in North America and Europe, much interest has arisen to understand the role of the honeybee, Apis mellifera, in the different ecosystems where it lives. This interest results from the fact that A. mellifera is a species of great importance for humans for providing goods such as honey, wax, pollen, propolis and other derivatives of the colony3,4, as well as for its role as a crop pollinator5. Currently, the commercial, cultural, nutritious and medicinal value of honey has caused that, of the eleven species existing in the genus Apis, the species A. mellifera (Apidae: Apini), known as honeybee or in some localities as swarms or European bee, due to its origin, is the most valued worldwide6. In Mexico, A. mellifera, despite being an introduced species, has a great cultural and commercial value, heir to the importance that the Mesoamerican peoples gave to bees because they were part of their traditional activities7.
Given the environmental crisis of pollinators, the phenomenon of collapse in Latin American countries seems less accentuated, and the effects on bee populations respond to processes related to the type of beekeeping management, land use change and the type of agricultural practices8. In this context, it is necessary to review the current state of knowledge about this species in our country, in order to know its role in the productive aspects, its insertion in ecosystems, and analyze the threats to which they are subject. Likewise, identify the information gaps that exist and should be reviewed in the context of the “pollinator crisis” and climate change, as phenomena that represent threats to natural and managed populations of pollinators. Identifying these points would help establish better management and action strategies at the national level to support this important activity. This paper aims to assess what is known about A. mellifera in Mexico from an ecological and socioeconomic perspective from a review of the literature and official government data.
A bit of history
The presence of the honeybee, A. mellifera, in Mexico, and its importance as an ecological and social element within the country is the result of different processes that take to back to Mesoamerican cultures and the time of the Spanish colony, when, around 1760 and 1770, A. mellifera was introduced9,10. The management of meliponines or stingless bees (Apidae: Meliponini) represented an activity of important cultural value in different Mesoamerican peoples (e.g., Mayans, Nahuas and Totonacs;9-11. This biocultural relationship with stingless bees was also transferred to A. mellifera, replacing the products obtained from the colonies of stingless bees, although it did not completely displace them12.
Despite its presence since colonial times, beekeeping began as an activity of economic relevance until the mid-twentieth century13. Since then, different varieties of A. mellifera have inhabit practically the entire continent, and, in the warm areas, they are almost all Africanized, a process that has happened over more than half a century since their arrival on the continent in 195614. Due to the above and due to the use of the different floral resources where beekeepers move or keep their bees, A. mellifera is currently established in most of the ecosystems of this country15 (Figure 1).
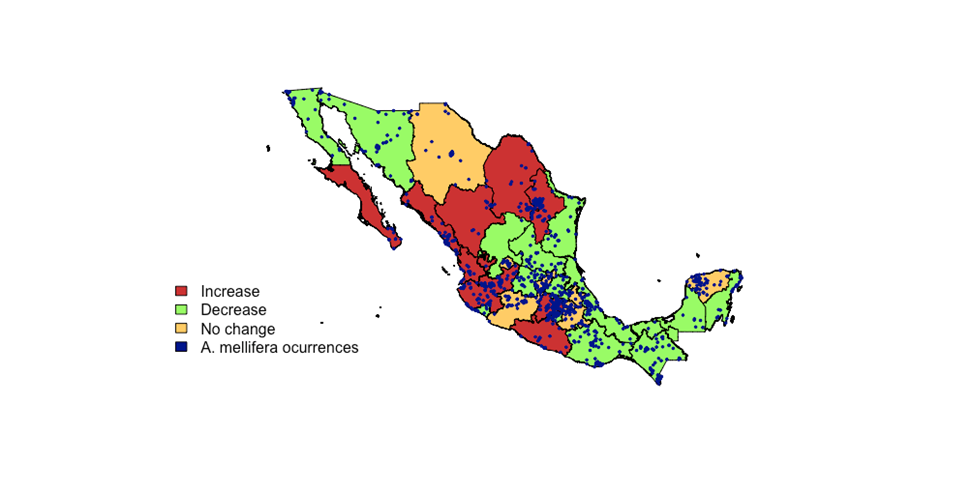
The colors of each state indicate whether in the period from 2009 to 2018 (SIAP) there has been a decrease (red), increase (green) or no change (yellow) in the number of hives recorded (negative binomial analysis)
Figure 1 Records of the presence of Apis mellifera in Mexico from data obtained in the Global Biodiversity Information Facility, GBIF (without duplicate data) and changes in beehives per state
Abundance and distribution of colonies
The success in the introduction of Apis in most of the world is because it is a generalist or polylectic species, that is, it can visit a great diversity of flowering plants to collect nectar, pollen and resins, and produces large amounts of honey that can be used by people. Considering that A. mellifera is an exotic species and of great economic importance in Mexico, its geographical distribution and abundance in the different ecosystems, both managed and wild colonies, still lacks precise information to analyze its spatiotemporal distribution.
A review of the official records obtained from the database of the National Commission for the Use and Knowledge of Biodiversity (CONABIO, for its acronym in Spanish) and the GBIF (Global Biodiversity Information Facility), shows us that, for Mexico, there are only 1900 records of A. mellifera (without duplicate data; Figure 1). This contrasts with the data from beekeepers’ associations and SAGARPA data, which, up to 2016, estimated around 45 thousand beekeepers who manage around 1.9 million hives throughout the country6,13, and 2018 censuses estimate around 2.172 million hives16. This discrepancy highlights the need to integrate productive information with biological information to have a better understanding of the situation of A. mellifera in Mexico, not only of its distribution in apiaries but also of those colonies that have escaped management.
In a review of the national agricultural survey developed by INEGI in 2016, was found that there are 7,080 apiaries with at least one hive, and they estimate an area of approximately 613,090.22 ha of land with apiaries, however, they do not report the number of hives that are kept per apiary, nor is the foraging area of bees clear. Despite having data on the number of hives and an approximation of the number of apiaries, it is still necessary to integrate the geographical information of the location of apiaries, both those that are moved to take advantage of the different flowerings and those with sedentary management. If the estimated area is divided by the number of hives reported by SAGARPA and SIAP (1.9 or 2.17 M), the information suggests that there are between 3 and 3.5 hives per hectare nationwide, which represents a low number. However, it is known know that there are five main beekeeping regions in Mexico (Altiplano, Pacific Coast, North, Gulf and Yucatán Peninsula), and that they do not have the same national representation in honey production, so it is necessary to better integrate the data on the number of hives by territory (region, state, municipality) as well as the foraging area of bees. Likewise, the saturation of hives is influenced by the types of flowering, whether natural or from crops, for example, orange or orange blossom honey that is produced in citrus-growing areas or butter honey from the Mexican plateau and have a greater number of associated colonies6. Therefore, the nonhomogeneous spatial distribution of hives, as well as the interest of beekeepers in seeking certain flowerings to increase the value of honey, results in regions with a higher density of bees and others that are underutilized.
In the context of land use change and high levels of deforestation in the national territory17, it is of vital importance to have information on the ecological quality of the foraging territories that support bee populations, both A. mellifera and native bees. This information could be very valuable to inform the policy that regulates beekeeping by knowing which territories are more favorable to locate apiaries and manage the movement of hives, while promoting better coordination between the associations of the different beekeeping areas of the country. This is particularly for those beekeepers who seek organic certification, which is increasingly demanded by the market, and therefore has been increasing and requires particular environmental conditions for the foraging of bees. On the other hand, very little is known about the wild colonies of A. mellifera, and it is not known if CONABIO records include this type of colonies, which in general belong to Africanized colonies that have escaped human management and have become feral18,19.
The loss of hives due to different factors such as diseases, pesticides, climatic phenomena such as frosts, hurricanes, lack of food due to alterations in flowering, as well as absconding (when bees leave the nest and migrate elsewhere) and swarming (when the colony divides and a large part of the bees leave the nest to form a new one) processes, is a problem little studied in Mexico, despite being commonly mentioned, mainly in the media. A study conducted by Medina-Flores20, who interviewed 196 beekeepers from 14 states of the republic, revealed that during the winter of 2015-2016, of the total of 41,907 hives they managed, about 33 % were lost. The reasons for this loss were attributed to bad weather, diseases, pesticide use, absconding and swarming. On the other hand, this paper analyzes the official data on the number of hives by state from 2009 to 201816 (negative binomial model: number of hives by state ~ year) in order to know if there is evidence of a significant reduction in hives. The result of the analysis revealed that, on the contrary, for 16 states there is a significant increase in the number of hives (Figure 1), while a significant decrease was observed only in 9 states and no change is observed in 7 states. The states with the highest number of hives coincide with the states with the highest honey production (Yucatán, Campeche, Quintana Roo; Table 1). Although beekeeping has grown in some regions and has remained stable in others, this does not necessarily mean that there have been no decreases in the number of colonies, but rather that these could have been replaced or that there is an increase in the number of beekeepers.
Table 1 Results from negative binomial analyses per state evaluating the increase or decrease of beehives in ten years (2009, 2018; SIAP)
| SATE | Deviance # Hives~Year |
P (Chi-square) | |
|---|---|---|---|
| Aguascalientes | 0.08456 | 0.000212 | *** |
| Baja_California | 7.8445 | 0.005097 | ** |
| Baja_California_Sur | 11.114 | 0.0008569 | *** |
| Campeche | 5.9368 | 0.01483 | * |
| Coahuila | 12.716 | 0.0003626 | *** |
| Colima | 24.87 | 6.13E-07 | *** |
| Chiapas | 36.623 | 1.43E-09 | *** |
| Chihuahua | 0.85191 | 0.356 | |
| CDMX | 0.59024 | 0.4423 | |
| Durango | 12.886 | 0.000331 | *** |
| Guanajuato | 19.71 | 9.01E-06 | *** |
| Guerrero | 15.982 | 6.39E-05 | *** |
| Hidalgo | 15.921 | 6.60E-05 | *** |
| Jalisco | 5.5714 | 0.01826 | * |
| México | 12.899 | 0.0003287 | *** |
| Michoacán | 0.44123 | 0.5065 | |
| Morelos | 111.34 | 2.20E-16 | *** |
| Nayarit | 5.887 | 0.01525 | * |
| Nuevo_León | 13.428 | 0.0002478 | *** |
| Oaxaca | 49.825 | 1.68E-12 | *** |
| Puebla | 3.509 | 0.06104 | |
| Querétaro | 0.029103 | 0.8645 | |
| Quintana_Roo | 83.473 | 2.20E-16 | *** |
| San_Luis_Potosí | 143.68 | 2.20E-16 | *** |
| Sinaloa | 64.141 | 1.16E-15 | *** |
| Sonora | 38.018 | 7.01E-10 | *** |
| Tabasco | 88.249 | 2.20E-16 | *** |
| Tamaulipas | 25.088 | 5.48E-07 | *** |
| Tlaxcala | 0.26805 | 0.6046 | |
| Veracruz | 20.788 | 5.13E-06 | *** |
| Yucatán | 0.090502 | 0.7635 | |
| Zacatecas | 26.363 | 2.83E-07 | *** |
Analyses were performed in R 3.5 (R Development Core Team, 2011) with MASS package (Venables and Ripley; 2002+). Asterisks indicate significant effect of year over the number of beehives.
+Venables WN, Ripley BD (2002). Modern Applied Statistics with S, Fourth edition. Springer, New York. ISBN 0-387-95457-0,
Honey production in Mexico
Current beekeeping is present to a greater or lesser degree in the 32 states that Mexico comprises, according to data from the Secretariat of Agricultural, Rural Development and Fisheries 15. The benign climate in much of Mexico makes it possible for the colonies of A. mellifera remain active throughout the year14, as well as the great diversity of plants and ecosystems that allow a great variation in the quantity and quality of the honey that is produced, which sometimes gives an added value to this product21. Due to the diversity of these ecosystems and the socioeconomic characteristics of beekeepers, the activity is carried out under two schemes 1) Fixed or sedentary beekeeping, where the apiaries that contain the hives are kept in the same place throughout the year, and 2) Transhumance or mobile beekeeping. In this, the apiaries are moved to different sites throughout the year, according to the flowerings of interest of the beekeeper22. Fixed beekeeping is favored in places where floral resources remain more or less constant throughout the year or in cases where beekeepers decide to make fewer annual harvests, of a smaller scale. Transhumance is favored in places with greater seasonality or fewer floral resources and is a strategy used to increase the number of annual harvests22. In any of its two forms of use of nectar-polliniferous resources, the beekeeper learns to know the behavior of the flowering seasons and schedules the harvest times, so that, depending on the place and management techniques used, one, two or even three or more annual harvests can be achieved23. Thus, the amount of honey produced per hive depends in part on the nectar-polliniferous resources present in the different beekeeping areas of the country, although there are other factors such as the time of year, the ecosystem, diseases, as well as the investment capital of beekeepers24.
For approximately 30 years, the management of the Apis colonies has led to Mexico being among the ten most important countries in honey production worldwide25. Among the most important regions in terms of honey production are the Yucatán Peninsula (Campeche, Yucatán and Quintana Roo), Jalisco and Veracruz16.
Historical data on honey production indicate that the number of hives and total honey production increased substantially from the 60s and were on the rise before 1986, when the Africanized bee was first recorded in the south of our country (a hybrid of European varieties with African varieties;26,27; (Figure 2). Nevertheless, Mexico ranked third worldwide in 1991, with 63,886 t26. However, honey production has been decreasing since 1986 and Mexico ranked ninth in honey production in 2017, with 51,066 t25 (Figure 3).

The red line indicates the year that was recorded as the beginning of the process of Africanization of the European bee in Mexico.
Figure 2 Historical data on the number of hives, total honey production per year (tons) and honey production per hive (kg) in Mexico from 1961 to 2018 (FAOSTAT, 2020)
On the other hand, honey exports before 1990 represented a very low percentage of total production (between 21-33 %). However, as of 1990, the average export of honey increased to 52.5 %, with around 30,333 t per year between 1991 and 201725 (Figure 4). Thus, in some years, exports represented around 40 % of total production, while 2015, which was one of the best years for export, represented 68.1 % of total production25.

The dotted line indicates the year in which the record of Africanization began in Mexico
Figure 4 Honey production and export from 1986 to 2017 (FAOSTAT, 2020)
This despite the increase in restrictions and demands of the international market, and the market fluctuations that are affected by different aspects, both internal and external28. The main importing countries of Mexican honey have been Germany, the United States and the United Kingdom, countries with a long tradition of consumption. In contrast, domestic consumption of honey is very low28, and the percentage of losses for beekeepers from production that is not exported and not consumed locally is unknown.
In summary, despite Africanization, Mexico has positioned itself as one of the largest producers of honey and its production has increased compared to decades prior to the entry of African bees. However, the decrease in hives and the declines in honey production in recent decades have been attributed to multiple reasons that together have affected beekeeping activity6,13. One of the main reasons was the arrival of Africanized bees, which produce less honey, swarm easily, and having a more defensive behavior have generated economic losses due to damage caused, which caused many beekeepers to abandon the activity27. Despite this, Africanization has not had the same effects in all regions and, in some, it could even have benefited beekeeping, because they are better adapted to tropical environments and because they became a source of colonies (collected from the field) for beekeepers29. Since the Africanization process began in the country, in the 1980s, the National Program for the Control of the African Bee was developed to counteract these negative effects and integrate its presence into management30.
On the other hand, the decrease in production in the mid-90s coincides with the presence of the mite Varroa destructor, first reported in 1992 in Mexican territory31. This parasite infests the hives and feeds on the hemolymph of the bees, promoting the entry of other diseases associated with different viruses32, affecting the reproduction and population of the colony, which means a lower production of honey and, in extreme cases, its death33. This disease is currently controlled with the application of drugs based on components such as thymol, oxalic acid and formic acid, among others34. However, there are controversies regarding their use and abuse, and even organic certification measures limit the type of drugs that can be used23,35. Another recent problem has been the proliferation of the small hive beetle (SHB) Aethina tumida Murray 1867, since it was first reported in Mexico in 200736 or the case of fungi of the genus Nosema, which have been found in the country since 196537. The consequences of such diseases should not only be measured in the context of the loss of colonies of A. mellifera, but they could have ecological consequences when transmitted to other pollinating insects.
Another aspect that may be relevant in honey production in Mexico is the frequent presence of natural phenomena, such as hurricanes and storms, which can be further altered by the effects of climate change38,39. Changes in flowering times, resulting from drought events or alterations in rainfall patterns, are another destabilizing factor for beekeeping40; because beekeepers need to coordinate and anticipate the flowering time to ensure that hives are ready for honey harvest time23. Among the regions with the highest incidence of these phenomena, the Yucatán Peninsula and Veracruz stand out, which together represented 45 % of the total honey production in 2018, and the Pacific coasts, especially the states of Jalisco, Guerrero, Michoacán, Chiapas and Oaxaca, that represented 26 % of production. Future analyses should try to consider how these climatic phenomena alter beekeeping activity in the country.
Finally, the availability of resources for honey production depends on the vegetation cover in the different ecosystems. The change of land use towards agricultural uses with intensive and industrial management and the consequent loss of floral diversity mean fewer floral resources for bees41. In addition to the problems with the use of toxic agricultural chemicals and even the use of genetically modified organisms that affect the health of bees42. However, the interaction of forest cover loss or change and its effects on both native and introduced bees is another situation that has been little studied in Mexico43,44.
All these factors contribute to the fluctuations in production and number of hives reported in this study. In addition, the variation in the quality of honey, its floral origin, associated with the variation in honey production, are directly related to its commercialization, since the standards in the regulation of honey for export must be met (see NOM-004-SAG/GAN-2018). According to Soto-Muciño and collaborators6, in Mexico, beekeepers with high commercial power have decreased and beekeepers with small and medium production have increased. This could represent a window of opportunity to promote beekeeping in regions where the management of Apis mellifera is a complementary activity within the agricultural and livestock practice, as is already the case in the north of the country, as well as to promote beekeeping, especially under agroecological management, and that it is a source of income and family employment.
An important part to promote the care of bee colonies is to improve production conditions and have a better assessment of the state of beekeeping in Mexico and have better data on the number of hives, the socioeconomic characteristics of beekeepers in different regions, management statistics, and identify their threats in different contexts. Likewise, knowledge about apiary distribution areas, flowering calendars, and coordination between beekeepers to avoid oversaturation of foraging areas are required.
Characterization of honeys and botanical origin
Despite the commercial value of Mexican honey, which is recognized in the Official Mexican Standard (NOM-004-SAG/GAN-2018), and that there is a general characterization of some honeys at the regional level (e.g., multifloral honeys from coffee plantations, or monofloral such as that of orange blossom from citrus flowering (Citrus sp.)), there are relatively few studies that evaluate the organoleptic characteristics of honeys (color, taste, smell, etc.)45, as well as the botanical composition of honeys46,47 (Table 1). These studies are important not only to provide commercial added value to honey in each of the regions, but also to know the interactions of A. mellifera with the plants it visits and its possible role in ecosystems.
From a non-exhaustive review of the literature on the melliferous and polliniferous floras of A. mellifera, considering papers, book chapters, theses and publications in congresses, about 30 studies that characterize the diversity of plants visited by honeybees were found for Mexico (Table 2). Many of these studies are based on melissopalynological studies, that is, the analysis of pollen grains contained in honey to determine the species used by bees. However, some works are based on literature and direct observations of visits and interviews with beekeepers to learn about the plants in the region that bees visit to obtain nectar and pollen. From this review, it can be said that A. mellifera visits an average of 43 plant species per locality, for which the diversity of plant families varies in a range of 16-60 families. Although the number of species visited seems to be high, still not known what percentage it represents of the total native flora in each region and whether honeybees provide them with an effective pollination service. Only for the Yucatán Peninsula, it is estimated that Apis visits 40 % of the total flora48. Another important aspect is that A. mellifera does not always collect pollen from the plants visited and only goes for nectar, so nectariferous plants may be underrepresented in melissopalynological studies49.
Table 2 Records of studies on melliferous floras in different states of Mexico for Apis mellifera from 1994 to 2019
| State | Method | Local (n) |
Sample (n) |
Family (n) |
Species /types (n) |
Ref. |
|---|---|---|---|---|---|---|
| QR | Palynological analysis of nectar | 1 | 44 (22 and 22) | - | 148 | 49 |
| QR | Analysis of pollen taken from the hive | 2 | 206 | 41 | 168 | 50 |
| CAM | Interviews with beekeepers and field observations | 1 | - | 35 | 146 | 51 |
| TAM | It does not describe methodology (the whole state) | 1 | - | - | 50 | 52 |
| COL | Literature review and observations of visit | 1 | - | 45 | 140 | 53 |
| VDM | Melissopalynological | 2 | 2 | 15 | 19 | 54 |
| MOR | Melissopalynological | 3 | 3 | 23 | 41 | 55 |
| YUC, CAM, QR | Melissopalynological | 40 (total) | 78 | 15 | 250 | 56 |
| YUC, CAM QR | Melissopalynological | 17 (total) | 168 | 36 | 238 | 57 |
| 10 YUC) | 56 | 26 | 92 | |||
| 5 (CAM) | 56 | 19 | 66 | |||
| 2 (QR) | 55 | 36 | 80 | |||
| YUC CAM QR OAX | Melissopalynological | 4 | 39 | 29 | 64 | 58 |
| TAB | Melissopalynological | 4 | 40 | 36 | 129 | 59 |
| TAB | Melissopalynological | 3 | 12 | 32 | 63 | 60 |
| GUER | Literature review and observations of visit | - | - | 53 | 143 | 61 |
| GUER | Melissopalynological | 2 | 12 | 27 | 43 | 62 |
| DUR | Melissopalynological | 3 | 3 | 16 | 22 | 63 |
| BC | Melissopalynological | 13 | 52 | 33 | 150 | 64 |
| TAM | Melissopalynological | 11 | 27 | 60 | 215 | 65 |
| Páztcuaro, MICH | Direct observation of visits and interviews | 1 | 4 | 33 | 93 | 66 |
| Hopelchén, CAM | Interview and Herbarium review | 1 | 40 produces | 26 | 56 | 67 |
Local= municipality, region, state.
The role of A. mellifera as a pollinator
Although A. mellifera is very productive in terms of its establishment and honey production, it turns out that, from the perspective of plants, it is not necessarily the most efficient pollinator1,68. This means that, despite transporting pollen from one flower to another, the amount and place where it deposits it is not necessarily the most suitable for the plant to maximize seed production, and they can even be nectar robbers, that is, they take the nectar without making contact with the androecium and gynoecium of the flower. Despite this, it has been shown that A. mellifera is one of the most important crop pollinators so that millions of bees are managed for this purpose globally69. Perhaps one of the best-known examples is that of almond cultivation in California68, where Apis is used as the main pollinator. It is for this reason that the importance of studying and knowing the efficiency of A. mellifera as a pollinator of both the local flora and the crops is highlighted. In Mexico, the Beekeeping Pollination Manual70 includes recommendations for the use of Apis for pollination of different crops (citrus, cucurbits, cotton, etc.). Despite being a species widely used in crops, its efficiency as a pollinator compared to other pollinators is unknown.
In Mexico, studies on the efficiency of pollination by A. mellifera in crops are still limited71-77. Some studies have even excluded the Apis data because it is very abundant, to focus on native bees, so its role in pollination is not known78. The studies reviewed reveal that, for some crops, A. mellifera is not the most effective pollinator, as in the case of tomatoes and habanero peppers73 or as in coffee79. In the case of the squash species Cucurbita moschata (Cucurbitaceae), although Apis is not the best pollinator (at each visit), its effectiveness is compensated by being very abundant71. In addition, it was found to be a very important pollinator during the time of year where the main pollinator is absent71. However, another study on pollination networks in other Cucurbitaceae species (melon, squash, cucumber and watermelon) did not report any visits by Apis80, indicating that the role of Apis as a pollinator is variable. In some cases, Apis turns out to be a pollinator as efficient as other native bee species81, and in others a very important one, as in avocado72, or even more efficient than other pollinators77; while in other crops it is irrelevant, as is the case of rambutan, where no visits by Apis were observed74. In addition, little is known about the effect of A. mellifera on species of economic importance that are not cultivated. For example, in different species of Agave, it was found that A. mellifera is a nectar stealer, that is, it consumes the nectar of the flower but does not pollinate, and in other species it turns out to be a secondary pollinator during the hours of the day when there is less production of pollen and nectar82. Finally, it can be said that the efficiency of Apis as an effective pollinator in native species of no commercial importance has been evaluated very little. An example is the work on Kallstroemia grandiflora, where it was found that Apis is as efficient as native pollinators83. Although there is information in the literature about the visit of Apis to non-cultivated plants, particularly studies on plant reproductive biology, this information deserves to be reviewed to complement the knowledge about the interaction of Apis with native plants, but it has not been the subject of this review.
Studies at the landscape level and its effects on pollination indicate that Apis mellifera can take advantage of landscapes modified for agricultural or urban uses. A study that compared the effect of shade and sun coffee plantations on bee diversity found that shade coffee plantations harbor greater diversity of bee species, including A. mellifera, but that it substantially preferred sun coffee plantations, which have less plant diversity and where the diversity of native bees was lower84. In European and South American landscapes, studies suggest that, due to its characteristics, Apis seems to adapt and is abundant in highly transformed landscapes, including urban areas and semi-natural forests or with little plant diversity85-87.
Finally, studies on pollination networks are important because they help to understand more comprehensively the role of A. mellifera as a floral visitor of different species and its possible interaction with other pollinators. Nevertheless, in Mexico, studies on pollination networks are very few80,88-92. These studies reveal that A. mellifera is a very abundant species and that it has a large number of connections within the network91. Given this evidence, there is a need for more studies to evaluate the role of A. mellifera in the different ecosystems of Mexico, in its role as a pollinator and in that of its interactions.
Competition with native bees
One aspect that should not be forgotten is that Apis is an introduced species and can therefore have negative effects on local fauna, particularly on other bees with which it might be competing for resources. This competence can occur in different ways, and at least seven have been described93. Of these, the most studied are the reduction of pollen and nectar in a community due to the presence of Apis; the exclusion of native bees due to prolonged foraging times in floral patches, forcing native bees to travel further in search for resources; the active movement of native bees, mainly in the case of Africanized bees and the transmission of parasites of Apis to native bees93. In Mexico, there are still relatively few studies on the matter12,79,83,94,95 and of which the work of Villanueva-Gutiérrez et al12 in Quintana Roo stands out, which showed that A. mellifera and the native bee Melipona beecheii, in a context of abundant floral resources, avoided competition by diversifying resources, that is, they avoided visiting the same plants. A similar result was found in studies of competition between Apis and three other native bees, among them Partamona bilineata (a stingless bee), in squash and watermelon crops in Yucatán95. Another study suggests that Apis displaces native pollinators in coffee plantations because it was found that the greater the presence of A. mellifera, the lower the richness of other bee species79.
Several studies in other parts of the world have shown that Apis mellifera is capable of displacing native bees; however, critics of these studies argue that competition has not been demonstrated because these studies have not explored the effects on the adequacy of native bees93. However, evaluating the success of native bees against interaction with Apis is very complicated if one considers that for most native bee species is know practically nothing about their natural history, even for most species only females have been described [personal communication from experts in the area96].
Finally, the arrival of the Africanized variety of Apis mellifera could have altered the relationship with other native bees, since these varieties are more aggressive when it comes to defending their hive and floral resources, in addition to the fact that the Africanized bee is more adapted to become feral than European varieties13,20. Future studies should evaluate the role of feral colonies of A. mellifera on pollination and on other native bees and insects.
Competition with other species is mainly for floral resources, although it is not dismissed that they also compete for nesting sites. Studies on this subject are needed. Considering the transformation of the landscape and the reduction of the floral supply in transformed or impoverished landscapes, and the high densities of European bees in some areas of Mexico, it wonders if there would be greater competition between native bees and A. mellifera for plant resources, or if in landscapes with greater floral diversity the competition is less. This point is relevant to the regulations regarding the management of A. mellifera, as well as to the conservation of native fauna and of which there is no evidence.
Conclusions
The evidence presented indicates that although A. mellifera is an extremely important species both culturally and economically for thousands of Mexican families, and that, compared to native bees, it has been much more studied, there is still a need for studies that address both productive aspects from the generation of better databases on production, management, diseases, etc., and ecological aspects, such as its interaction with local fauna. On the other hand, and under the current scenario of global change, including climate change, land use changes and pollution, among other aspects, there is a need to have more ecological studies on A. mellifera in Mexico. These studies will help to predict changes in honey production as well as understand and address threats to these pollinators, contributing to the generation of better management practices. On the other hand, it will help to understand more about its interactions with other bee and plant species, and to be able to have better conservation strategies that include the presence of A. mellifera. Likewise, it will provide valuable information that contributes to the management of other native bees in order to improve agricultural practices by considering the pollination efficiency of different pollinators, including A. mellifera.
Based on this review, it is concluded that studies of pollination ecology that integrate the role of A. mellifera not only in species of economic importance but in other groups of plants are very relevant. Likewise, more studies on the various threats to pollinators in general are needed. Although there are global patterns of the role of changes in the landscape on the loss of pollinators and the service of pollination, which can be extrapolated to the country, the ecological, orographic, and cultural complexity of Mexico demand a better characterization of the current state of pollinators and particularly of A. mellifera, due to its economic and biocultural importance.
Acknowledgements
The authors thank the Integralidad Gamma (i-Gamma) project funded by FORDECyT and CONACyT (project number 296842) for the funds granted for the study of beekeeping in the state of Veracruz from which this manuscript is derived. We also thank Ricardo Quiroz for his comments and suggestions during the literature review process.
REFERENCES
1. Kearns CA, Inouye DW, Waser NM. Endangered mutualisms: The conservation of plant-pollinator interactions. Annu Rev Ecol Syst 1998;29:83-112. [ Links ]
2. Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. Global pollinator declines: Trends, impacts and drivers. Trends Ecol Evol 2010;25(6):345-353. [ Links ]
3. Bradbear N. Bees and their role in forest livelihoods: a guide to the services provided by bees and the sustainable harvesting, processing and marketing of their products. Non-Wood For Prod 2009;19. [ Links ]
4. IPBES. Scenarios and models of biodiversity and ecosystem services. 2016. [ Links ]
5. Garibaldi LA, Requier F, Rollin O, Andersson GK. Towards an integrated species and habitat management of crop pollination. Curr Opin Insect Sci 2017;21(1):105-114. [ Links ]
6. Soto-Muciño LE, Elizarras-Baena R, Soto-Muciño I. Situación apícola en México y perspectiva de la producción de miel en el Estado de Veracruz. Rev Estrateg Desarro Empres 2017;3(7):40-64. [ Links ]
7. González Acereto JA. La importancia de la meliponicultura en México, con énfasis en la Península de Yucatán. Bioagrociencias 2012;5(1):34-42. [ Links ]
8. Vandame R, Palacio MA. Preserved honey bee health in Latin America: A fragile equilibrium due to low-intensity agriculture and beekeeping? Apidologie 2010;41(3):243-255. [ Links ]
9. Arnold N, Zepeda R, Vásquez-Dávila MA, Aldasoro Maya M. Las abejas sin aguijón y su cultivo en Oaxaca, México con catálogo de especies. 2018. [ Links ]
10. Negrín-Muñóz EN, Sotelo-Santos LES. Abejas nativas, señoras de la miel. Patrimonio cultural en el estado de Campeche. RICSH Rev Iberoam Las Cienc Soc Humanísticas 2016;5(9):162-185. [ Links ]
11. Guzmán M, Balboa C, Vandame R. Manejo de las abejas nativas sin aguijón: Melipona beecheii y Scaptotrigona mexicana. Colegio de la Frontera Sur. 2011. [ Links ]
12. Villanueva-Gutiérrez R, Roubik DW, Porter-Bolland L. Bee-plant interactions: competition and phenology of flowers visited by bees. In: Biodiversity and conservation of the Yucatán Peninsula. Springer; 2015:131-152. [ Links ]
13. Pérez de Ayala LR, Martínez- Puc JF, Cetzal-Ix WR. Apicultura: manejo, nutrición, sanidad y flora apícola. Universidad Autónoma de Campeche. 2017. [ Links ]
14. Guzmán-Novoa E, Benítez AC, Espinosa Montaño LG, Novoa GG. Colonización, impacto y control de las abejas melíferas Africanizadas en México. Vet Mex 2011;42(2):149-178. [ Links ]
15. Secretaría de Desarrollo Agropecuario, Rural y Pesca. 2018. Gobierno de Veracruz http://www.veracruz.gob.mx/agropecuario. [ Links ]
16. Servicio de Información Agroalimentaria y Pesquera, SIAP. http://infosiap.siap.gob.mx/gobmx/datosAbiertos. [ Links ]
17. Keenan RJ, Reams GA, Achard F, Freitas JV de, Grainger A, Lindquist E. Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment 2015. For Ecol Manag 2015;352:9-20. [ Links ]
18. Roubik DW. Foraging behavior of competing africanized honeybees and stingless bees. Published by: Ecological Society of America. Ecology 1980;61(4):836-845. [ Links ]
19. Roubik DW, Villanueva-Gutiérrez R. Invasive Africanized honeybee impact on native solitary bees: A pollen resource and trap nest analysis. Biol J Linn Soc 2009;98(1):152-160. [ Links ]
20. Medina-Flores CA, Esquivel-Marín NH, López-Carlos M, Medina-Cuellar SE, Aguilera-Soto JI. Estimación de la pérdida de colonias de abejas melíferas en el altiplano y el norte de México. Ecosistemas Recur Agropecu 2018;5(14):365. [ Links ]
21. Becerra-Guzmán F de J , Contreras-Escareño F. Historia de la apicultura en México. Imág Vet 2004;4(1):10-15. [ Links ]
22. Luna ChG, Roque PJG, Fernández EE, Martínez ME, Díaz ZUA, Fernández LG. Caracterización apícola en la región sierra centro-norte de Veracruz: contexto y trashumancia. Rev Mex Cienc Agríc 2019;10(6):1339-1351. [ Links ]
23. Coordinación General de Ganadería. Situación actual y perspectiva de la apicultura en México. Claridades Agropecu 2010;(199):3-34. [ Links ]
24. Crane E. Bees and beekeeping: Science, practice and world resources. Oxford: Heinemann Newnes; 1990. [ Links ]
25. FAOSTAT, 2020. https://www.fao.org/faostat/es/ [ Links ]
26. Fierro MM. The effects of the first year of Africanization on honeybee populations in Chiapas. Mexico. Am Bee J 1984;127(12):845. [ Links ]
27. Rubio U, Luis J, Novoa G, Greg J, Benítez C, Antonio J. Efecto de la africanización sobre la producción de miel, comportamiento defensivo y tamaño de las abejas melíferas (Apis mellifera L.) en el altiplano mexicano. Vet Mex 2003;34(1):47-59. [ Links ]
28. Magaña Magaña M, Moguel Ordoñez Y, Sanginés JR, Leyva Morales C. Estructura e importancia de la cadena productiva y comercial de la miel en México. Rev Mex Cienc Pecu 2012;3(1). [ Links ]
29. Quezada-Euán JJG, Echazarreta CM, Paxton RJ. The distribution and range expansion of Africanized honey bees (Apis mellifera) in the state of Yucatan, Mexico. J Apic Res 1996;35(3-4):85-95. [ Links ]
30. Programa Nacional para el Control de la Abeja Africana. Diario Oficial de la Federación. 1984. [ Links ]
31. Amparán DC, Ávalos LMR, Rodríguez-Dehaibes SR. Presencia en Veracruz, México del acaro Varroa jacobsoni, causante de la varroasis de la abeja melífera (Apis mellifera L.). Tec Pecu Mex 1992;30(2):123-135. [ Links ]
32. Ratnieks FLW, Carreck NL. Clarity on honeybee collapse? Science 2010;327(5962):152-153. [ Links ]
33. Espinosa-Montaño L, Guzmán-Novoa E. Eficacia de dos acaricidas naturales, ácido fórmico y timol, para el control del ácaro Varroa destructor de las abejas (Apis mellifera L.) en Villa Guerrero, Estado de México, México. Vet Mex 2007;38(1):9-19. [ Links ]
34. Facultad de Medicina Veterinaria y Zootecnia , Departamento de Medicina y Zootecnia de Abejas C y OA. Buenas prácticas pecuarias en la producción primaria de miel. 2018. [ Links ]
35. Vandame R, Ayala R, Guzmán M, Balboa C, Esponda J, Merida J. Diversidad de abejas (Hymenoptera: Apoidea) de la Reserva de la Biosfera “La Sepultura” Chiapas, México. El Col Front Sur. 2012;97. [ Links ]
36. Bayona Celis A, Voldovinos-Flores C, Dorantes Ugalde JA, Sadaña Loza LM. Potenciales de aptitud del territorio y riesgo mayor de reproducción del Pequeño Escarabajo de la Colmena, Aethina tumida Murray (Coleoptera, Nitidulidae) en México. Realidad, Datos y Espacio. Rev Intern Estadíst Geog. 2018(9). [ Links ]
37. Martínez L, Martínez J, Rolando W. Apicultura: manejo nutrición sanidad y flora apícola. 2017. Universidad Autónoma de Campeche, Campeche. [ Links ]
38. Rosengaus-Moshinsky M, Jiménez-Espinosa M, Vázquez-Conde MT. Atlas climatológico de ciclones tropicales en México. 2002. CENAPRED. [ Links ]
39. Comisión Nacional del Agua. https://www.gob.mx/conagua. [ Links ]
40. Magaña MAM, Cortés MET, Salazar LL, Sanginés JR. Productividad de la apicultura en México y su impacto sobre la rentabilidad. Rev Mex Cienc Agríc 2016;7:1103-1115. [ Links ]
41. Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C. Landscape perspectives on agricultural intensification and biodiversity - Ecosystem service management. Ecol Lett 2005;8(8):857-874. [ Links ]
42. Gómez GI. A honey-sealed alliance: Mayan beekeepers in the Yucatan peninsula versus transgenic soybeans in Mexico’s last tropical forest. J Agrar Change 2016;16(4):728-736. [ Links ]
43. Calvillo LM, Ramírez VM, Parra-Tabla V, Navarro J. Bee diversity in a fragmented landscape of the Mexican neotropic. J Insect Conserv 2010;14(4):323-334. [ Links ]
44. Vides-Borrell E, Porter-Bolland L, Ferguson BG, Gasselin P, Vaca R, Valle-Mora J, et al. Polycultures, pastures and monocultures: Effects of land use intensity on wild bee diversity in tropical landscapes of southeastern Mexico. Biol Conserv 2019;236:269-280. [ Links ]
45. González SM. La melisopalinología como una estrategia para la caracterización y regionalización de la miel en Tamaulipas México [tesis licenciatura]. Universidad Autónoma de Tamaulipas: México; 2017. [ Links ]
46. Alfaro Bates RG, Ortiz Díaz JJ, González Acereto JA. Biodiversidad y desarrollo humano en Yucatán.: “Plantas melíferas: melisopalinología.” Biodiver Desarro Hum Yuc. Durán R, Méndez M. editores. 2010;(7):346-358. [ Links ]
47. Nordin A, Sainik NQAV, Chowdhury SR, Saim AB, Idrus RBH. Physicochemical properties of stingless bee honey from around the globe: A comprehensive review. J Food Compos Anal 2018;73:91-102. [ Links ]
48. Cetzal-Ix W. Flora melífera de la península de Yucatán, México: Estrategia para incrementar la producción de miel en los periodos de escasez de alimento de Apis mellifera L. Desde El Herb CICY. 2019;11(5):172-179. [ Links ]
49. Villanueva-Gutiérrez R. Nectar sources of European and Africanized honeybees (Apis mellifera L.) in the Yucatán Peninsula, Mexico. J Apic Res 1994;33(1):44-58. [ Links ]
50. Villanueva GR. Polliniferous plants and foraging strategies of Apis mellifera (Hymenoptera: Apidae) in the Yucatán Peninsula, Mexico. Rev Biol Trop 2002;50(3-4):1035-1044. [ Links ]
51. Porter-Bolland L. La apicultura y el paisaje maya. Estudio sobre la fenología de floración de las especies melíferas. Mex Stud 2003;19(2):303-330. [ Links ]
52. Villégas DG, Bolaños MA, Miranda SJA, García AJ, Galván GOM. Floral nectarífera y polinífera del estado de Tamaulipas. SAGARPA. 2003:109. [ Links ]
53. Roman L, Palma JM. Árboles y arbustos tropicales nativos productores de néctar y polen en el estado de Colima, México. Av Investig Agropecu 2007;11(3):3-24. [ Links ]
54. Piedras Gutiérrez B, Quiróz García DL. Estudio melisopalinológico de dos mieles de la porción sur del Valle de México. Polibotánica 2007;23:57-75. [ Links ]
55. Quiroz García D, Arreguín Sánchez M. Determinación palinológica de los recursos florales utilizados por Apis mellifera L. (Hymenoptera: Apidae) en el estado de Morelos, México. Polibotánica 2008;(26):159-173. [ Links ]
56. Villanueva-Gutiérrez R, Moguel-Ordóñez YB, Echazarreta-González CM, Arana-López G. Monofloral honeys in the Yucatán Peninsula, Mexico. Grana 2009;48(3):214-223. [ Links ]
57. Alfaro BRG, Acereto GJA, Ortiz JJ, Viera CFA, Burgos PAI, Martínez HE, et al. Caracterización palinológica de las mieles de la Península de Yucatán. Universidad Autónoma de Yucatán-Comisión Nacional para el Conocimiento y Uso de la Biodiversidad; Mérida, Yucatán, México. 2010. [ Links ]
58. Ramírez-Arriaga E, Navarro-Calvo LA, Díaz-Carbajal E. Botanical characterisation of Mexican honeys from a subtropical region (Oaxaca) based on pollen analysis. Grana 2011;50(1):40-54. [ Links ]
59. Castellanos-Potenciano BP, Ramírez AE, Zaldivar-Cruz JM. Análisis del contenido polínico de mieles producidas por Apis mellifera L. (Hymenoptera: Apidae) en el estado de Tabasco, México. Acta Zool Mex NS 2012;28(1):13-36. [ Links ]
60. Córdova C, Ramirez E, Hernández E, Zaldívar J. Botanical characterisation of honey (Apis mellifera L.) from four regions of the state of Tabasco, Mexico, by means of melisopalynological techniques. Univ Cienc Tróp Húmedo 2013;29(2):163-178. [ Links ]
61. Librado Carranza G. Plantas nectaríferas y poliníferas en la Costa Chica de Guerrero y municipios aledaños de Oaxaca. Tlamati Sabiduría. 2016;7. [ Links ]
62. Ramírez-Arriaga E, Martínez-Bernal A, Maldonado NR, Martínez-Hernández E. Palynological analysis of honeys and pollen loads of Apis mellifera (Apidae) from the central and northern regions of the state of Guerrero, Mexico. Bot Sci 2016;94(1):141-156. [ Links ]
63. González-Castillo MP. Caracterización palinológica de miel colectada por Apis mellifera Linnaeus (Hymenoptera: Apidae) en tres localidades del municipio de Durango, Dgo., México. Entomol Mex 2017;4:79-83. [ Links ]
64. Alaniz-Gutiérrez LA, Ail-Catzim CE, Villanueva-Gutiérrez R, Delgadillo-Rodríguez J, Ortíz-Acosta ME, García-Moya E, et al. Caracterización palinológica de mieles del Valle de Mexicali, Baja California, México. Polibotánica 2017;0(43):255-283. [ Links ]
65. González SM. La melisopalinología como una estrategia para la caracterización y regionalización de la miel en Tamaulipas México. 2017;(7). [ Links ]
66. Araujo-Mondragón F, Redonda-Martínez R. Flora melífera de la región centro-este del municipio de Pátzcuaro, Michoacán, México. Acta Bot Mex 2019;(126):1-20. [ Links ]
67. Coh-Martínez ME, Cetzal-Ix W, Martínez-Puc JF, Basu SK, Noguera-Savelli E, Cuevas MJ. Perceptions of the local beekeepers on the diversity and flowering phenology of the melliferous flora in the community of Xmabén, Hopelchén, Campeche, Mexico. J Ethnobiol Ethnomedicine 2019;15(1):1-16. [ Links ]
68. Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, et al. Importance of pollinators in changing landscapes for world crops. Proc R Soc B Biol Sci 2007;274(1608):303-313. [ Links ]
69. Garibaldi LA, Garibaldi LA, Steffan-Dewenter I, Winfree R, Aizen MA, Bommarco R, et al. Set of crops regardless of honey bee abundance. Science 2013;339:1608-1612. [ Links ]
70 . SAGARPA. Manual de Polinización Apícola. 2000. [ Links ]
71. Delgado-Carrillo O, Martén-Rodríguez S, Ashworth L, Aguilar R, Lopezaraiza-Mikel M, Quesada M. Temporal variation in pollination services to Cucurbita moschata is determined by bee gender and diversity. Ecosphere 2018;9(11). [ Links ]
72. Ish-am G, Barrientos-Priego F, Castaneda-Vildozola A, Gazit S. Avocado (Persea americana Mill.) pollinators in its region of origin. Rev Chapingo Ser Hortic 1999;5:137-143. [ Links ]
73. Macías-Macías O, Chuc J, Ancona-Xiu P, Cauich O, Quezada-Euán JJG. Contribution of native bees and Africanized honey bees (Hymenoptera: Apoidea) to Solanaceae crop pollination in tropical México. J Appl Entomol 2009;133(6):456-465. [ Links ]
74. Rincón-Rabanales M, Roubik DW, Guzmán MA, Salvador-Figueroa M, Adriano-Anaya L, Ovando I. High yields and bee pollination of hermaphroditic rambutan (Nephelium lappaceum L.) in Chiapas, Mexico. Fruits 2015;70(1):23-27. [ Links ]
75. Romero MJ, Quezada-Euán JJG. Pollinators in biofuel agricultural systems: The diversity and performance of bees (Hymenoptera: Apoidea) on Jatropha curcas in Mexico. Apidologie 2013;44(4):419-429. [ Links ]
76. Vergara CH, Badano EI. Pollinator diversity increases fruit production in Mexican coffee plantations: The importance of rustic management systems. Agric Ecosyst Environ 2009;129(1-3):117-123. [ Links ]
77. Zapata II, Villalobos CMB, Araiza DS, Solís ES, Martínez OA, Wallace R. Efecto de la polinización de la fresa por Apis mellifera L. y Chrysoperla carnea S. sobre la calidad de los frutos. Nova Sci 2014;7:85-100. [ Links ]
78. Meléndez-Ramirez V, Magana-Rueda S, Parra-Tabla V, Ayala R, Navarro G. Diversity of native bee visitors of cucurbit crops (cucurbitaceae) in Yucatán, México. J Insect Conserv 2002;6(3):135-147. [ Links ]
79. Badano EI, Vergara CH. Potential negative effects of exotic honeybees on the diversity of native pollinators and yield of highland coffee plantations. Agric For Entomol 2011;13(4):365-372. [ Links ]
80. Parra-Tabla V, Campos-Navarrete MJ, Arceo-Gómez G. Plant-floral visitor network structure in a smallholder Cucurbitaceae agricultural system in the tropics: implications for the extinction of main floral visitors. Arthropod-Plant Interact 2017;11(5):731-740. [ Links ]
81. Romero MJ, Quezada-Euán JJG. Pollinators in biofuel agricultural systems: The diversity and performance of bees (Hymenoptera: Apoidea) on Jatropha curcas in Mexico. Apidologie 2013;44(4):419-429. [ Links ]
82. Trejo-Salazar RE, Scheinvar E, Eguiarte LE. ¿Quién poliniza realmente los agaves? Diversidad de visitantes florales en 3 especies de Agave (Agavoideae: Asparagaceae). Rev Mex Biodivers 2015;86(2):358-369. [ Links ]
83. Osorio-Beristain M, Domínguez CA, Eguiarte LE, Benrey B. Pollination efficiency of native and invading Africanized bees in the tropical dry forest annual plant, Kallstroemia grandiflora Torr ex Gray. Apidologie 1997;28(1):11-16. [ Links ]
84. Jha S, Vandermeer JH. Contrasting bee foraging in response to resource scale and local habitat management. Oikos 2009;118(8):1174-1180. [ Links ]
85. Alaux C, Allier F, Decourtye A, Odoux JF, Tamic T, Chabirand M, et al. A “Landscape physiology” approach for assessing bee health highlights the benefits of floral landscape enrichment and semi-natural habitats. Sci Rep 2017;(7):1-10. [ Links ]
86. Boscolo D, Tokumoto PM, Ferreira PA, Ribeiro JW, dos Santos JS. Positive responses of flower visiting bees to landscape heterogeneity depend on functional connectivity levels. Perspect Ecol Conserv 2017;15(1):18-24. [ Links ]
87. Carré G, Roche P, Chifflet R, Morison N, Bommarco R, Harrison-Cripps J, et al. Landscape context and habitat type as drivers of bee diversity in European annual crops. Agric Ecosyst Environ 2009;133(1-2):40-47. [ Links ]
88. Campos-Navarrete MJ, Parra-Tabla V, Ramos-Zapata J, Díaz-Castelazo C, Reyes-Novelo E. Structure of plant-Hymenoptera networks in two coastal shrub sites in Mexico. Arthropod-Plant Interact 2013;7(6):607-617. [ Links ]
89. de Santiago-Hernández MH, Martén-Rodríguez S, Lopezaraiza-Mikel M, Oyama K, González-Rodríguez A, Quesada M. The role of pollination effectiveness on the attributes of interaction networks: from floral visitation to plant fitness. Ecology 2019;100(10). [ Links ]
90. Domínguez-Alvarez A, Zenón-Cano S, Ayala-Barajas R. Estructura y fenología de la comunidad de abejas nativas (Hymenoptera: Apoidea). Divers Hábitats Ecol Comunidades 2007;(2):421-432. [ Links ]
91. Parra-Tabla V, Angulo-Pérez D, Albor C, Campos-Navarrete MJ, Tun-Garrido J, Sosenski P, et al. The role of alien species on plant-floral visitor network structure in invaded communities. PLoS ONE 2019;14(11):1-19. [ Links ]
92. Ramírez-Flores VA, Villanueva-Gutiérrez R, Roubik DW, Vergara CH, Lara-Rodríguez N, Dáttilo W, et al. Topological structure of plant-bee networks in four Mexican environments. Sociobiology. 2015;62(1):56-64. [ Links ]
93. Cane JH, Tepedino VJ. Gauging the effect of honeybee pollen collection on native bee communities. Conserv Lett 2017;10(2):205-210. [ Links ]
94. Anna-Aguayo AIS, Schaffner CM, Golubov J, López-Portillo J, García-Franco J, Herrera-Meza G, et al. Behavioral repertoires and interactions between Apis mellifera (Hymenoptera: Apidae) and the native bee Lithurgus littoralis (Hymenoptera: Megachilidae) in flowers of opuntia huajuapensis (Cactaceae) in the Tehuacán desert. Fla Entomol 2017;100(2):396-402. [ Links ]
95. Pinkus-Rendon MA, Parra-Tabla V, Meléndez-Ramírez V. Floral resource use and interactions between Apis mellifera and native bees in cucurbit crops in Yucatán, México. Entomol Soc Can 2005;137:441-449. [ Links ]
96. Reyes-Novelo E, Méléndez-Ramírez V, Ayala R, Delfín-González H. Bee faunas (Hymenoptera: Apoidea) of six natural protected areas in Yucatan, Mexico. Entomol News 2009;120(5):530-544. [ Links ]
Received: March 09, 2021; Accepted: September 03, 2021











 texto en
texto en 



