Articles
Home-range analyses and habitat use by white-tailed deer females during the breeding season
-
Publication dates-
January , 2018
Electronic publication (usually web, but also includes CD-ROM or other electronic only distribution) - Article in PDF
- Article in XML
- Automatic translation
- Send this article by e-mail
- Share this article +
Abstract:
There is little knowledge about the behavior strategies used by white-tailed deer (Odocoileus virginianus texanus) females with fawns. This study analyzed the variation in home range and the use of vegetation types by eight females during the breeding season in a brushland in northeastern Mexico and compared the strategies of females with and without fawns. Eight females were captured and radiotelemetry collars were fitted to them. We sampled two to three 24-hour cycles at 1-hour intervals monthly from April to October 1997 and 1998. In 1997 all females had fawns, whereas in 1998 some of these females had no fawns. This allowed comparing the strategies of females with and without offspring. The home range was significantly larger in females with fawns. In particular, no monthly variations in the home range were observed when females had fawns, while the home range was larger in August in females without fawns. Females preferred two of the seven habitat types (plant associations) studied, the ones dominated by Acacia-Prosopis and by Leucophyllum frutescens. Monthly variations were noted in both home-range size and use of plant associations when females were raising fawns. These data allow a better understanding of the behavioral strategies of females, which may contribute to the development of management strategies.
Key words::
activity patterns, Nuevo León, Odocoileus virginianus, radiotelemetry
Introduction
Females of the white-tailed deer Odocoileus virginianus (Zimmermann 1780) display complex behavioral patterns during pregnancy, birth and breeding, probably aimed at maximizing offspring survival (Kie and White 1985; Wallace 1990; Wallace and Krausman 1990; Schwede et al. 1993). Breeding fawns is likely to modify the type of areas used by females, as well as foraging and anti-predator strategies, and social responses (Gilliam and Fraser 1987; 1989; Holzenbein and Schwede 1989; Schwede et al. 1994; Main et al. 1996; Bowyer et al. 1998; Bongi et al. 2008; DeYoung and Miller 2011). It is generally accepted that parturition induces a temporary reduction in home-range size, habitat use, and social interactions (Nelson and Mech 1981; Ozoga et al. 1982; Holzenbein and Schwede 1989; Nixon et al. 1992; Zultowski 1992; Schwede et al. 1993; Fox and Krausman 1994). The only published study comparing female deer with and without fawns found no differences in home-range size (Bertrand et al. 1996).
-
Kie and
White 1985Population dynamics of white-tailed deer on the Welder Wildlife Refuge, TexasThe Southwestern Naturalist, 1985
-
Wallace 1990Neonatal elk habitat in central ArizonaThe biology of deer, 1990
-
Wallace and Krausman 1990Neonatal elk habitat in central ArizonaThe biology of deer, 1990
-
Schwede et al. 1993Social and spatial organization of female white tailed deer, Odocoileus virginianus, during the fawning seasonAnimal Behavior, 1993
-
Gilliam and Fraser
1987Habitat selection under predation hazard: test of a model with foraging minnowsEcology, 1987
-
1989Strong effects of foraging minnows on a stream benthic invertebrate communityEcology, 1989
-
Holzenbein and Schwede 1989Activity and movements of female white-tailed deer during the rutJournal of Wildlife Management, 1989
-
Schwede et al. 1994Early mother-young relations in white-tailed deerJournal of Mammalogy, 1994
-
Main et al.
1996Sexual segregation in ungulates: new directions for researchesJournal of Mammalogy, 1996
-
Bowyer et al. 1998Habitat selection by neonatal black-tailed deer: climate, forage, or risk of predation?Journal of Mammalogy, 1998
-
Bongi et al. 2008Anti-predator behavior, space use and habitat selection in female roe deer during the fawning season in a wolf areaJournal of Zoology, 2008
-
DeYoung and Miller 2011White-tailed deer behaviorBiology and Management of White-tailed Deer, 2011
-
Nelson and Mech 1981Deer social organization and wolf predation in northeastern MinnesotaWildlife Monography, 1981
-
Ozoga et al. 1982Parturition behavior and territoriality in white-tailed deer: impacts on neonatal mortalityJournal of Wildlife Management, 1982
-
Holzenbein and Schwede 1989Activity and movements of female white-tailed deer during the rutJournal of Wildlife Management, 1989
-
Nixon et al. 1992Stability of white-tailed doe parturition ranges on a refuge in east central IllinoisCanadian Journal of Zoology, 1992
-
Zultowski
1992Behavioral and spatial ecology of female white tailed deer in the everglades ecosystem, 1992
-
Schwede et al. 1993Social and spatial organization of female white tailed deer, Odocoileus virginianus, during the fawning seasonAnimal Behavior, 1993
-
Fox and Krausman 1994Fawning habitat of desert mule deerThe Southwestern Naturalist, 1994
-
Bertrand et al. 1996Effects of parturition on home ranges and social affiliations of female white-tailed deerJournal of Wildlife Management, 1996
In northeastern Mexico, a number of studies have compared the behavior of males and females of the subspecies O. v. texanus (Gallina et al. 2003), by analyzing the use of habitat between years, sexes and reproductive periods (Bello et al. 2001a, 2003), home range (Bello et al. 2001b), movements in relation to precipitation (Bello et al. 2004), distances traveled (Bello et al. 2006), energy expenditure (Gallina and Bello 2010), and activity (Gallina and Bello 2014). The current knowledge about pregnancy and fawning is limited. It is considered that home-range size and habitat use directly influence the deer population dynamics (Gallina 1981; Gallina et al. 1998; Green et al. 2017), reproductive patterns (gestation, number of offspring, time of parturition, fawn survival, size of mothers and fawns), and the behavior of mothers (Green et al. 2017). The objective of this study was to determine the behavioral strategies of females of white-tailed deer with and without fawns under conditions of semi-captivity, estimating home-range size and use of plant associations in a brushland.
-
Gallina et al. 2003El venado cola blanca: comportamiento en zonas semiáridas del Noreste de MéxicoManejo de Fauna silvestre en Amazonía y Latinoamérica-Criterios de Sostenibilidad, 2003
-
Bello et al.
2001aCharacterization and habitat preferences by white-tailed deer in MexicoJournal of Range Management, 2001
-
2003El venado cola blanca: uso del hábitat en zonas semiáridas y con alta disponibilidad de agua del Noreste de MéxicoManejo de Fauna Silvestre en Amazonía y Latinoamérica-Criterios de Sostenibilidad, 2003
-
Bello et al. 2001bHome range, core area and distance to water sources by white tailed deer in northeastern MexicoVida Silvestre Neotropical, 2001
-
Bello et al. 2004Movements of white tailed deer and their relationship with precipitation in the northeastern of MexicoInterciencia, 2004
-
Bello et al. 2006Distancias de desplazamiento del venado cola blanca y su relación con factores ambientales en el Noreste de MéxicoMemorias VI Congreso en Manejo de Fauna silvestre en Amazonia y Latinoamérica, 2006
-
Gallina and Bello 2010El gasto energético del venado cola blanca (Odocoileus virginianus texanus) en relación a la precipitación en una zona semiárida de MéxicoTherya, 2010
-
Gallina and Bello 2014Patrones de actividad del venado cola blanca en el noreste de MéxicoTherya, 2014
-
Gallina 1981Biology and population dynamics of White-tailed deer in northwestern MexicoDeer Biology, Habitat Requirements and Management in Western North America, 1981
-
Gallina et al. 1998Patrones de actividad del venado de cola blanca (Odocoileus virginianus texanus) en un matorra1 xerófilo de MéxicoBoletín de la Sociedad de Biología, 1998
-
Green et
al. 2017Reproductive characteristics of female while-tailed deer (Odocoileus virginianus) in the Midwestern USATheriogenology, 2017
-
Green et al. 2017Reproductive characteristics of female while-tailed deer (Odocoileus virginianus) in the Midwestern USATheriogenology, 2017
Materials and Methods
Study Area. This work was carried out at Rancho San Francisco, located between the municipalities of Lampazos and Progreso in the States of Nuevo León and Coahuila (27° 22’ N, -100° 40’ W; 27° 22’ N, -100° 36’ W; 27° 20’ N, -100° 40’ W; 27° 20’ N, -100° 36’ W). The study area stretches across 1,500 ha, including a 1,000 ha enclosed in a deer fence, with 33 artificial 1500-L water troughs in addition to three dams whose water level depends entirely on rainfall (Bello et al. 2001a). Maximum temperature can reach 40 °C, and the maximum mean temperature in July reaches only 29 °C (Briones 1984). The climate is warm and dry, with mean annual precipitation below 400 mm; the rainy season spans from May to September, showing interannual variations (Bello et al. 2001a; Bello et al. 2004). The estimated deer density is 8 to 10 individuals per km2, and between 80 and 100 deers are estimated to thrive in the area (Gallina and Bello 2010; Bello et al. 2001b).
-
Bello et al. 2001aCharacterization and habitat preferences by white-tailed deer in MexicoJournal of Range Management, 2001
-
Briones 1984Sinecología and florística de Lampazos de Naranjo, Nuevo León (México) con énfasis en la Gran Llanura, 1984
-
Bello et al. 2001aCharacterization and habitat preferences by white-tailed deer in MexicoJournal of Range Management, 2001
-
Bello et al. 2004Movements of white tailed deer and their relationship with precipitation in the northeastern of MexicoInterciencia, 2004
-
Gallina and Bello
2010El gasto energético del venado cola blanca (Odocoileus virginianus texanus) en relación a la precipitación en una zona semiárida de MéxicoTherya, 2010
-
Bello et al. 2001bHome range, core area and distance to water sources by white tailed deer in northeastern MexicoVida Silvestre Neotropical, 2001
The local vegetation is a xeric scrubland, including seven plant associations with variable cover: 1% medium-height thorny mezquite-acacia brushland, 3% toboso grassland (Hilaria mutica = Pleuraphis mutica), 6% thornless hojasen shrubland (Flourensia cernua), 10% chaparro prieto (Acacia-Celtis), 11% cenizo shrubland (Leucophyllum frutescen), 15% high thorny mezquite-acacia shrubland (Prosopis), and 54% medium sub-thorny mezquite-acacia-toboso-hojasen shrubland (Acacia-Prosopis; Bello et al. 2001b). Factors considered were availability of each association, species richness, cover protection and percent of food species in each association.
-
Bello et al. 2001Home range, core area and distance to water sources by white tailed deer in northeastern MexicoVida Silvestre Neotropical, 2001
Although the predation of adult females and fawns was not documented in this study, the presence of puma (Puma concolor), bobcat (Lynx rufus), feral dog (Canis lupus familiaris) and coyote (Canis latrans) was recorded in the study area, all being potential predators (Cook et al. 1971; Ozoga and Verme 1982; Messier and Barrette 1985). Breeding coyote couples predate on deer fawns, mostly during July and August (Lopez-Soto and Badii 2000).
-
Cook et al. 1971Mortality of young white-tailed deer fawns in South TexasJournal of Wildlife Management, 1971
-
Ozoga and Verme 1982Parturition behavior and territoriality in white-tailed deer: impacts on neonatal mortalityJournal of Wildlife Management, 1982
-
Messier and Barrette 1985The efficiency of yarding behavior by white-tailed deer as an antipredator strategyCanadian Journal of Zoology, 1985
-
Lopez-Soto and Badii 2000Depredación en crías de venado cola blanca (Odocoileus virginianus texanus) por coyote (Canis latrans) en una Unidad de Manejo y Aprovechamiento del norte de Nuevo León, MéxicoActa Zoológica Mexicana, 2000
Capture and Radio Tracking. Females were captured using a 15 x 15 m drop net with a 20 cm mesh modified for capturing deer, with a manual trigger that is activated from a distance of approximately 30 m. Corn grains were used as bait. When a deer was captured, eyes were covered (to keep it calm); then it was untangled from the net and the legs were tied to facilitate handling. This procedure is commonly used in the UMAS located in the States of Nuevo León, Coahuila and Tamaulipas. Each deer was weighed with a Pesola 100 kg dynamometer and was fitted with a 180 g radio transmitter collar with activity sensor (Model 400, Telonics, Inc. Meza, Arizona). No anesthetic were administered to avoid complications in deer handling (Bello et al. 2001a).
-
Bello et al. 2001aCharacterization and habitat preferences by white-tailed deer in MexicoJournal of Range Management, 2001
Eight females were captured, six in 1997 and two in 1998 (Table 1). Three were captured within the 1,000 ha confinement area, and five outside of this area. The six females monitored in 1997 were raising fawns. The six females monitored in 1998 had no fawns.
Table 1
Year of capture and number of monthly locations of females of Texan white-tailed deer in Rancho San Francisco, Nuevo León.
Year of capture and number of monthly locations of females of Texan white-tailed deer in Rancho San Francisco, Nuevo León.
| Female | Year |
Number of locations |
Number of locations |
||||||||
| Apr | Jun | Aug | Oct | Total | May | Jun | Aug | Oct | Total | ||
| 1 | 1997 | 14 | 29 | 45 | 31 | 119 | 46 | 30 | 41 | 28 | 145 |
| 2 | 1997 | 10 | 20 | 12 | 33 | 75 | 36 | 21 | 33 | 25 | 126 |
| 3 | 1997 | 16 | 35 | 38 | 30 | 119 | 47 | 41 | 43 | 30 | 161 |
| 4 | 1997 | - | 30 | 52 | 30 | 112 | 30 | - | 25 | 25 | 80 |
| 5 | 1997 | 42 | 19 | 20 | 19 | 100 | - | - | - | - | - |
| 6 | 1997 | - | - | 56 | - | 56 | - | - | - | - | - |
| 7 | 1998 | - | - | - | - | - | 33 | 15 | 14 | 34 | 96 |
| 8 | 1998 | - | - | - | - | - | 18 | - | 17 | 13 | 48 |
| Total | 82 | 133 | 223 | 143 | 581 | 240 | 107 | 209 | 180 | 736 | |
All the females studied were located using radio telemetry (White and Garrot, 1990). Female locations were recorded in May, July, August and October 1997, and in April, July, August and October 1998, corresponding to the breeding season (Table 1). Data were obtained at 1-h intervals, performing two to three 24 hour cycles each month. Each deer specimen was radio-located by triangulation using TR2 and TR4 receptors (Telonics Inc., Meza, Arizona;) and H- and Yaguis-type portable antennas (Telonics Inc., Meza, Arizona) from two or three fixed stations (Samuel and Fuller 1994) fitted with a Garmin georeferencing instrument, performing simultaneous readings. We used a Suunto KB-14 (Finland) compass to obtain the directions for each triangulation.
-
White and Garrot, 1990Analysis of wildlife radio-tracking data, 1990
-
Samuel and Fuller 1994Wildlife Radiotelemetry in Research and Management Techniques for Wildlife and Habitats, 1994
Estimated Home Range. To obtain the location of each female deer, degrees were transformed to UTM coordiates for each triangulation using the software Tripoly 2 (Laundré 1990), considering a magnetic deviation of 9.15° and an error of 0.7 ha. The home-range size of each individual deer female was estimated with the CALHOME Program (California Home Range; Kie et al. 1994) using a 95% minimum convex polygon model (White and Garrot, 1990). A total of 1,317 locations were recorded (581 in 1997 and 736 in 1998). The number of locations used to calculate the home range was similar for each individual female in the two years (Table 1).
-
Laundré 1990Tripoly, 1990
-
Kie et al. 1994CALHOME home range analysis program electronic user´s, 1994
-
White and Garrot, 1990Analysis of wildlife radio-tracking data, 1990
To perform the analysis, the breeding season was divided into four stages regardless of the female reproductive status: 1) pre-breeding stage (April to June), corresponding to the late gestation period. 2) First breeding stage (July), when fawns are completely dependent on the mother. 3) Second breeding stage (August), when fawns start becoming independent. 4) Third breeding stage (October), corresponding to the total weaning of fawns. The results were analyzed at two levels: a) variations in each breeding stage considering the home range of the eight females captured, and b) variations in each stage at the individual level, considering only three of the four females monitored in 1997 (with fawns) and 1998 (without fawns). The fourth female was excluded from the analysis because no records of its activity were noted during April (1997) and June (1998).
The data were not normally distributed, nor the variances were homogeneous, so that non-parametric analyzes were used (Zar 1996). The variations in home-range size was explored for each stage at the sample level (n = 8) between females with and without fawns using Kruskal-Wallis and Mann-Whitney tests (Zar 1996). The monthly home-range size at the individual level for the three females monitored during the two years was analyzed using Friedman tests. The home-range size of females with and without fawns was compared using Wilcoxon tests (Zar 1996).
-
Zar 1996Biostatistical Analysis, 1996
-
Zar
1996Biostatistical Analysis, 1996
-
Zar 1996Biostatistical Analysis, 1996
Use of and Preference for Plant Associations. Only the three females that were observed with and without fawns were considered. An analysis was made throughout the entire breeding season and another for each breeding stage, considering the availability of each plant association in both Rancho San Francisco and the home range of each female. We used the ArcView geographic information system software to obtain a digitized map of the home range of each female, derived from the locations recorded through radio telemetry for each plant association. The habitat use and preference were derived for each female from the number of locations in each plant association. The use of associations according to availability was assessed with X2 tests (Zar 1996) and Bonferroni intervals (Byers et al. 1984).
-
Zar 1996Biostatistical Analysis, 1996
-
Byers et al. 1984Clarification of the technique for analysis of utilization-availability dataJournal of Wildlife Management, 1984
Results
Home Range. Home-range size varied between females with and without fawn across the stages analyzed (H = 16.84, P = 0.01). In females with fawns, it was largest in the pre-breeding and second-breeding stages (U = 2, P = 0.05; U = 1.0, P = 0.01, respectively). The home-range size between stages was similar in females with fawns (H = 4, P = 0.20). Females without fawns (H = 9, P = 0.02) showed a smaller home range from the pre-breeding stage to the first and second breeding stages (Figure 1).
Thumbnail
Figure 1
Home range of females with and without fawns of Texan white-tailed deer during the breeding season.
Home range of females with and without fawns of Texan white-tailed deer during the breeding season.
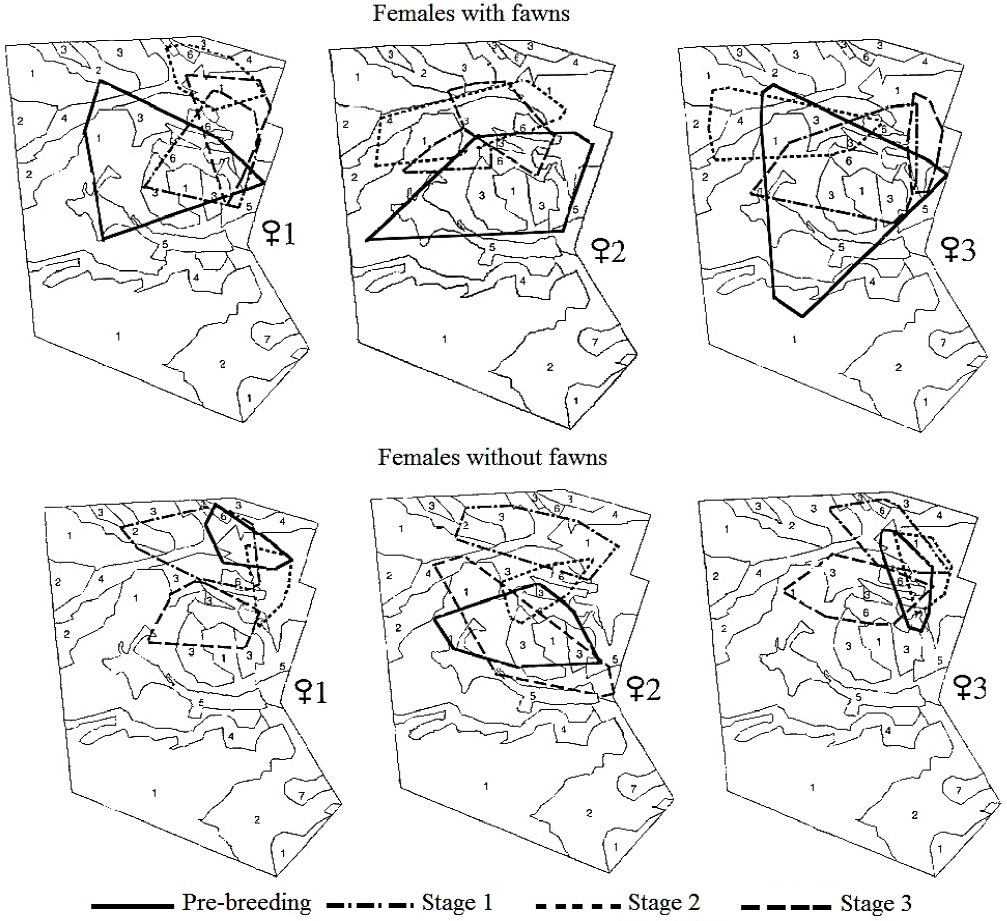
At the individual level, the three females monitored in 1997 versus 1998 showed a similar home-range size across stages. However, there was a trend to display larger home-range sizes when raising fawns and in the pre-breeding stage, that did not reach statistical significance (n = 3; Xr2 = 12, P > 0.05). A similar size across stages was observed in both females with fawns (Xr2 = 5, P > 0.05) and females without fawns (Xr2 = 7, P > 0.05). These females relocated their home range in each stage, showing a different overlap between each breeding stage (Figure 2).
Thumbnail
Figure 2
Location of the home range of females of Texan white-tailed deer with and without fawns during the breeding season. Plant associations: Acacia-Prosopis (1); Prosopis (2); Leucophyllum (3); Acacia-Celtis (4); Flourensia (5); Hilaria (6); Opuntia (7).
Location of the home range of females of Texan white-tailed deer with and without fawns during the breeding season. Plant associations: Acacia-Prosopis (1); Prosopis (2); Leucophyllum (3); Acacia-Celtis (4); Flourensia (5); Hilaria (6); Opuntia (7).
Use of and Preference for Plant Associations. Irrespective of their reproductive status, females included six of the seven plant associations in Rancho San Francisco within their areas of activity: Acacia-Prosopis, Leucophyllum frutescens, Prosopis, Acacia-Celtis, Flourensia cernua and Hilaria mutica (Figure 2). The number of observations corresponding to each plant association differed for each of the females, within an interval that ranged from 0 locations in Opuntia up to 91 locations in Acacia-Prosopis (Figure 3).
Thumbnail
Figure 3
Use of and preference for plant associations by females in each breeding stage: w/f with fawns and w/of without fawns. Symbols: greater-than expected use (+); lower-than-expected use (-); expected use (unsigned); association not included in the home range (Ni).
Use of and preference for plant associations by females in each breeding stage: w/f with fawns and w/of without fawns. Symbols: greater-than expected use (+); lower-than-expected use (-); expected use (unsigned); association not included in the home range (Ni).
During each breeding stage, females displayed a differential use of the plant associations. females with fawns (female 1: X2 = 16, d. f. = 8, P = 0.001; female 2: X2 = 33, d. f. = 8, P = 0.001; female 3: X2 = 41, d. f. = 10, P = 0.001) and without fawns (female 1: X2 = 30, d. f. = 10, P = 0.001; female 2: X2 = 37, d. f. = 10, P = 0.001; female 3: X2 = 37, d. f. = 10, P = 0.001). The proportion of home-range use differed between stages and females showed a preference for Acacia-Prosopis only (Figure 3). No difference was found in the behavior of females with and without fawns in the three breeding stages (Females 1, 2 and 3; P < 0.03).
Discussion
Females with fawns showed larger home ranges in the pre-breeding and the second breeding stages, which is consistent with the report by Bertrand and collaborators (1996). This may be due to the increased energy metabolism in the last gestation stage (30 to 45%) rather than in lactation (Gallina and Bello 2010). In Rancho San Francisco during the breeding season, females increase their energy expenditure to 100 Kcal/ind/day (Gallina and Bello 2010), as well as the time dedicated to food search and feeding to consume herbaceous and shrub sprouts (Gallina et al. 1998). By contrast, the lack of fawns may foster optimal resource use within smaller areas, as females restrict their activity sites (Shipley and Spalinger 1995). Females with and without fawns relocated their home range in each breeding stage, which is common in deer, according to resource distribution and abundance, as well as social factors (Mackie 1970; Riley and Dood 1984; Tierson et al. 1985).
-
Bertrand and collaborators (1996)Effects of parturition on home ranges and social affiliations of female white-tailed deerJournal of Wildlife Management, 1996
-
Gallina and Bello 2010El gasto energético del venado cola blanca (Odocoileus virginianus texanus) en relación a la precipitación en una zona semiárida de MéxicoTherya, 2010
-
Gallina and Bello 2010El gasto energético del venado cola blanca (Odocoileus virginianus texanus) en relación a la precipitación en una zona semiárida de MéxicoTherya, 2010
-
Gallina et al. 1998Patrones de actividad del venado de cola blanca (Odocoileus virginianus texanus) en un matorra1 xerófilo de MéxicoBoletín de la Sociedad de Biología, 1998
-
Shipley and Spalinger 1995Influence of size and density of browse patches on intake rates and foraging decisions of young moose and white-tailed deerOecología, 1995
-
Mackie 1970Range ecology and relations of mule deer, elk and cattle in the Missouri River Breaks, MontanaWildlife Monographs, 1970
-
Riley and Dood 1984Summer movements, home range, habitat use, and behavior of mule deer fawnsThe Journal of Wildlife Management, 1984
-
Tierson et al. 1985Seasonal movements and home ranges of white-tailed deer in the AdirondacksJournal of Wildlife Management, 1985
Females with fawns do not show a significant change in home-range size between pre-breeding and the three breeding stages, as reported in other studies (Zultowsky 1992; Beltrand et al. 1996). As reproduction was successful, all females seemingly obtained the resources needed simply by relocating their home ranges. The area used is likely the minimum area that allows them to obtain the resources needed to meet their energy demands and those of fawns in each breeding stage.
-
Zultowsky 1992Behavioral and spatial ecology of female white tailed deer in the everglades ecosystem, 1992
-
Beltrand et al. 1996Effects of parturition on home ranges and social affiliations of female white-tailed deerJournal of Wildlife Management, 1996
The most important plant associations for females during the breeding season are Acacia-Prosopis and Leucophyllum frutescens. These associations show an intermediate cover (39.5% and 34% respectively), and a near-to-highest species richness (10 sp), surpassed only by Flourensia cernua (11 sp). Acacia-Prosopis showed an intermediate proportion of species consumed by deer (64.7%), while Leucophyllum frutescens had the lowest percentage of such species ( 28.4%; Bello et al. 2003). Therefore, habitat selection by females is seemingly determined by an intermediate cover value coupled with a high diversity of plant species.
-
Bello et al. 2003El venado cola blanca: uso del hábitat en zonas semiáridas y con alta disponibilidad de agua del Noreste de MéxicoManejo de Fauna Silvestre en Amazonía y Latinoamérica-Criterios de Sostenibilidad, 2003
Females use areas of intermediate plant cover during the breeding season, maybe as an anti-predator strategy. This cover allows fawns to hide in the vegetation while still facilitating a quick escape (Nelson and Mech 1981; Kie and White 1985; Schwede et al. 1994; Bowyer et al. 1998; Mandujano et al. 2004; DeYoung and Miller 2011); in addition, fawns obtain protection from high sun radiation and rain-fall (Messier and Barrett 1985; Illius and Gordon 1987; Fox and Krausman 1994; Bowyer et al. 1998).
-
Nelson and Mech 1981Deer social organization and wolf predation in northeastern MinnesotaWildlife Monography, 1981
-
Kie and White 1985Population dynamics of white-tailed deer on the Welder Wildlife Refuge, TexasThe Southwestern Naturalist, 1985
-
Schwede et al. 1994Early mother-young relations in white-tailed deerJournal of Mammalogy, 1994
-
Bowyer et al. 1998Habitat selection by neonatal black-tailed deer: climate, forage, or risk of predation?Journal of Mammalogy, 1998
-
Mandujano et al. 2004Variación estacional del uso y preferencia de los tipos vegetacionales por el venado cola blanca en un bosque tropical de JaliscoActa Zoológica Mexicana, 2004
-
DeYoung and Miller 2011White-tailed deer behaviorBiology and Management of White-tailed Deer, 2011
-
Messier and Barrett 1985The efficiency of yarding behavior by white-tailed deer as an antipredator strategyCanadian Journal of Zoology, 1985
-
Illius and Gordon 1987The Allometry of food intake in grazing ruminantsJournal of Animal Ecology, 1987
-
Fox and Krausman 1994Fawning habitat of desert mule deerThe Southwestern Naturalist, 1994
-
Bowyer et al. 1998Habitat selection by neonatal black-tailed deer: climate, forage, or risk of predation?Journal of Mammalogy, 1998
Nursing females apparently carried out an optimal foraging strategy, as observed in other sites (Marchinton and Hirth 1984; Hofmann 1989). They choose plant associations with high species diversity (Arceo et al. 2004), seeking food quality rather than abundance (Weckerly and Kennedy 1992; Weckerly 1994; Main et al. 1996; Kammermeyer and Larry 1997; Gallina et al. 1998), and inhabit areas with low food biomass if these increase the safety of their offspring against predation (Miquelle et al. 1992).
-
Marchinton and Hirth 1984BehaviorWhite-tailed deer ecology and management, 1984
-
Hofmann 1989Evolutionary steps of ecophysiological adaptation and diversification of rumiants: a comparative view of their digestive systemOecología, 1989
-
Arceo et al. 2004Diversity of diet of white-tailed deer in a Mexican tropical forestMammalia, 2005
-
Weckerly and Kennedy 1992Examining hypotheses about feeding strategies of white-tailed deerCanadian Journal of Zoology, 1992
-
Weckerly 1994Selective feeding by black-tailed deer: forage quality or abundance?Journal of Mammalogy, 1994
-
Main et al. 1996Sexual segregation in ungulates: new directions for researchesJournal of Mammalogy, 1996
-
Kammermeyer and Larry 1997Seasonal change in circadian activity of radio-monitored deerJournal of Wildlife Management, 1997
-
Gallina et al. 1998Patrones de actividad del venado de cola blanca (Odocoileus virginianus texanus) en un matorra1 xerófilo de MéxicoBoletín de la Sociedad de Biología, 1998
-
Miquelle et al. 1992Sexual segregation in Alaskan mooseWildlife Monographs, 1992
Acknowledgments
Logistical support was provided by DUMAC. The Consejo Nacional de Ciencia y Tecnología (CONACYT) provided financial support under Project No. 225260-5-2480BP. The U.S. Fish and Wildlife Service awarded a Master’s degree scholarship to the first author. The Master’s Degree in Wildlife Management was part of the Latin American Partnership of Training in Wildlife Management. The support provided by the U.S. Fish and Wildlife Service supplements of Conservation for the Protection of Nature. María Elena Sánchez-Salazar translated the manuscript into English.
Literature cited
- Arceo, G. S., S., Mandujano, and L. A., Perez-Jimenez. 2005. Diversity of diet of white-tailed deer in a Mexican tropical forest. Mammalia 69:159-168. Links
- Bahnak, B. R., J. C., Holland, L. J., Verme, and J., Ozoga. 1979. Seasonal and nutritional effects on serum nitrogen constituents in white-tailed deer. Journal of Wildlife Management 43:454-467. Links
- Bello, J., S., Gallina, and M., Equihua. 2001a. Characterization and habitat preferences by white-tailed deer in Mexico. Journal of Range Management 54:537-545. Links
- Bello, J., S., Gallina, M., Equihua, S., Mandujano, and C., Delfín- Alfonso. 2001b. Home range, core area and distance to water sources by white tailed deer in northeastern Mexico. Vida Silvestre Neotropical 10:30-37. Links
- Bello, J., S., Gallina, and M., Equihua. 2003. El venado cola blanca: uso del hábitat en zonas semiáridas y con alta disponibilidad de agua del Noreste de México. Pp. 67-76 in Manejo de Fauna Silvestre en Amazonía y Latinoamérica-Criterios de Sostenibilidad (Polanco-Ochoa, R., ed.). CITES, Fundación Natura. Bogotá, Colombia. Links
- Bello, J., S., Gallina, and M., Equihua. 2004. Movements of white tailed deer and their relationship with precipitation in the northeastern of Mexico. Interciencia 29:357-361. Links
- Bello, J., S., Gallina, and M., Equihua. 2006. Distancias de desplazamiento del venado cola blanca y su relación con factores ambientales en el Noreste de México. Memorias VI Congreso en Manejo de Fauna silvestre en Amazonia y Latinoamérica:137-142 Links
- Beltrand, M., A. J., Denicola, S. R., Beissinger, and R. K., Swihart. 1996. Effects of parturition on home ranges and social affiliations of female white-tailed deer. Journal of Wildlife Management 60:899-909. Links
- Bongi, P., S., Ciutis, S., Grignolio, M., Del Frate, S., Simi, And D., Gandelli, and M., Apollonio. 2008. Anti-predator behavior, space use and habitat selection in female roe deer during the fawning season in a wolf area. Journal of Zoology 276:242-251. Links
- Bowyer, R. T., J. G., Kie, and V., Ballenberghe. 1998. Habitat selection by neonatal black-tailed deer: climate, forage, or risk of predation? Journal of Mammalogy 79:415-425. Links
- Briones, V. O.. 1984. Sinecología and florística de Lampazos de Naranjo, Nuevo León (México) con énfasis en la Gran Llanura. Tesis de Licenciatura. Universidad Autónoma de Nuevo León, México. Links
- Byers, C. R, R. K., Steinhorst, and P. R., Krausman. 1984. Clarification of the technique for analysis of utilization-availability data. Journal of Wildlife Management 48:1050-1053. Links
- Cook, R. S., M., White, D. O., Trainer, and W. C., Glazner. 1971. Mortality of young white-tailed deer fawns in South Texas. Journal of Wildlife Management 35:47-56. Links
- DeYoung, R. W., and K. V., Miller. 2011. White-tailed deer behavior. D. G. Hewitt. Biology and Management of White-tailed Deer. CRC Press. Boca Raton. Florida, U. S. A. Links
- Fox, K. B., and P. R., Krausman. 1994. Fawning habitat of desert mule deer. The Southwestern Naturalist 39:269-275. Links
- Gallina, S.. 1981. Biology and population dynamics of White-tailed deer in northwestern Mexico. Pp. 79-108 in Deer Biology, Habitat Requirements and Management in Western North America (Ffolliott, P. F., and S. Gallina, eds.). Instituto de Ecología. Ciudad de México, México. Links
- Gallina, S., A., Pérez-Arteaga, and S., Mandujano. 1998. Patrones de actividad del venado de cola blanca (Odocoileus virginianus texanus) en un matorra1 xerófilo de México. Boletín de la Sociedad de Biología, Concepción, Chile 69:221-228. Links
- Gallina, S., P., Corona, and J., Bello. 2003. El venado cola blanca: comportamiento en zonas semiáridas del Noreste de México. Pp. 165-173 in Manejo de Fauna silvestre en Amazonía y Latinoamérica-Criterios de Sostenibilidad (Polanco-Ochoa, R., ed.). CITES, Fundación Natura. Bogotá, Colombia. Links
- Gallina, S., and J., Bello. 2010. El gasto energético del venado cola blanca (Odocoileus virginianus texanus) en relación a la precipitación en una zona semiárida de México. Therya 1:9-22. Links
- Gallina, S., y J., Bello. 2014. Patrones de actividad del venado cola blanca en el noreste de México. Therya 5:423-436. Links
- Gilliam, F. H., and D. F., Fraser. 1987. Habitat selection under predation hazard: test of a model with foraging minnows. Ecology 68:1856-1862. Links
- Gilliam, F. H., and D. F., Frazer. 1989. Strong effects of foraging minnows on a stream benthic invertebrate community. Ecology 70:445-452 Links
- Gittlemann, J. L., and P. H., Harvey. 1982. Carnivore home-range size, metabolic needs and ecology. Behavioral Ecology and Sociobiology 10:57.63. Links
- Green, L. M., A. C., Kelly, D., Satterthwait-Phillips, M. B., Manjerovic, P., Shelton, J., Novakofski, and N., Matheus-Pinilla. 2017. Reproductive characteristics of female while-tailed deer (Odocoileus virginianus) in the Midwestern USA. Theriogenology 94:71-78. Links
- Hall, R.. 1981. The mammals of North America. Vol. I. John Wiley & Sons. New York, U. S. A. Links
- Holzenbein, S., and G., Schwede. 1989. Activity and movements of female white-tailed deer during the rut. Journal of Wildlife Management 53:219-223. Links
- Hofmann, R. R.. 1989. Evolutionary steps of ecophysiological adaptation and diversification of rumiants: a comparative view of their digestive system. Oecología 78:443-457. Links
- Illius, A. W., and I. J., Gordon. 1987. The Allometry of food intake in grazing ruminants. Journal of Animal Ecology 56:989-999. Links
- Kie, J. G., and M., White. 1985. Population dynamics of white-tailed deer on the Welder Wildlife Refuge, Texas. The Southwest Naturalist 30:105-118. Links
- Kie, J. G., J. A., Baldwin, and C. J., Evans. 1994. CALHOME home range analysis program electronic user´s. U. S. Forest Service, Pacific Southwest Research Station. Albany, U. S. A. Links
- Kammermeyer, K. E., and R., Larry. 1997. Seasonal change in circadian activity of radio-monitored deer. Journal of Wildlife Management 41:315-317. Links
- Laundré, J. W.. 1990. Tripoly. Intermountain Wildlife Research Institute. University of Idaho. Idaho, U. S. A. Links
- López-Soto, J. H., and M. H., Badii. 2000. Depredación en crías de venado cola blanca (Odocoileus virginianus texanus) por coyote (Canis latrans) en una Unidad de Manejo y Aprovechamiento del norte de Nuevo León, México. Acta Zoológica Mexicana (n. s.) 81:135-138. Links
- Mandujano, S., S., Gallina, G., Arceo, and L. A., Pérez-Jiménez. 2004. Variación estacional del uso y preferencia de los tipos vegetacionales por el venado cola blanca en un bosque tropical de Jalisco. Acta Zoológica Mexicana (n. s.) 20:45-67. Links
- Mackie, R. J.. 1970. Range ecology and relations of mule deer, elk and cattle in the Missouri River Breaks, Montana. Wildlife Monographs 20:1-79. Links
- Main, M. B., and B. E., Coblentz. 1990. Sexual segregation among ungulates: a critique. Wildlife Society Bulletin 18:204-210. Links
- Main, M. B., F. W., Weckerly, and V. C., Bleich. 1996. Sexual segregation in ungulates: new directions for researches. Journal of Mammalogy 77:449-461. Links
- Marchinton, R. L., and D. H., Hirth. 1984. Behavior. Pp. 129-168 in White-tailed deer ecology and management (Halls, L. K. ed.). Stackpole Books. Pennsylvania, U. S. A. Links
- Messier, F., and C., Barrette. 1985. The efficiency of yarding behavior by white-tailed deer as an antipredator strategy. Canadian Journal of Zoology 63:785-789. Links
- Miquelle, D. G., J. M., Peek, and V., Van Ballenberghe. 1992. Sexual segregation in Alaskan moose. Wildlife Monographs 122:1-57. Links
- Nelson, M. E., and L. D., Mech. 1981. Deer social organization and wolf predation in northeastern Minnesota. Wildlife Monography 77:1-53. Links
- Nixon, C. M., L. P., Hansen, P. A., Brewer, and E., Chelsvig. 1992. Stability of white-tailed doe parturition ranges on a refuge in east central Illinois. Canadian Journal of Zoology 70:968-973. Links
- Ozoga, J. J., L. J., Venne, and C. S., Bienz. 1982. Parturition behavior and territoriality in white-tailed deer: impacts on neonatal mortality. Journal of Wildlife Management 46:1-11. Links
- Kie, J. G., and M., White. 1985. Population dynamics of white-tailed deer on the Welder Wildlife Refuge, Texas. The Southwestern Naturalist 30:105-118. Links
- Riley, S. D., and A. R., Dood. 1984. Summer movements, home range, habitat use, and behavior of mule deer fawns. The Journal of Wildlife Management 48:1302-1310. Links
- Samuel, D. M., and M. R., Fuller. 1994. Wildlife Radiotelemetry in Research and Management Techniques for Wildlife and Habitats (A. Bookhout, ed.). The Wildlife Society. Maryland, U. S. A. Links
- Smith, W. P.. 1991. Odocoileus virginianus. Mammalian Species 388:1-3. Links
- Schwede, G., H., Hendrichs, and W., McShea. 1993. Social and spatial organization of female white tailed deer, Odocoileus virginianus, during the fawning season. Animal Behavior 45:1007-1017. Links
- Schwede, G., H., Hendrichs, and C., Wemmer. 1994. Early mother-young relations in white-tailed deer. Journal of Mammalogy 75:438-445. Links
- Shipley, L. A., and D. E., Spalinger. 1995. Influence of size and density of browse patches on intake rates and foraging decisions of young moose and white-tailed deer. Oecología 104:112-121. Links
- Tierson, W. C., G. F., Mattfield, R. W., Sage, and D. R., Behrend. 1985. Seasonal movements and home ranges of white-tailed deer in the Adirondacks. Journal of Wildlife Management 49:760-769. Links
- Villarreal, J. G.. 2013. Ganadería diversificada: importancia ecológica, cinegética y económica de los venados mexicanos. SEGARPA. Monterrey, México. Links
- Wallace, M. C., and P. R., Krausman. 1990. Neonatal elk habitat in central Arizona. Pp. 69-75 in The biology of deer (R. D. Brown, ed.). New York, U. S. A. Links
- Weckerly, F. W.. 1994. Selective feeding by black-tailed deer: forage quality or abundance? Journal of Mammalogy 75:905-913. Links
- Weckerly, F. W., and M. L., Kennedy. 1992. Examining hypotheses about feeding strategies of white-tailed deer. Canadian Journal of Zoology 70:432-439. Links
- White, G. C., and R. A., Garrot. 1990. Analysis of wildlife radio-tracking data. Academic Press, Inc. California, U. S. A. Links
- Zar, J. H.. 1996. Biostatistical Analysis. Second Edition. Prentice-Hall. New Jersey, U. S. A. Links
- Zultowsky, J. M.. 1992. Behavioral and spatial ecology of female white tailed deer in the everglades ecosystem. Master of Science Thesis. Department of Wildlife and Range Sciences. University of Florida. Florida, USA. Links