Introduction
Mammal recording in Bolivia has been conducted over a long time (Tarifa 2014), with the work of Sydney Anderson as a cornerstone for contemporary mammalogy in the country. Sydney Anderson conducted studies from 1964 to 1993; his efforts, which spanned over nearly three decades, resulted in a reference book for Bolivian mammalogy (Anderson 1997). In subsequent decades, the study of mammals has increased significantly, as regards not only taxonomic aspects (Aguirreet al. 2019) but also investigating ecological (Wallace et al. 2010) and conservation features (Tarifa and Aguirre 2009). Anderson was also the first to set the grounds for the study of bats in Bolivia, publishing a preliminary list of 79 species (Andersonet al. 1982). This information provided the basis for multiple publications on bats, including compilations of the current state of knowledge (Aguirre 2007) and taxonomic revisions (e. g., Siles et al. 2013), up to complex ecological studies (e. g., Aguirre et al. 2016; Siles et al. 2007). From the work of Anderson et al. (1982), the richness of bat species known to Bolivia has increased significantly to 138 species currently reported (Aguirre et al. 2019), equivalent to approximately two records of new species per year over a decade (Aguirreet al. 2009).
Bats, which account for some 50 % of the mammalian fauna of tropical rainforests (Patterson et al. 2003), play a central role as pollinators, seed dispersers, and natural pest controls in crops (Cleveland et al. 2006; Bracamonte 2011; Kunz et al. 2011). Also, they have a huge potential as indicators of habitat disturbance levels (Kunz et al. 2011; Castro-Luna et al. 2007; Park 2015) and provide a broad view of the health of ecosystems, as they exploit different trophic resources in Neotropical forests (García-Moraleset al.2013). This considerable importance contrasts with the lack of information on the current status of their roosts (Kunz 1982; Arita and Vargas 1995; Aguirre 2007; Siles et al.2007) and the misconception or erroneous beliefs about them, leading to ungrounded extermination of individuals or colonies (Hutson et al. 2001; Lizarroet al.2010).
Bats need roosts to shelter from adverse weather conditions and predators (Kunz and Fenton 2003), where breeding, nursing, or temporary roosting colonies are also established between migrations (Altringham 2011). Therefore, roost characteristics and roost-related processes play a central role in the ecology and evolution of different bat species (Kunz 1982).
There is little knowledge in Bolivia about the availability of roosts for bats, given the few studies carried out on this topic. Of the 138 bat species registered (Aguirre et al.2019), at least 35 use caves as primary or alternative roosts (Aguirre 2007; Moya et al. 2007; Siles et al. 2007) and at least 12 can be considered as strict cave dwellers (Aguirre 2007). Bat species inhabiting these roosts may be listed under a threatened category, mainly because of the specificity of the sites uses and for having small populations, which is aggravated by the destruction or misuse of caves, indiscriminate killing, and because of negative misbeliefs and myths (Galarza and Aguirre 2007). Knowing and protecting the natural roosts of bats, especially those of threatened species, are essential for the conservation of key elements of biodiversity (Aguirreet al.2010). This study aims to contribute basic information on the characteristics of cave roosts that are regularly or occasionally inhabited by bats, as well as on the use of caves (position, resting sites, and interactions), in the east of the department of Santa Cruz, Bolivia. With the data obtained, we expect to set biological bases for the implementation of conservation strategies that ensure the survival of bat populations.
Materials and Methods
Study Area. We carried out a comprehensive search of caves and caverns in the east of the department of Santa Cruz, Bolivia, corresponding to the Brazilian-Paranense biogeographic region (Navarro 2011), starting with a literature review and gathering incidental information from local inhabitants. Each of the caves was located and georeferenced in the localities (Figure 1). The localities where caves and caverns with the presence of bat species were recorded are Huanchaca Plateau, San Matías, Santiago de Chiquitos, Puerto Suárez, Roboré, Ascension de la Frontera, and San Ignacio de Velasco (Figure 1).
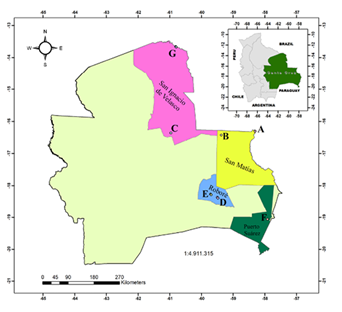
Figure 1 Location map of the 7 study sites where the 19 caves were located in the department of Santa Cruz, Bolivia. Caves are grouped according to closeness, marked with different letters (A: CU1, CU2; B: AS1; C: CY1; D: MS1, PM1, PM2; E: MN1, MN2, MN3; F: MT1; G: TR1, TR2, TR3, TG1, CR1, HJ1, TM1, TM2; see Table 1 for names).
Characterization of caves. The fieldwork was conducted from June 2008 to March 2009, with a three-day visit to each cave. The environmental-structural characteristics of each cave were recorded using a methodology adapted from Siles et al. (2007). We measured the distance at both sides (width) and to the roof (height) at one-meter intervals and recorded the temperature and humidity during the same period of time in each cave (9:00 to 14:00 hrs). Since the environmental variables measured may be biased by seasonal variations, the results include the month when each cave was evaluated (Table 1), in the understanding that the data obtained characterize each cave at a particular time point. In addition, notes were made regarding the illumination in sites where individuals were recorded or the distance from the entrance to the cave.
The particular location of species within caves during the day, resting sites, and interspecific interactions were recorded on days when neither measurements were taken nor bats were captured, for a similar period of time for each cave. The size of each colony was estimated according to the number of bats observed during daytime visits: for small colonies (< 30 individuals/colony), all individuals could be counted directly; for larger colonies, photographs were taken and individuals were counted on the printout. No attempt was made to estimate absolute population sizes from catch data; instead, this information was used for validating species identification and estimating relative abundance.
Table 1 Summary of the environmental-structural characteristics of the 19 caves in the department of Santa Cruz, Bolivia. (Lat: Latitude, Long: Longitude, LT: Overall Length, H: Height, W: Width, Ent: Entrance, RH: Relative humidity, T: Temperature, : Average, SD: Standard Deviation, Max: Maximum, Min: Minimum).
| Cave | Month | Lat. | Long. | LT (m) | H (m) (Max) | H (m) (Min) | W (m) (Max) | W (m)(Min) | N° Ent | %RH () | %RH (SD) | %RH (Max) | %RH (Min) | T (°C) () | T(°C) (SD) | T (°C) (Max) | T (°C) (Min) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Curicha (CU1) | June | -16.30397 | -58.30754 | 12.0 | 2.5 | 0.8 | 4.4 | 1.5 | 1 | 68.4 | 1.8 | 70.5 | 64.0 | 26.7 | 0.8 | 27.8 | 26.4 |
| Curicha 2 (CU2) | June | -16.30418 | -58.30721 | 19.0 | 3.1 | 1.8 | 17.9 | 1.9 | 1 | 67.5 | 3.1 | 73.0 | 63.0 | 22.6 | 1.2 | 24.8 | 21.3 |
| Cueva Ascensión (AS1) | June | -16.31121 | -59.44959 | 5.0 | 2.8 | 2.5 | 1.5 | 0.6 | 1 | 71.8 | 5.5 | 79.0 | 66.0 | 24.7 | 2.0 | 26.9 | 21.5 |
| Cueva del Yeso (CY1) | June | -16.35668 | -60.96482 | 18.0 | 6.0 | 1.0 | 17.3 | 3.2 | 1 | 74.8 | 2.7 | 77.0 | 68.0 | 17.5 | 0.4 | 18.0 | 16.3 |
| Miserendino (MS1) | August | -18.36037 | -59.50118 | 208.0 | 40.0 | 1.4 | 7.5 | 0.3 | 3 | 93.8 | 4.8 | 98.0 | 65.0 | 23.9 | 0.9 | 28.5 | 23.0 |
| Puente Mono 1 (PM1) | February | -18.38711 | -59.48085 | 56.0 | 10.0 | 4.5 | 7.5 | 1.5 | 1 | 87.8 | 4.9 | 97.0 | 79.0 | 26.5 | 1.2 | 28.4 | 25.1 |
| Puente Mono 2 (PM2) | February | -18.38944 | -59.48050 | 65.0 | 16.0 | 6.5 | 6.0 | 1.0 | 2 | 86.5 | 3.2 | 94.0 | 83.0 | 26.9 | 0.8 | 27.7 | 24.6 |
| Mono 1 (MN1) | August | -18.26006 | -59.71580 | 13.0 | 1.9 | 1.4 | 12.0 | 2.5 | 2 | 52.5 | 6.7 | 61.0 | 45.0 | 32.2 | 4.3 | 37.1 | 28.0 |
| Mono 2 (MN2) | August | -18.27911 | -59.69929 | 17.0 | 9.5 | 6.0 | 2.5 | 0.4 | 1 | 57.3 | 2.8 | 61.0 | 52.0 | 25.3 | 0.3 | 25.6 | 24.9 |
| Mono 3 (MN3) | August | -18.28369 | -59.69608 | 14.0 | 11.0 | 10.5 | 4.5 | 0.3 | 1 | 53.5 | 1.5 | 56.0 | 52.0 | 26.3 | 0.5 | 26.9 | 25.5 |
| Motacucito (MT1) | February | -19.05967 | -57.91698 | 81.0 | 10.0 | 0.9 | 13.0 | 1.1 | 1 | 93.7 | 5.3 | 99.0 | 79.0 | 29.3 | 1.4 | 36.9 | 27.7 |
| Torres 1 (TR1) | December | -13.66872 | -60.79697 | 40.0 | 10.0 | 0.4 | 6.1 | 0.2 | 1 | 81.0 | 3.9 | 87.0 | 74.0 | 31.0 | 0.2 | 31.0 | 30.4 |
| Torres 2 (TR2) | December | -13.66942 | -60.79706 | 9.0 | 2.7 | 0.5 | 1.2 | 0.5 | 1 | 79.7 | 1.8 | 83.0 | 78.0 | 31.3 | 0.1 | 31.5 | 31.1 |
| Torres 3 (TR3) | December | -13.66984 | -60.79703 | 10.0 | 2.5 | 0.3 | 1.6 | 0.1 | 1 | 66.7 | 6.0 | 77.0 | 60.0 | 31.7 | 0.9 | 33.0 | 30.2 |
| Tigre (TG1) | December | -13.65209 | -60.81296 | 35.0 | 3.5 | 0.4 | 1.7 | 0.2 | 3 | 86.3 | 3.4 | 92.0 | 83.0 | 26.0 | 0.0 | 26.0 | 26.0 |
| La Cruz (CR1) | December | -13.67011 | -60.79615 | 48.0 | 12.0 | 0.5 | 2.0 | 0.3 | 2 | 87.2 | 3.1 | 94.0 | 79.0 | 26.0 | 0.0 | 26.0 | 26.0 |
| Hojarasca (HJ1) | December | -13.66910 | -60.79611 | 17.0 | 12.0 | 0.5 | 2.0 | 0.4 | 1 | 82.4 | 1.4 | 84.0 | 80.0 | 28.4 | 0.2 | 28.7 | 28.1 |
| Torre Mediana (TM1) | December | -13.66678 | -60.79638 | 10.0 | 7.0 | 0.7 | 2.3 | 0.2 | 1 | 77.2 | 1.6 | 81.0 | 75.0 | 30.4 | 0.2 | 30.7 | 30.2 |
| Torre Mediana 2 (TM2) | December | -13.66706 | -60.79678 | 9.0 | 9.0 | 1.0 | 1.0 | 0.4 | 1 | 74.8 | 3.1 | 79.0 | 70.0 | 29.4 | 0.4 | 29.8 | 28.8 |
To confirm the identity of all bat species, present in each cave, individuals were captured following the methodology by Vargas et al. (2006). Mist nets were placed in narrow areas of the caves to ensure the capture of bats; afterward, these individuals were identified using field keys (Emmons and Feer 1999; Aguirreet al. 2009) and then released.
Relationship between bat abundance and seasonality. To determine if the number of records of bat species in caves is related to the season of the year, a Chi-Square test (Zar 1999) was performed between the number of records of each species in the dry season (April to September) and the rainy season (October to March). The analysis excluded Chrotopterus auritus because of the small number of records for this species.
Interspecific association in caves. The association between the bat species captured in caves was analyzed using presence-absence data to calculate the interspecific association index V for pairs of species (Arita and Vargas 1995). Values for this index range from -1 (complete segregation), 0 (no association), to 1 (perfect positive association).
Relationship between roost characteristics and bat diversity. To characterize the caves according to the variables evaluated in roosts, a linear correlation analysis (Zar 1999) was conducted between temperature and humidity and structural variables, i.e., cave length (long), number of arms or galleries (passages of varying dimensions), number of entrances (cavities in the ceiling, on the side, or at the bottom of the cave), and structural variation (presence of structures in the roof or walls formed by the dissolution of the parental material, with cracks or cavities), in addition to the biological variables of species richness and diversity. The complexity parameters of caves were defined based on the presence of galleries, entrances, and variation (1 = Nil, 2 = Medium, 3 = Complex, 4 = Highly Complex). Accordingly, a cave with a complexity value of 1 has a single gallery, few entries (≤ 5), and no structural variation; a cave that scored 2 has two galleries, few entries (> 5, ≤ 10) and little variation in the structure of the roof; a cave that scored 3 has three galleries, entrances (> 10, ≤ 20) and little variation in the roof and walls; a cave that scored 4 has more than three galleries, entrances (> 20) and a great variation in roof and walls. The relationship of environmental and structural variables with species was explored with a Canonical Correspondence Analysis (CCA), including a permutation test to evaluate the significance of the variables in the analysis (Tel-Braak 1986). The statistical analyses used in the present work were carried out with the program R (v. 3.2.2).
Results
Seven localities were visited during the study period, finding 19 caves that were georeferenced (Figure 1). Of these, 12 were identified for the first time and named according to their particular characteristics or locations. The mean temperature and humidity, as well as the structural features of each cave, are described in Table 1.
As regards the species composition of roosting bats, we recorded the presence of seven species in three families: Phyllostomidae (4), Natalidae (1), and Emballonuridae (2). The family Phyllostomidae was represented by four subfamilies: Carolliinae, Desmodontinae, Lonchorhininae, and Phyllostominae. The species recorded (ranked from higher to lower frequency of occurrence in caves, in parenthesis) were Peropteryx macrotis (12 caves), Carollia perspicillata (7), Desmodus rotundus and Natalus macrourus (3), Peropteryx kappleri (2), and Chrotopterus auritus and Lonchorhina aurita (1; Figure 2).
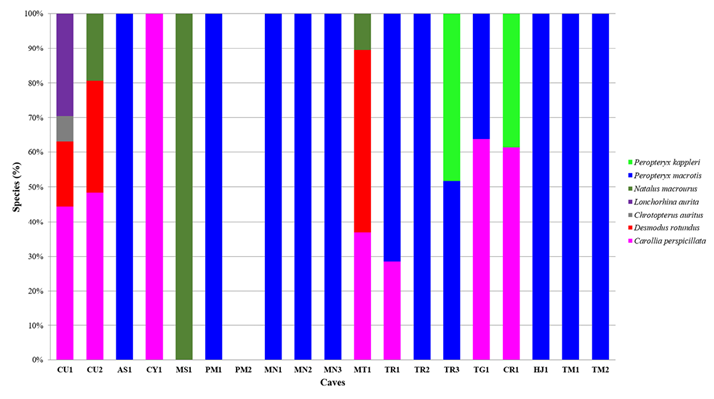
Figure 2 Bat species present in the caves evaluated in the eastern portion of the department of Santa Cruz, Bolivia. Percentages represent the relative abundance of species.
The observed abundance data are summarized in Table 2, showing that cave MT1 had the highest abundance for three species (C. perspicillata, D. rotundus, and N. macrourus). Two individuals of C. auritus and eight of L. aurita were found in cave CU1. Caves AS1 and MN1 showed the smallest number of individuals (n = 5) in both caves, with a single species (P. macrotis). On the other hand, cave HJ1 attained the highest number of individuals of P. macrotis. It should be mentioned that no individuals were found in cave PM2.
Table 2 Total abundance of bat colonies in caves, as calculated by direct daytime observations and photographs. Cper = C. perspicillata, Drot = D. rotundus, Caur = C. auritus, Laur = L. aurita, Nmac = N. macrourus, Pmac = P. macrotis, Pkap = P. kappleri.
| Caves | Cpe | Dro | Cau | Lau | Nma | Pma | Pka | Total |
|---|---|---|---|---|---|---|---|---|
| Curicha (CU1) | 12 | 5 | 2 | 8 | 0 | 0 | 0 | 27 |
| Curicha 2 (CU2) | 15 | 10 | 0 | 0 | 6 | 0 | 0 | 31 |
| Cueva Ascensión (AS1) | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 5 |
| Cueva del Yeso (CY1) | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 15 |
| Miserendino (MS1) | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 25 |
| Puente Mono 1 (PM1) | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 8 |
| Puente Mono 2 (PM2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mono 1 (MN1) | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 5 |
| Mono 2 (MN2) | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 8 |
| Mono 3 (MN3) | 0 | 0 | 0 | 0 | 0 | 22 | 0 | 22 |
| Motacucito (MT1) | 35 | 50 | 0 | 0 | 10 | 0 | 0 | 95 |
| Torres 1 (TR1) | 10 | 0 | 0 | 0 | 0 | 25 | 0 | 35 |
| Torres 2 (TR2) | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 10 |
| Torres 3 (TR3) | 0 | 0 | 0 | 0 | 0 | 15 | 14 | 29 |
| Tigre (TG1) | 7 | 0 | 0 | 0 | 0 | 4 | 0 | 11 |
| La Cruz (CR1) | 19 | 0 | 0 | 0 | 0 | 0 | 12 | 31 |
| Hojarasca (HJ1) | 0 | 0 | 0 | 0 | 0 | 31 | 0 | 31 |
| Torre Mediana (TM1) | 0 | 0 | 0 | 0 | 0 | 20 | 0 | 20 |
| Torre Mediana 2 (TM2) | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 11 |
| Total | 113 | 65 | 2 | 8 | 41 | 164 | 26 | 419 |
There was a significant relationship between the number of species records in caves and the time of year (Pearson’s Chi-square = 72.4; d.f. = 5; P = 0.01). In general, we recorded more individuals in caves during the wet season, although the proportions vary according to the species.
As regards interspecific associations in caves, we calculated the V index for all potential pairs of the seven species found in caves, for a total of 21 combinations. This resulted in 12 cases of negative interspecific association; of these, none showed an absolute value of -1, i.e., the species involved are not completely segregated. There was no case where with a V value of zero (i.e., no association). On the other hand, we found nine cases of a positive association, with a V value of 1 (perfect positive association) only in three cases, namely L. aurita-C. perspicillata, L. aurita-C. auritus, and C. auritus-C. perspicillata (Table 3).
Table 3 Association index values (V) calculated for pairs of species present in the caves of the department of Santa Cruz, Bolivia.
| Species | Cpe | Dro | Cau | Lau | Nma | Pma | Pka |
|---|---|---|---|---|---|---|---|
| P. kappleri (Pka) | 0.1 | -0.1 | -0.1 | -0.1 | -0.1 | -0.1 | |
| P. macrotis (Pma) | -0.5 | -0.6 | -0.3 | -0.3 | -0.6 | ||
| N. macrourus (Nma) | 0.3 | 0.6 | -0.1 | -0.1 | |||
| L. aurita (Lau) | 1.0 | 0.5 | 1.0 | ||||
| C. auritus (Cau) | 1.0 | 0.5 | |||||
| D. rotundus (Dro) | 0.6 | ||||||
| C. perspicillata (Cpe) |
The assessments of caves revealed that the colonies of P. macrotis were compact groups, in contact with each individual, and separated from all other species. We also observed that D. rotundus roosts in hard-to-access areas of the cave but can coexist in the proximity of individuals of C. perspicillata, L. aurita, C. auritus, and N. macrourus.
The linear correlation analysis (Table 43) shows that long caves are characterized by higher HR; this environmental parameter is re3lated to the structural complexity of the cave. Similarly, caves with a complex structure show a relationship with the number of galleries.
According to the Canonical Correspondence Analysis (Figure 3), the first two axes explain 22 % of the variation in the data. This analysis shows that C. perspicillata prefers complex caves with more galleries (MT1 and CR1); N. macrourus prefers structurally long caves with high RH (MS1 and MT1); P. macrotis is found in simple caves that record high temperatures (average 28.6 °C); and D. rotundus prefers structurally long caves with high RH.
Discussion
Caves are vital resources allowing the existence of species that are key for ecosystems, such as bats (Altringham 2011). This contrasts with the scarce information on cave bat roosts in Bolivia, given the few studies conducted in this country to date. Thus, to date, only four speleological zones had been recorded for the country: Sorata (La Paz), Carrasco National Park (Cochabamba), Torotoro (Potosí), and Puerto Suárez (Santa Cruz), all including caves that harbor bat species (Aguirre 2007; Miranda-Chumacero 2007). Therefore, the 19 caves identified in the present study are an important contribution to the knowledge of cave roosts in Bolivia, with different species of bats found in 95 % of these shelters.
Regarding the abundance of bats in caves, Arita (1996) mentions that the largest caves shelter the highest numbers of individuals. However, it should be noted that cave size is also related to structural complexity, as larger caves tend to have more chambers, tunnels, and bifurcations. Sherwinet al. (2000) show that simple caves are less populated by bats than those of greater complexity, while Siles et al. (2007) report that caves with high structural complexity tend to shelter a higher abundance of bats than less complex caves of similar size. In our case, cave MT1 follows the same trend: it has the most complex structure, which is probably the reason for the higher abundance of bat species that make good use of the various structural areas to roost.
Some species appear to prefer particular structures in caves. For example, D. rotundus roosted in darker and narrower cavities, consistent with the observation reported in this same species by Siles et al. (2007). The use of deeper chambers in caves by compact groups of bats has been reported for Artibeus jamaicensis (Arita and Vargas 1995; Ortega and Arita 1999), C. perspicillata (Trajano and Gimenez 1998; Reis et al. 2007), Phyllostomus hastatus (McCracken and Bradbury 1981), Pteronotus parnellii and Diphylla ecaudata (Arita and Vargas 1995). In the present study, this pattern was observed for C. perspicillata, forming compact groups at the bottom of all caves where it was found. Similarly, our data indicate that N. macrourus prefers to roost in caves with very high humidity, since the colonies of this species were always located in narrow passages of low height. This same characteristic was observed by Torres-Flores and López-Wilchis (2010) in Natalus stramineus roosting in different caves of Mexico.
One of the major drivers for the presence of bats in caves is microclimate (Kunz 1982), which depends on the length, number of entrances, and depth of the cave, as well as on the temperature and humidity inside it (Siles et al. 2007). The temperature and humidity records showed differences between the 19 caves evaluated. The roosts with the greatest variations in temperature and relative humidity inside caves were MN1, TR3, AS1, MT1, PM1, and MS1 (Table 1), likely as a result of their structural complexity. These features probably provide bats with habitat conditions that best suit their metabolic or energy requirements (Lewis 1995; Sedgeley and O’Donnell 1999). Siles et al. (2007) detected no relationship between temperature and species composition across caves. In the 19 caves explored, bat species probably choose roosting sites based on structural rather than environmental characteristics, although both features (i.e., structure and microclimate) should not be separated, as they are often related (Lewis 1995). Nonetheless, it is challenging to derive generalizations with these data, as temperature was recorded only once in each cave; thus, it should not be used as an indicator of the microclimate inside a cave. Thus, we recommend recording continuous readings over long periods of time (Sherwin et al. 2000). Therefore, continuous monitoring of these caves should be conducted to accurately determine the role of this abiotic factor on the presence and abundance of bats.
On the other hand, the calculated V index values may not show the existence of a particular interspecific interaction, since the fact that two species co-occur in the same roost (cave) does not necessarily imply that both are positively associated. An improved analysis of interspecific associations in caves should consider whether pairs of species occupy the same area within roosting caves. Caves offer a wide variety of sites for bat species to choose from (Hill and Smith 1992; Altringham 1996). Our study evidenced the segregation of some species within their roosts (e. g., P. macrotis and P. kappleri), which remain clearly separated from all other bat species in the caves where they were recorded. Therefore, we agree with Swift and Acey (1983), who indicate a nonexistent interspecific interaction when a pair of species occupying the same roosting cave use separate areas within it, leaving and arriving at different times, foraging in different areas, and feeding on different preys. Also, in this study, it was observed that D. rotundus shelters in hard-to-access sectors of the cave but might occupy perch sites close to individuals of C. perspicillata. These observations are consistent with those reported by Siles et al. (2007), who mention that no interaction between these two species was observed. All our combinations between L. aurita, C. perspicillata, and C. auritus showed a positive association. However, it should be noted that very few individuals were observed or captured; moreover, only these three species co-occur within a single cave. Graham (1988) pointed out that cases where pairs of species roost in the same site are exceptional, being the only ones that clearly showed positive associations between species.
The analysis of the conservation status of bats in Bolivia shows that, of the 138 species present in the country, 12 face some risk level. In the present study, we found L. aurita and N. macrourus, which inhabit cave environments only (Lassieur and Wilson 1989; Emmons and Feer 1999) and are categorized as “Endangered” and “Vulnerable”, respectively (Vargas and Rocha 2009; Vargas et al. 2009). The records of both species in this study are relevant from a conservation standpoint. L. aurita is a species distributed from Mexico to southeastern Bolivia (Vargas 2008 Vargas et al. 2009) that may potentially live in mountain ranges in the Chiquitano and Cerrado forest ecoregions and areas adjacent to wetlands (Vargaset al. 2010). To date, the cave reported in San Matías is the only known in Bolivia where this species is present. For N. macrourus, the records confirm its presence in caves MT1 and MS1, as previous reports involving these caves came from personal observations (Aguirre 2007; Vargas 2008). Thus, the cave CU2 is a new record of a cave inhabited by this species, considering that only confirmed reports about the distribution of this species in Brazil were available (Delgado-Jaramillo et al. 2018). In this way, caves can constitute important shelters for these species classified as threatened in Bolivia (Aguirre et al. 2010).
Most of the 19 caves evaluated in Santa Cruz represent new records of this type of important environments for bats in Bolivia and thus serve as references for advancing the knowledge about natural history and as key tools for future research on cave ecology.
In this work, we found bat species that could be susceptible to local extinctions, given their highly specialized use of caves. Because of the importance of new bat records in caves, we suggest that the entities involved should manage the conservation of the sites mentioned, in addition to coordinating actions to establish a system of protected areas called Areas or Sites Important for the Conservation of Bats (AICOMs/SICOMs).
This work aims to contribute solid elements to advance the knowledge of cave bat species and their roosting requirements, laying the grounds for future research. Caves are sources of valuable information on population structure, reproduction, behavior, and interspecific association of bat species; in addition, the information in this study contributes to identify shelters requiring conservation measures in our country.











 nueva página del texto (beta)
nueva página del texto (beta)



