Introduction
Monogamous mating systems have long puzzled behavioral ecologists given that males - and in many species females - appear to gain fitness benefits by mating with multiple members of the opposite sex (Bateman 1948; Trivers 1972). Studies that integrate behavioral and molecular data have revealed that monogamy consists of two distinct but related components. Social monogamy is characterized by the formation of an exclusive behavioral bond between a male and a female (Kleiman 1977). In contrast, genetic monogamy refers to the number of partners whose gametes contribute to production of an individual’s offspring (Dolotovskaya et al. 2020; Kappeler 2019). The extent to which these two forms of monogamy coincide varies, as evidenced by interspecific differences in the frequency of extra-pair copulations and fertilizations in socially monogamous taxa (Waser et al. 1994; Sillero-Zubiri et al. 1996; Girman et al. 1997; Goossens et al. 1998; Fietz et al. 2000; Solomon et al. 2004; Cohas and Allainé 2009, 2009; Huck et al. 2014; Dolotovskaya et al. 2020). The duration of monogamous relationships also varies, ranging from a single round of reproduction to lifetime reproductive partners (Kleiman 1977; Lukas and Clutton-Brock 2013). Quantifying these sources of variation is critical to identifying the combination of selective factors favoring monogamous mating systems across diverse species.
Social monogamy is often inferred from behavioral data (e. g., spatial relationships, evidence for pair bonds; Ribble and Salvioni 1990; Sabol et al. 2018). In contrast, demonstrating genetic monogamy typically requires molecular data regarding the parentage of young (Lambert et al. 2018). Studies that incorporate both types of information suggest that truly monogamous - that is, both socially and genetically monogamous - systems are rare among vertebrates (Lambert et al. 2018). Among mammals, only a handful of species are thought to be truly monogamous (3 to 5 % (Kleiman 1977), including the Malagasy giant jumping rat (Sommer and Tichy 1999), Kirk’s dik-dik (Brotherton et al. 1997), and some populations of coyotes (Hennessy et al. 2012). Consistent with this designation, genetic analyses confirm that in each of these taxa all offspring are sired by a female’s social partner.
One of the best-studied examples of monogamy in mammals is the California deermouse (Peromyscus californicus). This species was first described as socially monogamous by Ribble and Salvioni (1990), who used a combination radiotelemetry and fluorescent powder tracking to demonstrate that members of a male-female pair share a home range and a nest site but do not typically overlap spatially with neighboring pairs of animals. Subsequent analyses based on multi-locus DNA fingerprinting (Ribble 1991) revealed no evidence of extra-pair parentage, suggesting that social partners are genetically monogamous. Building on this foundation, studies of California mice have been used to examine the ecological, life history, endocrine, and neural correlates of mammalian monogamy (e. g.Gubernick and Nordby 1993; Bester-Meredith et al. 1999; Trainor and Marler 2001; Campi et al. 2013; Johnson et al. 2015; Pultorak et al. 2015; Petric et al. 2021).
Characterization of free-living P. californicus as socially and genetically monogamous is based on data collected from a single population studied in oak savannah habitat in Monterey County, California. The geographic distribution of this species, however, extends from the San Francisco Bay area south to Baja California and encompasses habitats ranging from mesic coastal woodlands to considerably more arid chaparral (Grinnell and Swarth 1913; Grinnell and Orr 1934; King 1968). Given this geographic and ecological variation and given intraspecific variability in rates of extra-pair paternity in other socially monogamous species (Cohas and Allainé 2009), we chose to explore the occurrence of monogamy in populations of P. californicus from multiple locations in California. Specifically, we used a combination of live-trapping, fluorescent powder tracking, and microsatellite analyses of parentage to determine if male-female pairs identified on the basis of spatial relationships were the genetic parents of offspring reared by the female in each pair. These analyses generate important new insights into the occurrence of extra-pair young in this species, thereby contributing to efforts to understand the adaptive bases for social versus genetic monogamy in free-living populations of mammals.
Materials and methods
Field sites, trapping, and marking of animals. Mice were captured at 4 localities - 2 in the northern and two in the southern portion of the range of P. californicus (Figure 1). The northern sites sampled were at the Hastings Natural History Reservation, Carmel Valley, California and the Landels-Hill Big Creek Reserve, Big Sur, California. The two southern sites were located at the Emerson Oaks Reserve, Temecula, California and the Torrey Pines State Natural Reserve, La Jolla, California. These are the same locations sampled by Melendez-Rosa et al. (2020). As described by these authors, the two northern sites are characterized by greater annual rainfall; for both northern and southern localities, the more coastal site receives greater rainfall than the more inland site. Collectively, these sampling localities span much of the range of habitats and environmental conditions in which P. californicus is known to occur (Meléndez-Rosa et al. 2020).
All trapping of mice was conducted between February and April 2016. At each sampling locality, animals were captured using Sherman live-traps baited with rolled oats and containing a small ball of synthetic batting that the animals used as nesting material. A total of 180 traps per locality were set, with traps placed in pairs at 10 m intervals to create a grid measuring 150 m x 60 m and containing 90 trap stations (pairs of traps). At each sampling locality, traps were opened at 16:00 hrs and closed 3:00 hrs for 20 consecutive nights. Individuals captured were identified to species using standard pelage and body size characters (Jameson and Peeters 2004). At the time of first capture, each animal was permanently marked by attaching a uniquely numbered metal tag (Monel 1005-1, National Band and Tag Company, Inc.) to the right ear pinna. In addition, each animal was weighed and its sex and reproductive status were assessed based on the appearance of the external genitalia. Upon completion of these procedures, each animal was released at the location at which it had been caught.
Field identification of male-female pairs via pigment transfer. Putative male-female pairs were identified based on capture localities and transfer of fluorescent pigment between individuals. A male and female were considered probable reproductive partners if they were captured in adjacent (paired) traps on more than three occasions during the same 20-night trapping effort. Physical contact between putative partners was confirmed using fluorescent powder tracking (Ribble and Salvioni 1990; Kalcounis-Rüppell et al. 2001). Previous studies of P. californicus have demonstrated that when a female whose pelage has been coated with fluorescent powder returns to her nest, some of the powder is transferred to the adult male with which she lives (Ribble and Salvioni 1990; Ribble 1991). By recapturing the female and her partner on the following night, transfer of powder can be detected visually (either directly or with a hand-held black light; Figure 1: photo A), thereby confirming physical contact between the adults in question. Accordingly, the female in each putative pair was covered from neck to tail in one of six colors of non-toxic Eco PigmentsTm (DayGlo, Cleveland, OH) fluorescent powder just prior to release at the point of capture. In the few cases in which the male was not caught the following night, additional powder was applied to the female and the process was repeated. A male displaying significant powder transfer was determined to be a female’s putative reproductive partner; significant transfer was defined as powder that was visible without the assistance of a UV light. Although transfer of powder could be detected on any part of a male’s body, it was most common on the pinnae, muzzle, and around the eyes as well as on the feet, tail, and genitalia. As a final check on our assignments of individuals to reproductive pairs, the fluorescent powder tracking process was repeated for each putative pair using a different color of powder.
All fieldwork involving mice was approved by the Animal Care and Use Committee at the University of California, Berkeley, and was consistent with the Guidelines for the Use of Wild Mammals in Research published by the American Society of Mammalogists (Sikes and the Animal Care and Use Committee of the American Society of Mammalogists 2016).
Microsatellite analyses of paternity. Prior to this study, genetic monogamy had been assessed only for P. californicus at HNHR (Figure 1) based on multi-locus DNA fingerprinting (Ribble 1991). To confirm the reported genetic monogamy of this population and to determine patterns of parentage at the other localities sampled, we used microsatellite loci to genotype pregnant adult females, their embryos, and the adult males identified in the field as the partners of those females; we focused these analyses on pregnant females because use of known mother-offspring pairs increased our confidence in the associated assignments of paternity. At each locality, the subset of females that were determined to be pregnant and whose putative male partners had been identified using the trapping and powder transfer criteria described above were euthanized via overdose with Isoflurane, after which a sample of the female’s liver and each embryo were frozen separately in liquid nitrogen until they could be transferred to a -80o C freezer on the Berkeley campus. Similarly, we euthanized and collected liver samples from all putative adult male partners.
Allelic variation was assessed at nine microsatellite loci. Primers for four loci (PO-9, PO-88 PO-26, PO-16) had been developed for P. polionotus by Prince et al. (2002). Primers for the remaining five loci (5477, 5411, 5142, 5466, 5334), were developed for Peromyscus by Weber et al. (2010). PCR amplification of loci isolated from P. polionotus was accomplished using the same master mix employed by Meléndez‐Rosa et al. (2019) to amplify the cyt-b locus from our study populations; thermocycling conditions were the same as those described by Prince et al. (2002). The master mix for the remaining loci consisted of 6.76μL of ddH2O, 1.25μL of 10x buffer, 1μL of MgCl2 (25μM), 1.25μL of BSA (Bovine Serum Albumin), 0.1875μL of dNTPs (10μM), 0.475μL (20pmol) of each primer (fluorescently tagged forward primer; Table 1), 0.10μL of Taq polymerase (New England Bio Labs), and 1μL of the DNA template. Amplification conditions for these loci consisted of an initial denaturation at 95 °C for 4:00 min followed by 40 cycles of denaturation at 95 °C for 0:30 min, annealing at 55 to 56 °C for 0:30 min, and extension at 72 °C for 0:30 min. Specific annealing temperatures for each locus are provided in Table 1.
To assess allelic variability at each microsatellite locus, amplicons were electrophoresed on an ABI 3730 sequencer, with 500 LIZ size standard (GeneScan) included in each lane. Allele sizes were determined using Geneious 7.1.7 (Kearse et al. 2012), after which estimates of allelic diversity, heterozygosity, and polymorphic information content (PIC) were generated and departures from Hardy-Weinberg expectations (HWE) were assessed using CERVUS 3.0.7. Pairwise estimates of linkage disequilibrium (LD) were calculated for all loci using GENEPOP v4.7.5 (Raymond and Rousset 1995; Rousset 2008). To determine the paternity of individual fetuses, genotypes for females, their offspring, and all males sampled were compared using CERVUS 3.0.7 (Kalinowski et al. 2007). This software package calculates likelihood ratio scores (LOD) for each candidate sire, after which the difference in LOD scores between the two most likely sires is used to assign parentage at a 95% confidence level.
Results
Identification of social partners. A total of 23 male-female pairs (n = 46 individuals) were identified based on trapping locations and the transfer of fluorescent powder from females to males. The number of pairs identified per trapping locality was 7 at BCR, 5 at EOR, 7 at HNHR, and 4 at TPSNR (Figure 1). In no case did we capture non-paired individuals in adjacent traps or detect pigment transfer from a female to more than one male.
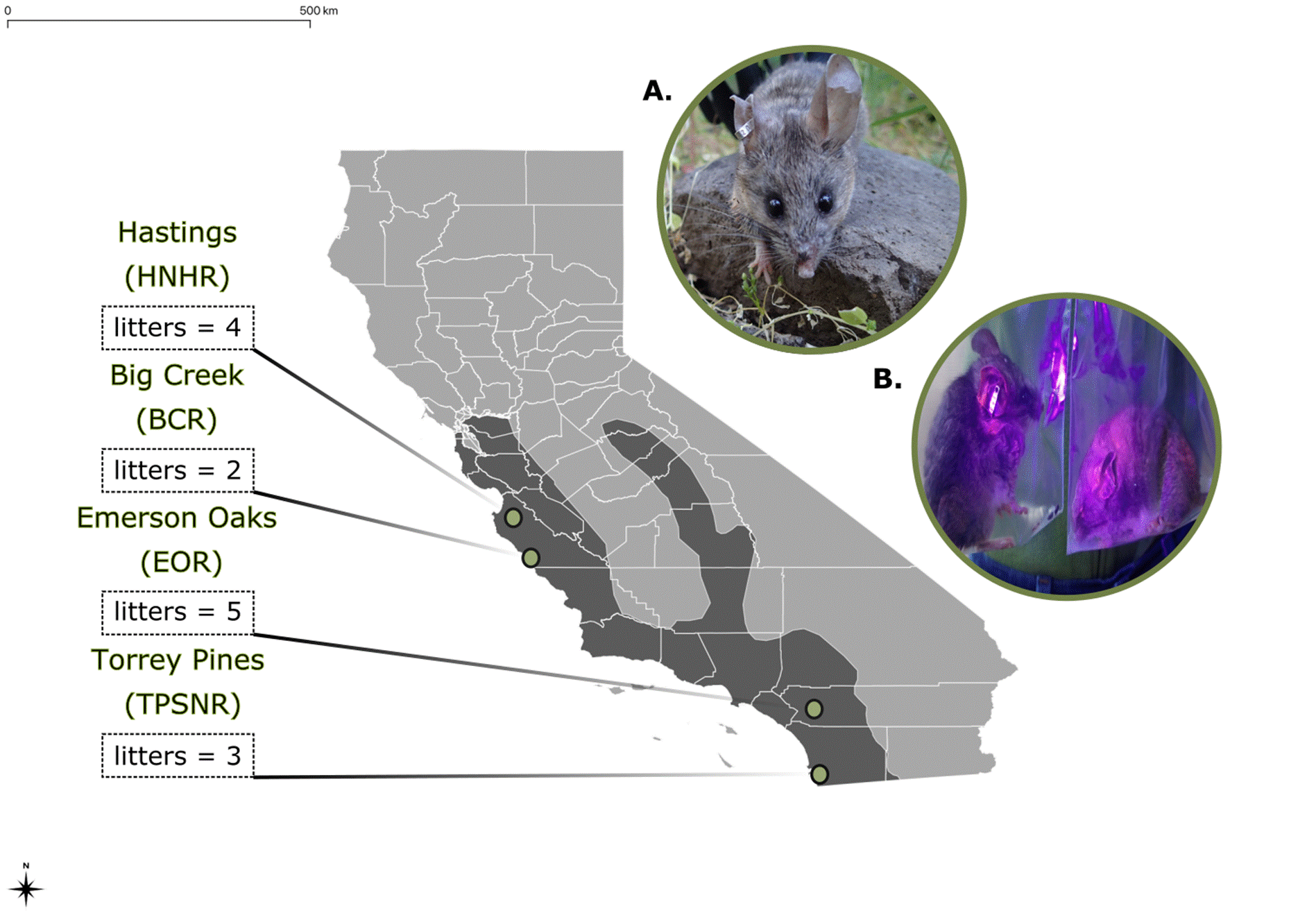
Figure 1 Locations of the populations of Peromyscus californicus sampled during this study. The geographic distribution of this species is shown in dark gray. The sites sampled were: Hastings Natural History Reservation (HNHR), Big Creek Reserve (BCR), Emerson Oaks Reserve (EOR), and Torrey Pines State Natural Reserve (TPSNR). For each site, the total number of litters (= number of male-female pairs) for which paternity was determined is indicated. Photo A: adult P. californicus from HNHR. Photo B: male (left) and female (right) P. californicus demonstrating transfer of fluorescent pigment; pigment revealed using a handheld UV lamp.
Microsatellite genotyping. Of the 23 females for which a male partner was identified, 14 (60.8 %) were determined to be pregnant. This included 2 females at BCR, 5 at EOR, 4 at HNHR, and 3 at TPSNR. Based on the number of embryos detected, mean litter size was 2.2 ± 0.6 offspring per female (range = 1-3 embryos, n = 31 embryos recovered from 14 females). The ages of embryos varied, with the result that litters for three females (21.0 %; all from HNHR) contained offspring that were too small to yield DNA that was not contaminated with maternal tissue. As a result, genotypes were generated for 11 females and their 26 offspring (Table 2).
All nine microsatellite loci employed were variable, with the number of alleles per locus ranging from 3 to 16 (mean = 9.4 + 4.7; Table 1). CERVUS was unable to evaluate departures from HWE expectations for five loci due to the limited number of individuals genotyped (Table 1); two of the remaining loci revealed significant departures from Hardy-Weinberg expectations (Table 1). Given the demonstrated effects of small samples sizes on accurate estimates of departures from HWE (Elston and Forthofer 1977; Wittke-Thompson et al. 2005), all loci were retained in downstream analyses of paternity because they were variable and informative. Only one pair of loci revealed potential LD for samples from EOR (loci PO-16 and 5466; p = 0.045); no significant LD was detected for any other pairwise comparisons of loci (p > 0.05; mean p = 0.73) and thus, again, all loci were retained in downstream analyses. Locus-specific estimates of polymorphic information content (PIC) ranged from 0.343 to 0.908 (mean = 0.742 + 0.180), indicating highly polymorphic fragments appropriate for paternity testing (Table 1).
Table 1 Summary of microsatellite markers used to determine paternity for embryonic litters of P. californicus. For each locus, the annealing temperature used in PCR amplifications is indicated, as is the fluorescent dye used during screening of variability at each marker. A total of 60 individuals were genotyped using these markers; for each locus, the number of alleles detected in this sample is given, as are the values for observed heterozygosity, expected heterozygosity, and the polymorphic information content (PIC). Significant departures from Hardy-Weinberg expectations are indicated (NS = not significant at p < 0.05; *** p < 0.001); departures from expectation could not be evaluated (ND = not estimated) for 5 loci due to the limited number of individuals genotyped.
| Locus | Annealing temperature | Dye | No. of alleles | Ho | He | PIC | HWE | Source |
|---|---|---|---|---|---|---|---|---|
| PO-9 | 55 | HEX | 13 | 0.8194 | 0.8573 | 0.839 | NS | Prince et al. (2002) |
| PO-88 | 55 | 6-FAM | 16 | 0.9583 | 0.9206 | 0.908 | ND | |
| PO-26 | 58 | VIC | 7 | 0.2083 | 0.3564 | 0.343 | ND | |
| PO-16 | 58 | 6-FAM | 3 | 0.338 | 0.6668 | 0.588 | *** | |
| 5477 | 56 | 6-FAM | 5 | 0.4722 | 0.7583 | 0.713 | *** | Weber et al. (2010) |
| 5411 | 55 | NED | 7 | 0.6806 | 0.8153 | 0.781 | ND | |
| 5142 | 55 | PET | 7 | 0.8451 | 0.7881 | 0.749 | NS | |
| 5466 | 55 | VIC | 11 | 0.7361 | 0.8721 | 0.852 | ND | |
| 5334 | 55 | PET | 16 | 0.5352 | 0.9173 | 0.904 | ND |
Paternity analyses. For each of the 26 embryos genotyped, only a single sire was identified with > 95% confidence. Based on these paternity assignments, 8 (72.3 %) of the litters genotyped were sired exclusively by the mother’s social partner (Table 2). Litters belonging to two (18.2 %) other females were sired by more than one male; in both cases the social partner was assigned as the sire of at least one embryo. The final litter examined was sired entirely by a male who was not the social partner of the litter’s mother (Table 2). Thus, overall, three (27.3 %) of 11 litters contained young that were not sired by the mother’s social partner, with a total of three (11.5 %) of the embryos genotyped being sired by extra-pair males. This included litters from three of our four sampling localities, indicating that extra-pair paternity was not restricted to a single population. For each of the litters containing extra-pair young, both the mother’s social partner and the extra-pair sire of her offspring were captured during this study; in all cases the extra-pair sire was trapped within 300 m of the capture localities for the female and her social partner.
Table 2 Results of paternity assignment analyses for P. californicus. Data are based on microsatellite analyses (n = 9 loci) of 11 embryonic litters obtained from females whose social partner had been identified based on live capture and fluorescent marking data. For each litter, the identity of the mother and putative sire (female’s social partner) are indicated, as are the LOD score and delta score for all candidate sires identified by CERVUS. The results for each litter are summarized with respect to the number (multiple paternity, yes or no) and identities of sires (social partner or other male). Data are organized by study site to facilitate comparisons of results across the populations sampled.
| Site | Mother ID | Social partner ID | Offspring ID | Candidate sire ID | LOD score | Delta Score | Multiple paternity | Sire type |
|---|---|---|---|---|---|---|---|---|
| EOR | A | 1 | A1 | 1 | 4.18 | 2.59 | N | partner |
| A2 | 2.79 | 2.79 | ||||||
| B | 2 | B1 a | 2 | 3.46 | 3.46 | N | partner | |
| B2 | 6.82 | 6.82 | ||||||
| C | 3 | C1 | 3 | 2.55 | 2.55 | N | partner | |
| C2 | 3.00 | 3.00 | ||||||
| C3 | 3.66 | 3.66 | ||||||
| D† | 4 | D1 | 1 | 6.66 | 6.66 | Y | partner & other | |
| D2 | 4 | 5.81 | 1.47 | |||||
| D3 | 2 | 7.95 | 6.28 | |||||
| E | 5 | E1 | 5 | 5.65 | 5.65 | N | partner | |
| E2 | 6.44 | 6.44 | ||||||
| E3 | 7.82 | 7.82 | ||||||
| TPSNR | Fa | 6 | F1 | 6 | 5.10 | 5.10 | N | partner |
| F2 | 1.12 | 1.12 | ||||||
| G | 7 | G1 | 7 | 4.46 | 4.46 | N | partner | |
| G2 | 8.20 | 8.20 | ||||||
| G3 | 5.62-01 | 5.62-01 | ||||||
| H | 8 | H1 | 8 | 2.26 | 2.26 | N | partner | |
| HNHR | I† | 9 | I1 | 12 | 7.06 | 6.89 | Y | partner& other |
| I2 | 9 | 9.88 | 3.41 | |||||
| BCR | J | 10 a | J1 | 10 | 1.49 | 1.49 | N | partner |
| J2 | 1.49 | 1.49 | ||||||
| J3 | 4.29 | 4.29 | ||||||
| K† | 11 | K1 | 10 | 1.77 | 1.77 | N | other | |
| K2 | 1.77 | 1.77 |
a individuals typed at 8 out of 9 total loci.
†females with extra-pair paternity litters.
Discussion
Our analyses indicate that although P. californicus has been described as genetically monogamous (Ribble and Salvioni 1990; Ribble 1991), extra-pair paternity of young does occur. Despite our limited sample size, we detected extra-pair young in multiple litters, including a litter from HNHR, the site of the studies that led to the original description of P. californicus as genetically monogamous (Ribble and Salvioni 1990; Ribble 1991; Ribble and Stanley 1998). Overall, extra-pair young were detected at three of our sampling localities, indicating that this phenomenon was not population specific. The occurrence of extra-pair paternity in P. californicus, even at low frequency, raises intriguing questions regarding the factors contributing to both extra-pair mating and the maintenance of strong social male-female pair bonds in the absence of true genetic monogamy.
Variable rates of extra-pair paternity. Although we detected extra-pair paternity in several of the litters of P. californicus examined here, Ribble (1991) found no evidence of extra-pair young in the 28 litters of California mice that he analyzed. One factor that may have contributed to this apparent disparity in outcomes is the use of different molecular markers to determine parentage. Our analyses of paternity were based on microsatellite loci; in contrast, Ribble (1991) employed multi-locus fingerprinting of mini-satellite DNA regions. These markers differ with respect to multiple features, including the structure of the underlying genetic material, the associated rates of evolutionary change, and the molecular procedures used to detect variability (Flanagan and Jones 2019). Accordingly, it is possible that these markers differ in their ability to detect fine-scale genetic differences among individuals such as those typically used to determine paternity. In particular, given that microsatellite markers can detect single base pair differences in allele sizes, it is possible that these markers reveal more genotypic variation than traditional analyses of mini-satellite regions of DNA (Jones et al. 2010). Accordingly, use of microsatellite markers may have contributed to the discovery of extra-pair paternity in our data set but not in that of Ribble (1991).
At the same time, it is possible that the occurrence of extra-pair paternity is dynamic and varies temporally in response to changes in behavioral, ecological, and demographic conditions (Emlen and Oring 1977; Lambert et al. 2018). Each extra-pair sire identified during this study was resident near the female with which he produced offspring, suggesting that density- or resource-driven changes in home range size or overlap may influence access to non-partner females and thus the prevalence of extra-pair young (Westneat and Sherman1997; Mayer and Pasinelli 2013). Further, variation in adult sex ratios, in particular the occurrence of male-biased populations, may increase the probability of extra-pair encounters (Fromhage et al. 2005). Intra-specific variation in rates of extra-pair paternity has been reported for multiple species of socially monogamous birds (Griffith et al. 2008; Botero and Rubenstein 2012; Wan et al. 2013; Brouwer and Griffith 2019) and it seems reasonable to expect that similar variation occurs in mammalian species. Clearly, more extensive sampling - in particular sampling conducted over longer time periods - is required to assess potential temporal variation in the prevalence of extra-pair young.
Monogamy in Peromyscus. The genus Peromyscus contains at least two independent evolutionary origins of social monogamy. One consists of P. californicus and, potentially, its sister species, P. eremicus, both of which occur in the western US and México (Grinnell and Swarth 1913; Grinnell and Orr 1934; King 1968). The other is P. polionotus, which occurs in the southeastern US (King 1968; Foltz 1981). The occurrence of male-female pair bonds is well established in P. californicus and P. polionotus (Ribble 2003; Jašarević et al. 2013). In contrast, the characterization of P. eremicus as socially monogamous is more equivocal and is based on largely anecdotal information regarding spatial relationships among opposite-sex individuals (Wolff 1989; Kalcounis-Rueppell and Ribble 2007). No analyses of parentage have been conducted for P. eremicus and thus the genetic mating system of this species remains unknown. Based on allozyme analyses, P. polionotus has been described as ‘overwhelmingly monogamous,’ with an estimated frequency of extra-pair paternity of ~ 12 % of offspring (Foltz 1981). The frequency of extra-pair paternity in our dataset was similar, again with ~ 12 % of offspring sired by extra-pair males. Although a larger sample size for P. californicus is desirable, available data suggest that this species and P. polionotus are similar with respect to degree of genetic monogamy.
The occurrence of two convergent examples of monogamy within Peromyscus suggests that comparative studies of these species may offer important insights into the factors favoring this mating system. At the same time, comparisons between monogamous and closely related but polygamous or polygynandrous species of Peromyscus provide opportunities to explore the factors associated with the evolution of divergent mating systems. Mating systems theory predicts that monogamy will occur when individual males are unable to monopolize access to more than one potential mate, typically due to either the spatial distribution of females or the need for biparental care to ensure offspring survival (Emlen and Oring 1977; Clutton-Brock 1989; Lukas and Clutton-Brock 2012, 2013). Because this conceptual framework views monogamy as a default strategy that males are forced to adopt under certain ecological, demographic, or life history conditions, it seems reasonable to expect that monogamous animals will pursue extra-pair copulations when such opportunities arise. Future studies that compare P. californicus to both socially monogamous and polygynandrous congeners should prove particularly informative regarding the correlates of extra-pair mating and, hence, the adaptive bases for the maintenance of male-female pair bonds in the absence of true genetic monogamy.











 nueva página del texto (beta)
nueva página del texto (beta)


