Introduction
The coexistence of potentially competing species is one of the central topics in ecology and biogeography. It plays a major role in the dynamics of species populations, can profoundly influence the evolution of the ecological traits of interacting species as well as the evolution of species, and also influences underlying ecosystem processes (Broennimann et al. 2012; Penteriani et al. 2013; Nagy-Reis et al. 2019; Marinho et al. 2020; Segar et al. 2020). For instance, in a community in which closely related species coexist, allopatric mechanisms and some forms of interference (e. g., intraguild predation) are ecological forces with highly relevant implications in structuring communities, with potential effects on the ecology and evolution of the species involved (Rosenzweig 1966; Holt and Polis 1997; Palomares and Caro 1999; de Oliveira and Pereira 2014; Donadio and Buskirk 2006; Hunter and Caro 2008; Arias- Alzate et al. 2022). However, other mechanisms that facilitate the coexistence of potentially competing species remain poorly understood (Godsoe et al. 2015; Arias- Alzate et al. 2022).
It could be expected that, at the assemblages level (within the same geographic space), from the competitive exclusion standpoint, the species could coexist if they have distinctive morphological and behavioral adaptations, such as differences in size and diet, changes in habitat use or segregation at spatial or temporal levels (i. e., character segregation or niche differentiation; Case and Gilpin 1974; Schoener 1974a; Chesson and Grubb 1990; Hutchinson 1978; Hunter and Caro 2008; Penido et al. 2017; Porto et al. 2021; Arias- Alzate et al. 2022). This aspect is an essential part of the ecology of organisms since it provides key information; for example, the use of time directly influences how species interact with the environment, maximize the search for food, seek mating, avoid the risk of predation, and how they continuously cope with the regular changes in the ecosystem (Schoener 1974b; Kronfeld-Schor and Dayan 2003; Laundre et al. 2010; Pratas-Santiago et al. 2016; Karanth et al. 2017; Penido et al. 2017; Marinho et al. 2020; Vilella et al. 2020; Peral et al. 2022). Consequently, the daily activity pattern reflects the evolutionary and physiological adaptations that allow species to increase their adequacy while facilitating coexistence (Schoener 1974b; Laundre et al. 2010; Tambling et al. 2015: Pratas-Santiago et al. 2016).
The order Carnivora is one of the most important groups in ecological dynamics. They play a central role in ecosystems through a top-down effect that regulates the populations of their prey, either directly by predation or because of the fear they can produce in the ecosystem (i. e., landscapes of fear; Treves and Karanth 2003; Roemer et al. 2009). However, it is one of the most sensitive groups to environmental imbalance, generated mainly by an anthropic effect (Crooks 2002). This group includes a great diversity of species in terms of morphology and function; most are small to medium-sized species (i.e., less than 15 kg), commonly known as mesocarnivores (Roemer et al. 2009; Bu et al. 2016; Easter et al. 2020; Marinho et al. 2020).
These species form a diverse group as regards their ecology and behavior; they can be solitary or highly social, with varied diets, and their trophic level is generally just below the top predators (Marinho et al. 2020). In general, it has been suggested that mesocarnivores are vitally important in ecosystem dynamics, facilitating nutrient flows between adjacent ecosystems and maintaining the populations of their potential prey in a state of equilibrium (Hunter and Caro 2008; Roemer et al. 2009; Penido et al. 2017; Marinho et al. 2020). In addition, unlike larger carnivores, these species can fulfill unique roles, such as direct or secondary seed dispersal (Hunter and Caro 2008; Roemer et al. 2009). Therefore, they play a major role in shaping ecological communities and, therefore, in local and regional ecosystem processes (Hunter and Caro 2008; Roemer et al. 2009). For these species, activity patterns may be partly determined by the climate, habitat structure and preference, and anthropogenic influences. On the other hand, these patterns are also driven by interspecific interactions, especially with larger dominant species, where the risk of predation plays a central role and can strongly influence the spatial distribution of resources and the foraging behavior of these species (Crooks and Soule 1999; Bu et al. 2016; Ramírez-Mejía and Sánchez 2016; Penido et al. 2017; Mpemba et al. 2019; Easter et al. 2020; Mendes et al. 2020). To this end, mesocarnivores must adjust their behaviors and activities in the face of the pressures exerted on these ecosystems while maximizing the search for food and reducing predation risks and antagonistic encounters with other species (Amarasekare 2002; Glen and Dickman 2005; Donadio and Buskirk 2006; Mpemba et al. 2019).
However, at a global level, many aspects of the natural history and ecology of mesocarnivores are still unknown, as they are one of the least studied groups about which there are huge gaps in information in the Neotropics (Andrade Ponce et al. 2016). For many of them, only their presence in certain regions is known (Andrade Ponce et al. 2016); however, little is known about how they interact with each other, their spatial or temporal patterns, and the segregation between them (Easter et al. 2020). For example, few studies have investigated the activity patterns of these species in Colombia (González-Maya et al. 2015; Cáceres-Martínez et al. 2016; Ramírez-Mejía and Sánchez 2016; García-R et al. 2019), with limited estimates in the Cordillera Central of Colombia (Delgado-V et al. 2011; Ramírez-Mejía and Sánchez 2016). In this region, there are still large information gaps about the ecological dynamics in the group, especially in relevant conservation areas. In the Aburrá Valley, 15 of the 34 carnivorous species registered for Colombia are currently recognized (Ramírez-Chaves et al. 2016; Arias-Alzate et al. 2021), accounting for 44.1 % of all species of the order Carnivora in Antioquía (Calle and Arango 2003). Among these species, mesocarnivores are one of the most affected groups in the region, mainly due to habitat fragmentation and roadkills (Delgado-V 2007; 2014; Arias-Alzate et al. 2015).
This work evaluated the daily activity patterns of mesocarnivore species in the southeast part of the Aburrá Valley (hereafter southeast Aburrá Valley) using non-invasive methods (i. e., tracking cameras). Particularly, we addressed the following questions: What are the activity patterns of the mesocarnivore species inhabiting the southeast Aburrá Valley? What is the degree of segregation in the temporal niche between these species? How does the lunar cycle influence these activity patterns? In functional terms, how similar are the mesocarnivore species living in the area? We hypothesized that mesocarnivores with similar functional characteristics exhibit greater temporal niche segregation, showing a low overlap in their activity patterns to reduce competition. A deeper knowledge of these ecological and natural history aspects of mesocarnivores will support better management and conservation strategies for these ecosystems immersed in landscapes where anthropic activity and urbanization are growing phenomena.
Materials and methods
Study Area. The study was conducted between the San Nicolás Valley and the southeast Aburrá Valley in the south-central department of Antioquia, north of the Central Cordillera of the Colombian Andes. This area harbors an ecosystem of cloud mountain forest or high Andean forest, corresponding to the Tropical Montane Humid Forest life zone (bmh-M, sensu Holdridge 1947). The area comprises mature secondary forests with tree ferns (Cythea arborea) and Chusquea spp. It stretches across an altitudinal range between 2,100 and 3,050 masl, with a mean annual temperature of 22 °C and precipitation between 1,400 and 3,000 mm (Hermelin 2007). The study area mainly included the municipalities of Envigado and its boundaries with the municipalities of Sabaneta, Caldas, Medellín, and El Retiro (Figure 1).
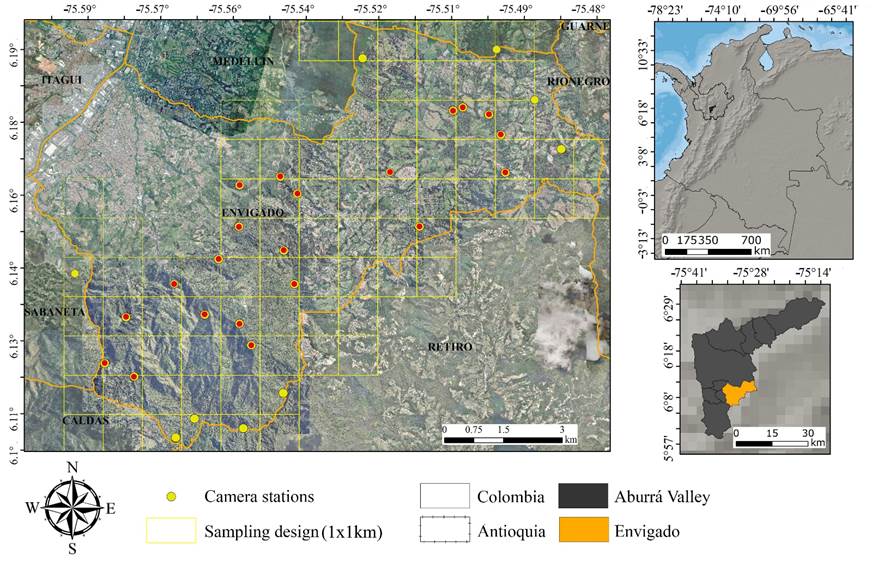
Figure 1 Sampling design for the tracking camera’s locations in the study area. A central red dot marks the same stations sampled between 2015 and 2018.
Sampling Design. Sampling records were obtained by installing tracking cameras (Bushnell Agressor Red-glow and Bushnell Trophy Cam Essential) in forest areas across the study area. For camera installation, the study area was divided into 1 km x 1 km (1 km2) quadrants, of which 30 sampling quadrants were selected randomly using the Repeating Shapes and Sampling Tools for ArcGIS 10.5 (ESRI 2018). At least 20 of these stations were sampled simultaneously with one camera per station, as follows: Twenty cameras were used from October to December 2015, 28 from August to November 2016, 20 from July to October 2017, seven from January to February 2018, 20 from April to August 2018, and six from December 2019 to December 2020. These monitoring stations were separated from one another by a minimum average distance of 850 m ± 630 m (Figure 1). Each monitoring station operated over a continuous 24-hour sampling period. For installing these cameras, we searched tracks or signs of the passage of species through existing trails or paths, which are frequently used by wildlife (especially felines, procyonids, and canids), to maximize the probability of capturing species (Navarro-Pelaez et al. 2021; Chávez et al. 2013; Marinho et al. 2018a). The cameras were set in video mode and affixed approximately 20 cm to 30 cm above the ground, with a slight downward-facing inclination and with the following settings: high HD-resolution videos, infrared auto-sensor for day and night, a 40-second video per event, and a 5-minute interval between videos.
Activity Patterns, Lunar Phase, and Temporal Niche Overlap. First, with the circular statistics program Oriana version 4.02 (Kovach 2011), we performed the Rayleigh test (95 % confidence level) to assess whether the species exhibit a regular daily activity pattern over a 24-hour period (Kovach 2011). Afterward, the daily activity patterns of the species were estimated, and their degree of overlap (i. e., temporal niche segregation) was assessed with the Overlap package (Ridout and Linkie 2009; Linkie and Ridout 2011; Meredith and Ridout 2018) in R (R Development Core Team 2013). Here, the probability density function of records was evaluated non-parametrically with the Kernel Density (DK) Estimation and the non-negative trigonometric sum (STN) distribution function. These distributions consider each record as a random sample of an underlying continuous distribution (Fernández-Durán 2004; Ridout and Linkie 2009), where individuals of any species have the same probability of being recorded at any time as long as they are active (Linkie and Ridout 2011; Pratas-Santiago et al. 2016). In this way, the bias of grouping the records in arbitrarily defined intervals is eliminated to determine the type of activity (Pratas-Santiago et al. 2016). To note, we did not consider a measure of independence between records since, according to De Solla et al. (1999), Blundell et al. (2001), and, recently, Peral et al. (2022), the independence assumption (i. e., eliminating the autocorrelation) substantially limits and skews the results, as it does not reflect the continuous activity state. By contrast, considering all data maximizes the performance and accuracy of estimates, for example, in activity pattern analyses.
It is worth mentioning that the tropical zone near the equator is characterized by little variation in the time of sunrise and sunset throughout the year. This situation makes the clock time to approach the solar time (Rowcliffe et al. 2014). Therefore, the records obtained were standardized at solar time. Subsequently, these were converted to radians (2π = 24 hrs) before performing the analyses with the Overlap package and following the proposals of Ridout and Linkie (2009), Linkie and Ridout (2011), and Vilella et al. (2020). Consecutively in Overlap, also following the proposal of Ridout and Linkie (2009) and Linkie and Ridout (2011), the daily activity pattern was estimated by splitting the 24-hr period into 4-time intervals, as follows: 00:00-6:00; 6:00-12:00; 12:00-18:00 and 18:00-24:00. In addition, given the little variation between sunrise and sunset times, the sunrise and sunset time are adjusted to π/2 and 3π/2 (Ridout and Linkie 2009; Bu et al. 2016). This allows for classifying the cycle into daytime (the hours elapsed from sunrise to sunset) and nighttime periods (the remaining time), considering sunrise as 06:00 hrs and sunset as 18:00 hrs, respectively (Bu et al. 2016; Ridout and Linkie 2009, Meredith and Ridout 2018, Porfirio et al. 2016; Rowcliffe et al. 2014). Twilight can be considered as the time elapsed one hour before sunrise (i. e., dawn) and one hour after sunset (i. e., dusk; Porfirio et al. 2016), corresponding specifically to the astronomical twilight for the study area (https://www.timeanddate.com/).
In this way, it is possible to identify the activity of species according to record density (e. g., Kernel density) identified at the time intervals, classified as diurnal (≥90 % of records during the day), nocturnal (≥90 % during the night), mainly diurnal (~70 % - 89 % during the day), and mainly nocturnal (70 % - 89 % during the night; Azevedo et al. 2018; Porfirio et al. 2016; Ridout and Linkie 2009). Each daily activity pattern by species was determined using a concentration parameter K = 1.5 for the DK function according to Meredith and Ridout (2018); for the STN function, it was identified based on the number of parameters according to the function implemented in Overlap (Ridout and Linkie 2009).
We calculated the overlap between pairs of species (i. e., probability density functions sp. 1: f (x) and sp. 2: g (x) using the overlap coefficient Δ, which estimates the degree of overlap between distributions, i. e., the magnitude of the difference between activity patterns (Linkie and Ridout 2011). This coefficient takes values between 0 (no overlap, i. e., different) and 1 (full overlap, i. e., identical; Ridout and Linkie 2009; Linkie and Ridout 2011). For the present analysis, 95 % confidence intervals were estimated through 1000 smoothed Bootstrap replicates,where values are taken from the estimated density function instead of using only those observed (Linkie and Ridout 2011; Meredith and Ridout 2018). For further mathematical and methodological details, please refer to Ridout and Linkie (2009) and Meredith and Ridout (2018). Last, we used the overlap coefficient Δ1 for small samples (<50 records) and Δ4 for samples greater than 50 records (Ridout and Linkie 2009). To avoid subjective interpretations of the overlap in activity periods between species, we used the classification proposed by Monterroso et al. (2014): low overlap (Δ ≤ 0.5), moderate overlap (0.5 < Δ ≤ 0.75), and high overlap (Δ > 0.75).
The effect of the lunar phase and light intensity on activity patterns was explored. To this end, the lunar phase was assigned to each species record that showed a mainly nocturnal activity pattern according to the abovementioned analyses. Subsequently, whether there is a uniform distribution related to the lunar phases was evaluated using the Rayleigh test (at a 95 % confidence level) in the circular statistics program Oriana version 4.02 (Kovach 2011). These data were treated as circular data (i. e., scaled to radians) based on the date of each record. These dates are converted to angular data in Oriana by calculating the duration of the lunar month and the day of the lunar cycle according to the time zone of the study area (i. e., GMT-5), where, for example, day one represents the first day of the new moon with 0 % luminosity, so that zero corresponds to the new moon, π/2 to the first quarter, π to the full moon and 3π/2 to the last quarter (Kovach 2011, Pratas-Santiago et al. 2016).
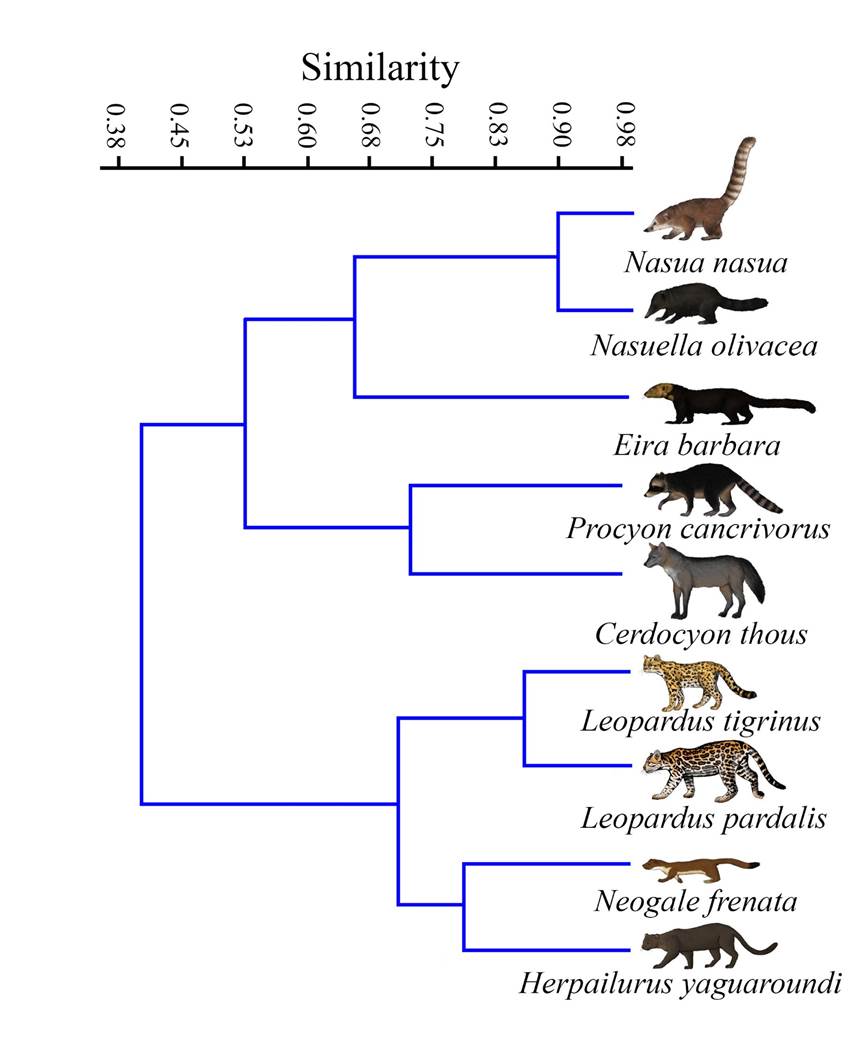
Functional Similarity Analysis. The similarity between the nine mesocarnivore species in the study area was evaluated using their ecological traits and constructing a binary database with the following structure: size, general activity pattern, trophic realm, trophic guild, habit, and social structure (Appendix 1). This information was obtained from previous works (Jones et al. 2009; Wilson and Mittermeier 2009; González-Maya et al. 2016, 2017; Arias-Alzate et al. 2020). These six ecological traits have been identified as major drivers in ecosystem functioning and as important predictors of the competitive capacity between species (de Oliveira and Pereira 20142014; Fergnani and Ruggiero 2015; González-Maya et al. 2016; Arias-Alzate et al. 2020). Subsequently, the level of similarity between species was determined through a similarity cluster analysis using the Jaccard index. This index takes values between 0 and 1, where zero indicates a greater distance - and therefore a greater differentiation between the species - while values closer to one denote a smaller distance and, therefore, greater similarity. This analysis was performed in the statistical software PAST (Hammer et al. 2001).
Results
We obtained a total of 469 records belonging to nine mesocarnivore species with a sampling effort of 10,744 cameras/night (1,118 cameras/night in 2015; 2,852 in 2016, 1,394 in 2017, 1,099 in 2018, 2.211 in 2019, and 2,070 in 2020). Due to the low number of records obtained for three species (n = 1 for Procyon cancrivorus, n = 2 for Puma yaguaroundi, n = 2 and Leopardus pardalis, n = 4), these were excluded from the daily activity pattern analysis. Thus, a final total of 461 records were considered, corresponding to six species (Table 1). The species with the highest number of records was Cerdocyon thous (n = 286), followed by Eira barbara (n = 76). The species with the lowest number of records were Nasua nasua (n = 21) and Neogale frenata (n = 21; Table 1).
Activity Patterns, Temporal Niche Overlap and Lunar Phase. The activity patterns of the species were not uniform throughout the circadian cycle (Table 1). The crab-eating fox (Cerdocyo thous), the northern tiger cat (Leopardus tigrinus), and the western mountain coati ( Nasuella olivacea) show mainly nocturnal activity (Figure 2). The crab-eating fox had two peaks of main activity, before dawn at 06:00 hrs and after sunset at 18:00 hrs, with decreased activity near midnight and slight activity at noon (Figure 2). The northern tiger cat starts its activity after sunset, with a slight decrease toward midnight and increased activity before dawn (Figure 2). In the case of the western mountain coati, its activity shows two main peaks, one well-marked starting after sunset (18:00 hrs) with an increase toward midnight, followed by a second, much milder peak before dawn (06:00 hrs). This species also shows a slight activity around noon, recorded only under a highway bridge (Via El Escobero; Figure 2).
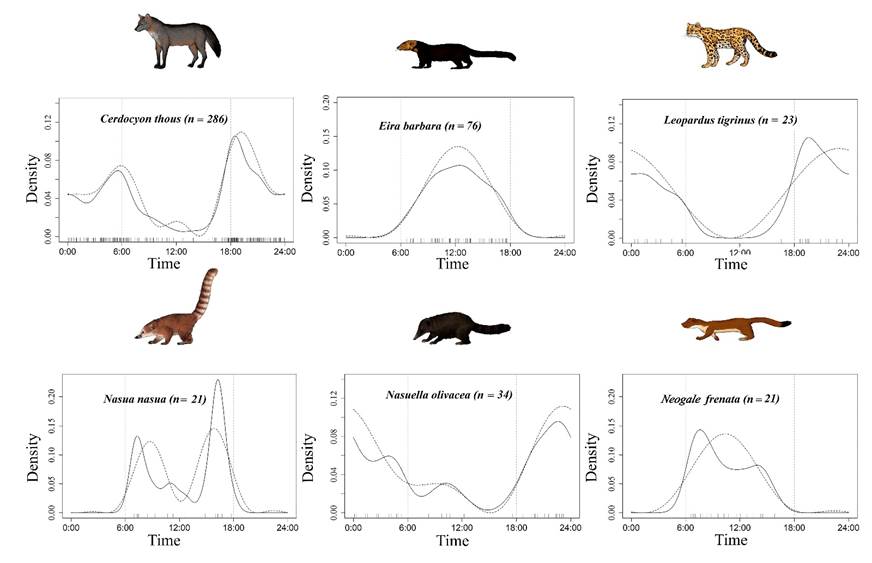
Figure 2 Daily activity patterns of mesocarnivores in the southeast Aburrá Valley. Records are marked as small vertical bars on the time axis. The dotted and solid lines represent estimates by non-negative trigonometric sum (STN) and Kernel density (DK), respectively. Vertical dotted lines mark the dawn (end of astronomical twilight) and dusk (beginning of astronomical twilight).
Tayra (Eira barbara), ring-tailed coati (Nasua nasua), and long-tailed weasel (Neogale frenata) exhibit a mainly diurnal activity pattern (Figure 2). The tayra begins its activity after dawn, with a peak toward noon, followed by a decrease before dusk (Figure 2). The ring-tailed coati has only two marked peaks of activity: one starting after dawn with a descent in activity near noon and a second peak around 14:00 hrs that decreases before dusk (Figure 2). The long-tailed weasel has a marked peak of activity after dawn (06:00 hrs) with a decrease toward noon (12:00 hrs), followed by a slight increase in the early afternoon hours (~14:00 hrs) and decreasing before dusk (Figure 2).
As regards the influence of the lunar phase, only the northern tiger cat shows significant activity around the crescent moon that decreases toward the new moon. The other two species (crab-eating fox and western mountain coati) show no variations in activity throughout the lunar phases (Table 1).
Table 1 Rayleigh test of uniformity for the six mesocarnivores activities and the lunar phase for mainly nocturnal species. n : Total number of records, * significant values at 95 % ( P < 0.05).
| Species | n | P | Lunar phase | P |
|---|---|---|---|---|
| Cerdocyon thous | 286 | 1.5E-11* | Crescent moon | 0.118 |
| Eira barbara | 76 | 1E-12* | ||
| Leopardus tigrinus | 23 | 0.000179* | Waning crescent-new moon | 0.000461* |
| Nasua nasua | 21 | 0.002* | ||
| Nasuella olivacea | 34 | 0.000713* | Waning half | 0.091 |
| Neogale frenata | 21 | 2.84E-6* |
We observed a variable overlap of activity patterns between pairs of mesocarnivore species, with a tendency to a low overlap, as in the case of C. thous and E. barbara (Figure 3). The greatest overlap levels were recorded between L. tigrinus and C. thous, and between L. tigrinus and N. olivacea (Figure 4). Moderate overlap levels were observed between E. barbara and Nasua nasua, E. barbara and N. frenata, N. nasua and N. frenata, and N. olivacea and C. thous (Figure 3).
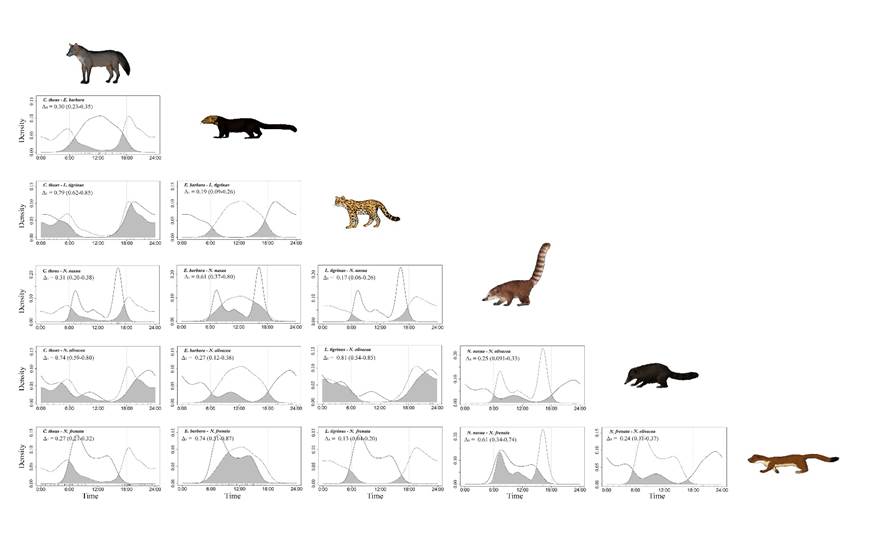
Figure 3 Daily activity patterns overlap between pairs of mesocarnivores species. The gray shaded area represents the overlap level. Δ1 and Δ4 represent the overlap coefficients; 95% confidence intervals are shown in parentheses. Low overlap level (Δ ≤ 0.5), moderate overlap (0.5 < Δ ≤ 0.75), and high overlap (Δ > 0.75). The dashed and solid lines represent the first and second species, respectively. Vertical dotted lines mark the dawn (end of astronomical twilight) and dusk (beginning of astronomical twilight).
Functional Similarity Analysis. The similarity analysis shows two main clusters. The first shows a high similarity between the coaties, followed by their similarity with the tayra. These three species were grouped with the crab-eating fox and the raccoon. Together, these five species show an intermediate degree of ecological similarity (Figure 4). The second cluster shows a high similarity between the tiger cat (~88 %), which is grouped with the yaguarundi and the long-tailed weasel at a similarity of around 70 %. These two main groups show a similarity of about 40 %; that is, they show important segregation in terms of their ecological traits (Figure 4).
Discussion
The species activity patterns may be partly determined by the climate, habitat structure and preference, and by interspecific interactions, where, for example, intra-guild predation plays a central role in the coexistence of potentially competing species (Ramírez-Mejía and Sánchez 2016; Mpemba et al. 2019; Easter et al. 2020). However, in landscapes surrounded by an important anthropic activity, these dynamics can be affected, disrupting how the different mesocarnivorous groups interact with each other (Easter et al. 2020; Mendes et al. 2020). In this sense, the low number of records for some species despite the great sampling effort may be associated with their cryptic nature, a low abundance associated with low ecological tolerance to human activity, or poor detectability of cameras located in wooded areas (Boron et al. 2019; García-R et al. 2019). However, although this study did not directly assess how development and anthropic disturbance can affect mesocarnivores activity and their interactions with each other, it is evident that the development processes have significantly fragmented and reduced the original extent of the habitat for these species, which probably influenced the activity patterns recorded here. For example, different works (Treves and Karanth 2003; Gaynor et al. 2018; Van Cleave et al. 2018; Wang et al. 2019) state that the activity of carnivores may vary to reduce the risk of conflict with humans in areas subjected to heavy anthropic pressure.
In general, the observed activity patterns are similar to those reported in other studies throughout the distribution ranges of these species (e. g., Sheffield and Thomas 1997; Tortato and Oliveira 2005; Faria-Corrêa et al. 2009; Delgado-V et al. 2011; Oliveira-Santos et al. 2012; González-Maya et al. 2015; Cáceres-Martínez et al. 2016; Ramírez-Mejía and Sánchez 2016; Penido et al. 2017; Marinho et al. 2018b; Dias et al. 2019; Mena y Yagui 2019; Nagy-Reis et al. 2019; Marinho et al. 2020; Villafañe-Trujillo et al. 2021). However, some important aspects are observed, likely associated with adjustments in the behavior, foraging, reduced risk of predation, and antagonistic encounters with other species (Penido et al. 2017; Mpemba et al. 2019). For instance, although E. barbara, N. frenata, and Nasua nasua show diurnal patterns, as suggested (Sheffield and Thomas 1997; Delgado-V et al. 2011; González-Maya et al. 2015; Cáceres-Martínez et al. 2016; Ramírez-Mejía and Sánchez 2016; Mena and Yagui 2019; Villafañe-Trujillo et al. 2021), the first two species show a single peak of activity, while N. nasua showed two peaks of activity not previously reported. It is worth noting that Sheffield and Thomas (1997) mention that the large-tailed weasel is active both day and night, contrasting with our observations in the present study. This is likely due to the low number of records, low abundance, lower nighttime activity given the weather conditions in the area, or limited camera detectability. However, the latter is less likely in our study site since we have recorded (at night) smaller mammal species, such as rodents, marsupials, and shrews with the same type of cameras.
With regard to species with a nocturnal activity pattern, although N. olivacea has been reported as a mainly nocturnal species (Cáceres-Martínez et al. 2016; Ramírez-Mejía and Sánchez 2016; Mena and Yagui 2019), in our study area, the species showed a small peak of activity in the morning hours before noon. These daytime records correspond to activity under a highway bridge, where low light intensity predominates. This could be related to a sense of safety and low predation risk for these individuals, which has important spatial and temporal implications for the species (Easter et al. 2020). However, Rodríguez-Bolaños et al. (2003) suggested a purely diurnal activity for N. olivacea from telemetry data of a single male individual marked in a high Andean forest. The difference in daily activity of the same species is likely due to the reduction of independent records, which may be affecting the effectiveness of activity estimations, for example, by eliminating intermediate or nighttime records (De Solla et al. 1999; Blundell et al. 2001; Peral et al. 2022).
On the other hand, the activity of C. thous partly agrees with reports for other regions throughout its range (Penido et al. 2017; Marinho et al. 2020). Here, north of the Andes, we observed a mainly nocturnal activity, with a slight daytime activity. This contrasts with the findings for the Atlantic Forest and the Catinga in Brazil, where the species is sympatric with other canids of similar size and shows a trend toward being nocturnal, while the other species (Lycalopex gymnocercus and L. vetulus) display a trend toward diurnal habits (Faria-Corrêa et al. 2009; Dias and Bocchiglieri 2016; Penido et al. 2017; Marinho et al. 2020). In this sense, sympatry can occur if species of similar size differ in one or more ecological traits, for example, in time activity or the primary food resources used (Easter et al. 2020; Godsoe et al. 2015, Arias- Alzate et al. 2022). Thus, similar, and potentially competitive sympatric species, such as mesocarnivores, should be segregated according to other ecological niche traits to avoid competition (i. e., competitive exclusion), producing more diffuse patterns of interspecific interaction (Chesson 2000; Arias- Alzate et al. 2022).
Regarding felids, although three species of small felines were recorded, only L. tigrinus had a sufficient number of records. The activity of this species is similar to that reported for Cerrado and the Catinga in Brazil, with nocturnal activity after sunset and before sunrise (Tortato and Oliveira 2005; Oliveira-Santos et al. 2012; Penido et al. 2017; Marinho et al. 2018b; Dias et al. 2019; Nagy-Reis et al. 2019; Marinho et al. 2020). However, these authors also report some daytime activity and suggest that this occurs when the species is found in sympatry with other felines. However, this feline inhabiting these two zones has been proposed as two new species (Leopardus guttulus and L. emilie; Do Nascimento and Feijo 2017). The only areas of Colombia with records of daytime activity for L. tigrinus are heavily fragmented areas disturbed by anthropic activities, where some individuals have been roadkilled (Arias-Alzate pers. obs.).
Three other species of felines (Leopardus pardalis, Puma concolor, and H. jaguaroundi) inhabit our study area; although we did not estimate their activity patterns due to the low number of records, they were recorded both at night and during the day. According to observations on these species from camera traps in unpublished information and literature reportes (e. g.,Ramírez-Mejía and Sánchez 2016; Penido et al. 2017; Massara et al. 2018; Marinho et al. 2018b; Dias et al. 2019; Nagy-Reis et al. 2019; Marinho et al. 2020), we hypothesize that there is a temporal segregation between these species, mainly between L. tigrinus and H. yaguaroundi, given their similar sizes and feeding habits (i. e., hypercarnivores). By contrast, the coexistence between the ocelot and the puma (a species recorded in the Aburrá Valley 10 years ago, Arias-Alzate et al. 2016) would be mediated by size differentiation and spatial segregation at the local level to avoid inter-guild predation to a large extent (i. e., fear landscapes created by larger predators; Treves and Karanth 2003; de Oliveira and Pereira 2014; Wang et al. 2019; Arias- Alzate et al. 2022; Zaman et al. 2022). Thus, the segregation of felines according to functional traits would be a driver of sympatry in these species (Penido et al. 2017; Massara et al. 2018; Marinho et al. 2018b; Dias et al. 2019; Nagy-Reis et al. 2019; Marinho et al. 2020; Arias- Alzate et al. 2022).
It should be noted that we found a significant trend in the activity of L. tigrinus during the lunar phase waning toward the new moon in the high Andean forests of the study area. This may be a strategy to avoid intra-guild predation when foraging in forested areas while maximizing energy gain by synchronizing their activity pattern with that of their potential prey, as proposed for other carnivorous species (Harmsen et al. 2011; Foster et al. 2013). However, Marinho et al. (2018a), in a study in the Catinga (lowlands) in Brazil, found no direct influence of the lunar cycle on this species, which, as mentioned above, has been proposed as a different species (Do Nascimento and Feijo 2017).
These activity patterns and the different trends in the overlap between these species seemingly result from the segregation of their functional ecological attributes (i. e., functional niche) to reduce interspecific competition, the predation risk, and, therefore, facilitate coexistence, as suggested in other works (Nagy-Reis et al. 2019; Marinho et al. 2020, Arias- Alzate et al. 2022). This segregation would be mediated mainly by size, i. e., species of similar size would become segregated in one or more functional attributes (Arias- Alzate et al. 2022).
Thus, time segregation appears as an additional mechanism to reduce and avoid interference competition between ecologically similar and potentially competing species (Monterroso et al. 2014; Massara et al. 2018; Nagy-Reis et al. 2019; Marinho et al. 2020; Arias- Alzate et al. 2022).
This hypothesis would be consistent with our findings, as more than 86 % of the pairs of species studied exhibited a low or moderate activity overlap. Only a few pairs of species showed a high overlap but displayed additional niche segregation, suggesting more diffuse interactions between these species (Chesson 2000, Arias- Alzate et al. 2022). For example, although N. nasua and N. olivacea showed a similar food pattern, they differ in size and are segregated in their temporal niche, as observed in areas where the two species are sympatric, mainly in high areas (Ramírez-Mejía and Sánchez 2016; Mena and Yagui 2019). Likewise, species with intermediate (i. e., E. barbara and N. frenata) and high (i. e., C. thous, L. tigrinus, and N. olivacea) temporal overlap show segregation based on their traits, particularly size and dietary preferences (Dias and Bocchiglieri 2016; Ramírez-Mejía and Sánchez 2016; Mena and Yagui 2019; Marinho et al. 2020). This suggests that resource partitioning would also be recognized as another mechanism that facilitates coexistence (Hearn et al. 2018; Nagy-Reis et al. 2019). For instance, Bubadué et al. (2016) noted that when in sympatry with other canids from the south of the continent (e.g., Lycalopex vetulus and L. gymnocercus), C. thous tends to be far more carnivorous, unlike individuals of the same species inhabiting the north of the continent.
Although this study did not directly assess how development and anthropic disturbance can affect mesocarnivore activity and their interaction in peri-urban ecosystems, it is clear that development processes have caused a reduction of the original habitat of these species. In this sense, the low number of records obtained for some species may be associated not only with poor detectability but also with low abundance as a consequence of these anthropic pressures. To note, abundance would also be declining due to deaths from collisions with motor cars on roads adjacent to these ecosystems, where a large number of individuals of these species have been found (e. g., more than 14 individuals of northern tiger cat over the past ten years; Delgado-V 2007, 2014; Arias-Alzate et al. 2014, 2016).
Our results suggest that mesocarnivore species inhabiting the Aburrá Valley show variable temporal segregation, with a tendency to a low overlap, which would help mediate interactions and coexistence between these species. These results contribute to the knowledge about the ecology of these species and the interactions between them, mainly in ecosystems surrounding large cities north of the Andes. It is important to highlight the need to implement conservation strategies to generate tools and management strategies for preserving these ecosystems by identifying priority conservation areas or restructuring poorly functional protected ones. This would greatly favor the connection between populations of these and other species, both across the Aburrá Valley and at the regional level, as well as the conservation of ecological and evolutionary processes and, hence, of the environmental services of the territory.











 nueva página del texto (beta)
nueva página del texto (beta)