Introduction
Soursop fruit (Annona muricata L.) (Magnoliales) is one of the most important species of the Annona genus (Jiménez et al., 2016). In Mexico, the annual production of soursop fruits is 28,853 tons grew on a surface of 3,527 ha, where the state of Nayarit is the main producer, concentrating 71.7 % of the national production on 2,529 ha (SIAP, 2017). However, the production of soursop fruit is affected by insect pest such as hibiscus mealybug (Maconellicoccus hirsutus Green) (Hemiptera: Pseudococcidae), Annonaceae weevil (Optatus palmaris Pascoe) (Coleoptera: Curculionidae) the seed borer wasp (Bephratelloides maculicollis Cameron and B. cubensis Ashmead) (Hymenoptera: Eurytomidae) and Euphoria leucographa (Gory & Percheron) (Coleoptera: Melolonthidae) (Coto & Saunders, 2001; Hernández et al., 2013; Cambero et al., 2017). On the other hand, plant diseases are one of the most important issue in agriculture, since it may generate losses of up to 100 % of the production (Agrios, 1998). Soursop fruit can be affected by radicular rot (Rosellinia sp. and Phytophthora sp.) and stains on fruits by Phytophthora sp. (Baraona & Sancho, 1992), as well as fruits putrefaction by Rhizopus stolonifer (Ehrenb.) Vuill., Aspergillus niger P.E.L. van Tieghem, Penicillium sp. (Okigbo & Obire, 2009), Colletotrichum gloeosporioides (Penz) Penz. and Sacc., C. acutatum and Fusarium chlamydosporum Wollenweber and Reinking (Alberto & Otanes, 2016). Only few studied have been performed in Mexico for the diagnostic of diseases in this fruit. Nevertheless the association of fungi C. gloeosporioides and Lasiodiplodia theobromae (Patouillard) Griffon and Maublanc has been reported in Nayarit with dry and soft rots, respectively (Hernández et al., 2013) without any identification until now of the causal agent of each type of rot. The study of the diagnosis of causal agents involved in soursop fruit rot turns out to be of great economic impact due to losses incurred by fruit production, in terms of volume and quality, since generating knowledge on diseases causing pathogens in fruits is the first condition required to be able to establish strategies for controlling rot in further research. In this sense, the aim of the current study was to identify rot causing pathogens using molecular approaches in soursop fruit in San Blas and Compostela municipalities, Nayarit, Mexico.
Materials and Methods
Area of study and biological material sampling
Sampling was performed in a total of 10 commercial orchards located in Compostela and San Blas municipalities, Nayarit (Table 1), from which different orchards were visited twice monthly from January to November 2017, where soursop fruits were sampled with different physiological maturation levels presenting symptoms of dry rot (96 fruits) and soft rot (23 fruits). Rotten fruits were placed individually in paper bags and were transferred at room temperature to the Plant Parasitology Laboratory of the Multidisciplinary Center of Cientific Research (CEMIC 03) of the Autonomous University of Nayarit (UAN), where they were stored at 0°C until analysis.
Table 1 Soursop orchards sampled in Nayarit, Mexico, 2017.
| Municipality | Orchad | Coordinates | Altitude |
|---|---|---|---|
| Compostela | Chacala I | N 21°09’23” W 105°13’22” | 90 |
| Chacala II | N 21°010’26” W 105°10’59” | 39 | |
| Chacala III | N 21°09’52” W 105°12’07” | 96 | |
| Tonino I | N 21°04’05” W 105°12’51” | 335 | |
| Tonino II | N 21°02’45” W 105°11’08” | 217 | |
| Tonino III | N 21°03’29” W 105°11’08” | 80 | |
| San Blas | Tecuitata I | N 21°26’50” W 105°09’56” | 381 |
| Tecuitata II | N 21°27’38” W 105°09’19” | 382 | |
| Palmas I | N 21°31’50” W 105°10’08” | 183 | |
| Palmas II | N 21°32’04” W 105°10’33” | 242 |
Isolating and purifying microorganisms associated to fruit rots
Fruits presenting symptoms of dry and soft rots were previously washed with tap water, then pieces of 5 to 10 mm2 were cut in the transition zone (margin of the lesion of the disease progress) and were disinfected with a 2 % NaClO solution for 3 minutes. In a laminar flow hood (TELSTAR AH-100) previously cut pieces were washed with sterile distilled water (ADE) three times, then dried at room temperature on absorbing paper. Each four pieces were sown in a Petri dish (90 x 15 mm) with Potatoes Dextrose Agar (PDA) medium at equidistant points and then incubated in a NOVATECH Ei45 temperature chamber at 28 °C to obtain phytopathogens fungus (Villanueva et al., 2008).
Fungi that grew after seven days in the culture medium were transferred in a new Petri dish with PDA in order to purify isolates and then were incubated at 22 ± 4 °C, until sporulation, when monosporic cultures were obtained (Guigón & González, 2004).
Morphological identification of microorganisms
For morphological identification, 7 days mycelium samples were extracted by means of sterile dissection needles, were mounted on slides with lactophenol and covered with cover slips for their morphological identification at the level of genus, by means of a compound microscope Leica® (DME 13595XXX model) and with taxonomic and dichotomous keys of Barnett & Hunter (1998) and Watanabe (2002).
Pathogenicity tests and virulence degrees
A conidia suspension was obtained from each isolated fungus genus, placed into a Petri plate with 10 mL of ADE and 5 µL of Tween 80 and mycelium was scraped with a sterile handle, the suspension was then filtered through a sterile gauze pad. Conidia counting was performed by means of a Neubauer-improved countingchamber (MARIENFELD®) and a compound microscope (Ruiz et al., 2011). Later, apparently healthy soursop fruits were inoculated with each isolated fungus genus in order to reproduce the symptoms from which they were isolated and to discover the causal agent of rot. Apparently healthy soursop fruits were sampled with 6.21 cm in diameter and 8.25 cm in length up to around 20 cm in length and 15 cm in diameter for dry rot testing and healthy soursop fruits of 6.5 cm in diameter and 10 cm in length were sampled for soft rot testing. Biological material was placed in a cooler wrapped with a protective plastic and then transferred to the Plant Parasitology Laboratory. Fruits were washed with running water in the cooler and then disinfected by immersion in a 2 % NaClO solution for 2 minutes (Gutiérrez et al., 2002), moisture on fruits was absorbed with sterile gauze pads.
In the laminar flow hood, soursop fruits were inoculated without damage (six replicas and six controls) and with a superficial damage (six replicas and six controls) in epidermis of about 15 mm in length and 5 mm in depth, which was realized by means of a sterile bistoury. Ten µL of conidia suspension, obtained from the sporulation of each isolated fungus genus, were added to each soursop fruit group, by means of a Finnpipette® micropipette. Concentrations of each isolated fungus genus ranged between 11,200 conidia/mL and 1,000,000 conidia/ mL. In the case of soft rot isolated fungus genera, inoculation was performed with a 0.5 cc suspension with 11,200 conidia/mL by means of a hypodermic syringe infiltrated in the floral receptacle at a distance of 1 cm away from the peduncle (seven replicas and seven controls). Controls were firstly inoculated with sterile distilled water and fungi isolated from each symptom were later inoculated to avoid crossed contamination. Inoculated soursop fruits were incubated in a bioclimatic chamber (Thermo Scientific) at 28°C and with a 12:12 light/dark photoperiod and were systematically checked every 24h for 10 days to monitor symptomatology and to measure the degree of rot advance (virulence), by means of a digital Vernier (Truper Herramientas S.A de C.V). As a verification, a second isolation of inoculated fungus was performed on inoculated fungus to confirm the identity of the inoculated microorganisms (Dinh et al., 2003; Fraire et al., 2003; Muñoz et al., 2003; Than et al., 2008).
Molecular identification of rot causing fungi of in soursop fruit
Genomic DNA (gADN) was extracted for molecular identification of fungi that tested positive for pathogenicity. For that, an explant of each purified fungus was placed in a Petri plate with PDA medium and was incubated at 28 °C for 7 days. By means of a sterile spatula, mycelium was collected and placed in a sterile porcelain mortar with a buffer solution [200 nM Tris-HCl (pH=8), 250 mM NaCl, 25 mM EDTA, 0.5 % SDS] at 70 °C (Ruiz et al., 2017), then soften and the extraction was realized according to Raeder & Broda (1985). gDNA was visualized by electrophoresis in a 1 % agarose gel which was used to amplify rDNA internal transcribed spacers (ITS4 and ITS5), with ITS5 (5’-GGAAGTAAAAGTCGTAACAAGG-3’) e ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) primers (White et al., 1990; Ruiz et al., 2017). Amplification was performed according to the following conditions: an initial step of denaturalization at 94 °C for 5 minutes, 30 cycles of denaturalization at 94 °C for 30 s, alignment at 60 °C for 30 s, extension at 72 °C for 10 minutes (Ruiz et al., 2017). Amplification was visualized by electrophoresis in a 1 % agarose gel (Ochoa et al., 2012). PCR products were later sequenced by Macrogen Company (Rockville, Maryland, EUA). Resulting sequences were compared with those reported in NCBI GenBank database (National Center for Biotechnology Information, 2018) by means of BLAST software (Altschu et al., 1990) to check the identity percentage corresponding to the identified species.
Results and Discussion
Description of rot symptoms in soursop fruits in orchards
Symptoms of dry and soft rots were found to present irregularly, independent or associated distributions in orchards. However, soursop fruits with soft rot can be found at different size (from 6 cm in diameter and 8 cm in length), rots appear at any place of fruits epidermis, with slightly sunken stains of 2 to 3 cm in diameter, which can develop independently or join together to make up rots up to 10 cm with colors ranging from brown to black (Figure 1a). Symptom can include up to 10 mm, but when associated to B. cubensis, depth varies according to the position of the seed from where the adult pest emerged (Figure 1b), in some occasions acervuli were observed on the necrotic area. Fruits were able to reach maturity in an irregular way despite the rot. In the case of soft rot of soursop fruits, symptoms appear initially in the floral receptacle, and later moves to the pulp and skin of the fruit. In an advanced state of rot, the skin turns into a brown color and the floral receptacle begin to show an in intense black color (Figure 1c), the fruits are mummified and fail to, reach physiological maturity and remain attached to the tree which is confused in most cases with fruit abortion. Many mummified fruits present acervuli for Colletotrichum sp.
Isolation and morphological identification of fungi associated to soursop fruit rot
Of the soursop fruits with dry rot symptoms, fungi of the Lasiodiplodia sp. genus were isolated and morphologically identified with 25.53 % of incidence (4 isolates), Colletotrichum sp. with 41.18 % (7 isolates), Pestalotiopsis sp. with 1.76 % (2 isolates), Fusarium sp. with 5.88 % (1 isolate) and Cladosporium sp. with 17.65 % (3 isolates), while in soursop fruits with soft rot symptoms, fungi of the Rhizopus sp. genus were isolated with 16.67 % of incidence (3 isolated), Penicillium sp. with 11.11 % (2 isolates), Aspergillus sp. with 33.33 % (6 isolates), Fusarium sp. with 5.56 % (1 isolate) and Lasiodiplodia sp. with 33.33 % (6 isolates).
Pathogenicity tests
Of the 5 fungi genera isolated from dry rot symptoms, only Pestalotiopsis sp. y Colletotrichum sp. resulted positive in pathogenicity tests. Pestalotiopsis sp. was 100 % pathogenic with damage and 0 % without damage, the virulence reached in 7 days after inoculation (DAI) was of 3.91 mm, indicating that this fungus only causes a rot with mechanical damage and although it did not result so aggressive, the rot could be the cause of fruit post-harvesting losses. In the case of Colletotrichum sp. it was 100 % pathogenic with damage and 83 % without damage, the virulence reached on the tenth DAI was of 7.36 mm and 3.69 mm, respectively. On the other hand, of the five isolated genera of soft rot symptoms, only Lasiodiplodia sp. fungus genus causes rot in 100 % of the inoculated fruits. Virulence reached in 6 DAI was of 9.6 cm, indicating a very aggressive rot.
Symptoms generated by Pestalotiopsis sp. appeared at the second DAI, the lesion was only superficial and presented blackish colorations, with brown-reddish tones, a hard consistency and absence of mycelium, corresponding to dry rot symptoms (Figure 2).

Figure 2 a) Symptoms of dry rot by Pestalotiopsis sp., b) Control soursop fruit, c) Conidia of Pestalotiopsis sp.
Symptoms generated by Colletotrichum sp. fungus in the replicas without damage appeared at the fourth DAI, the lesion was only superficial with a dry consistency; for replicas with damage, symptoms appeared at the second DAI, where the advance of the rot was also observed in the pulp, lesions became of intense brown color, with a hard and dry consistency and a necrotic appearance and there were no presence of mycelium of the pathogen (Figure 3).

Figure 3 a) Symptom of dry damaged by Colletotrichum sp., b) Symptom of dry rot without damage, c) Control soursop fruit, d) Conidia corresponding to Colletotrichum sp.
Symptoms generated by Lasiodiplodia sp., appeared at the third DAI, a softening was observed in the area at the base of the floral receptacle. On day 6, fruit was totally soft and light brown stains appeared on all the pericarp. On day 9, skin was totally mummified and darkened into an intense brown color. On day 10, the pulp of the fruit softened and started to darken (Figure 4).
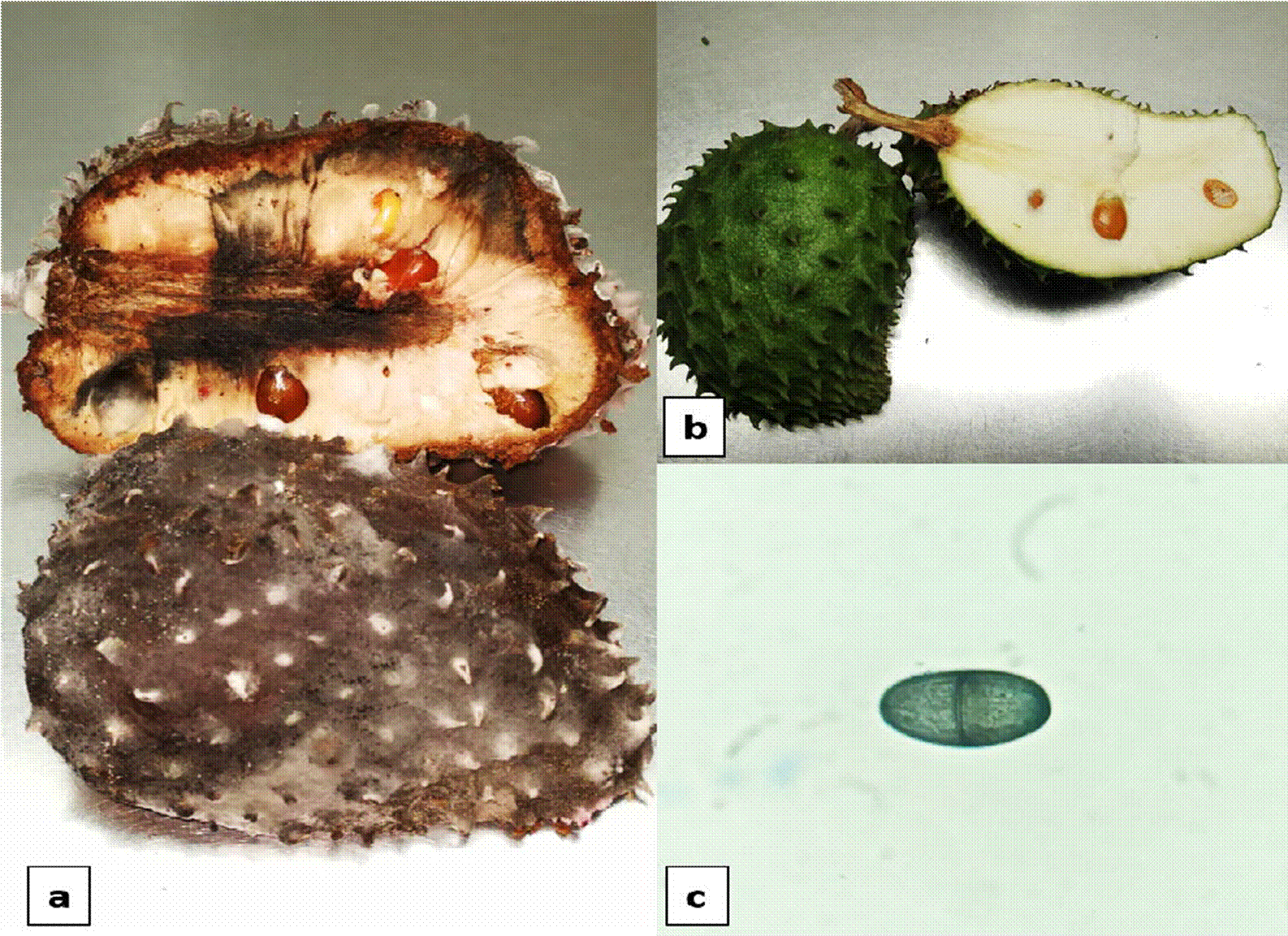
Figure 4 a) Symptoms of soft rot by Lasiodiplodia sp., b) Control soursop fruit, c) Conidium of Lasiodiplodia sp.
According to these results, C. gloeosporioides was confirmed for the first time in México to be the agent responsive for dry rot in soursop fruit in Nayarit. This coincides with results reported by Alberto & Otanes (2016), who mentioned that C. gloeosporioides, C. acutatum and F. clamydosporum are pathogenic in mature soursop fruits in Philippines. On the other hand, Andrades et al. (2009) associated Colletotrichum spp. With anthracnosis in soursop fruits in Venezuela, while Hernández et al. (2013) associated to C. gloeosporioides in A. muricata fruits in the state of Nayarit, Mexico. This fungus is also considered as the anthracnosis causing pathogen in avocado fruit (Persea americana Mill.) in the state of Michoacan, Mexico (Morales et al., 2009). Regarding Pestalotiopsis sp. fungus, it was not highly virulent; however, it was pathogenic by causing a light dry rot and it is the first time this microorganism is reported as responsible for damages in soursop fruits in Nayarit, Mexico. Montiel (1997) registered Pestalotiopsis sp. as the causal agent of necrosis in guava fruits (Psidium guajava L.) in Venzuela.
In Nayarit, Hernández et al. (2013)L. theobromae was identified as the responsible for soft rot in soursop fruits, however, in this research L. pseudotheobromae was found to be the causal agent of soft rot in soursop fruits and that represents the first record for Nayarit, Mexico. Nweke & Ibiam (2012) indicate this rot in soursop fruit is due to C. gloeosporioides and R. stolonifer in Nigeria; while Sandoval et al. (2013) reported L. pseudotheobromae as the responsible for the death of descendant branches and was associated to rot in mango peduncle (Mangifera indica L.), raised in Pacific coast of Mexico. Moreover, Awan & Akgül (2016) determined that L. pseudotheobromae is a highly virulent post-harvesting pathogen in lemon (Citrus limon L. Burm. f.) by damaging around 40 to 50 % of the area of the fruit, at 5 dai in Turkey.
According to these pathogenicity results, fungi from Lasiodiplodia sp., Fusarium sp. and Cladosporium sp. genera were determined to be associated with dry rot in soursop fruit, while Rhizopus sp., Fusarium sp. Penicillium sp. and Aspergillus sp. are associated to soft rot in soursop fruit.
Molecular identification of causal agents of rot in soursop fruit
Regarding molecular traits of rot causing fungi in soursop fruits, Pestalotiopsis sp. was identified with 99 % of identity with Cef-S6 strain (Accession Number KX960814.1), Colletotrichum gloeosporioides with 99 % of identity with Bpf2 strain (Accession Number KX960784.1) and Lasiodiplodia pseudotheobromae with 99 % of identity with CEF-9 strain (Accession Number MH062942.1) (NCBI, 2018).
Conclusion
Pestalotiopsis sp. and Colletotrichum gloeosporioides fungi are causal agents of dry rot in soursop fruits, while Lasiodiplodia pseudotheobromae is the causal agent of soft rot in soursop fruit and is moreover associated to dry rot. Besides, L. pseudotheobromae, Fusarium sp. and Cladosporium sp. were determined to be secondary dry rot-associated pathogens, while Rhizopus sp., Fusarium sp. Penicillium sp. and Aspergillus sp. were identified as saprophytic fungi in soft rot in soursop fruits. In the current work, Pestalotiopsis sp. and Colletotrichum gloeosporioides were recorded for the first time as causal agents of dry rot and L. pseudotheobromae as causal agent of soft rot in soursop fruits of A. muricata in Mexico.











 texto en
texto en 



