Introduction
Urinary tract infections (UTI) are severe public health problems, affecting around 150 million people each year worldwide (Sharma et al., 2013; Tibyangye et al., 2015). Among the most common causative agents are Escherichia coli, Klebsiella pneumoniae, Enterococcus faecalis, Proteus mirabilis, Pseudomonas aeruginosa, group B Streptococcus, Staphylococcus saprophyticus, Staphylococcus aureus and Candida spp (Flores-Mireles et al., 2015; Lagunas-Rangel, 2018). In Mexico, the most isolated pathogen from urinary infections is Escherichia coli (67.8 %), followed by E. faecalis (8 %), K. pneumoniae (3.4 %) and the yeast Candida spp. (2.7 %) (Lagunas-Rangel, 2018). Currently, these pathogens have acquired resistance to common antimicrobials used for their treatment (e.g. ampicillin, amoxicillin and ceftrixone, among others). Thus, the need to search for new natural alternatives, such as plant-based extracts since they contain multiple chemical compounds, to which bacterial, fungal, protozoal and viral diseases cannot develop resistance simultaneously (Tibyangye et al., 2015).
Turnera diffusa Willd. ex. Schult. (Passifloraceae) commonly known as damiana (Arbo, 2000; Szewczyk & Zidorn, 2014) is a small, wild shrub plant from arid and semi-arid regions of South America, Mexico and the United States (Alcaraz-Melendez et al., 2007; Zhao et al., 2007). Two varieties have been documented, T. diffusa var. aphrodisiac Ward Urb. (Tda) and T. diffusa Willd. ex Schult. var. diffusa Ward Urb. (Tdd) (Arbo, 2000). This small plant is traditionally used for the treatment of various diseases, including urinary tract infections, diabetes mellitus, malaria, diarrhea and peptic ulcers (Chevallier, 1996; Benson, 2008; FEUM, 2013) although the best known effect is as tonic and stimulant that has become popular as an aphrodisiac (Estrada-Reyes et al., 2016). The main bioactive components of T. diffusa include flavonoids, such as gonzalitosin I, apigenin and derived glycosides, luteolin and derived glycosides, cyanoglucoside tetrafillin B, arbutin a phenolic glycoside and the alkaloids damianin and caffeine. Additionally, its essential oil is composed of α-pinene, β-pinene, p-cymene, 1,8-cineol, which are obtained mainly from leaves and stems (Piacente et al., 2002; Kumar & Sharma, 2006; Zhao et al., 2007), the studies do not specify the variety analyzed.
It is known that both varieties have been used in traditional medicine, but to date and to our knowledge, no studies have demonstrated their pharmacognostic properties to correctly identify the two varieties or supported their biological activity as varieties. A few studies have shown the antimicrobial activity of different extracts of T. diffusa; however, they have not referred to the variety used since damiana varieties have been used interchangeably. For example, Hernández et al. (2003) demonstrated the antimicrobial activity of the hexanic and ethanolic extracts of damiana (T. diffusa) against gastrointestinal pathogens. Therefore, it is imperative to know the biological activities of the varieties of T. diffusa to ensure the benefit to consumers. Even though damiana of California (T. diffusa Willd. ex Schult. aphrodisiac Ward Urb.) is the one with higher demand as a herbal remedy compared to the other variety, no scientific studies are known that confirm its effectiveness yet. Due to the commercial importance of this plant and the lack of control for collecting practices, the government has established some programs to promote its cultivation and avoid deforestation since the wild plant is sold in local markets for medicinal purposes without any safety control (Gamez et al., 2010; Martinez de la Torre, 2013).
As a first step for the identification of the two varieties of damiana, our group has identified leaf morphology and anatomy of both varieties (Báez-Parra et al., 2018). Consequently, the aim of this study was to evaluate the antimicrobial effect of methanolic and hexanic crude extracts of Turnera diffusa Willd. ex Schult. var. aphrodisiaca Ward Urb. and Turnera diffusa Willd. ex Schult. var. diffusa Ward Urb., against pathogens related to urinary tract infections.
Material and Methods
Preparation of the strains
Positive controls of Klebsiella pneumoniae (ATCC 13883), Enterococcus faeccalis (ATCC 29212), Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922) and Candida albicans were used. The microorganisms were obtained from the Mexican National Laboratory for Food Safety Research and activated in culture medium of Trypticase Soy Broth (TSB) (BD Bioxon, Mexico) incubated at 37 °C for 24 h; subsequently, they were streaked on Trypticase Soy Agar (TSA) (BD Bioxon, Mexico) and incubated at 37 °C for 24 h (Microbank, Pro Lab Diagnostics, USA). Finally, the colonies obtained were used to prepare a suspension of 1.5 x 108 CFU/mL corresponding to McFarland scale standard 0.5 (CLSI, 2017).
Drugs and reagents
Reagents naringin, apigenin, chlorogenic acid, diosgenin, methanol, hexane, 2-aminoethyl diphenyl borate, anisaldehyde and dimethyl superoxide were obtained from Sigma-Aldrich (St. Louis, MO, USA); ethyl acetate, glacial acetic, formic, and sulfuric acids from J.T. Baker (Xalostoc, State of Mexico, Mexico); and polyethylene glycol 4000 from Alfa Aesar (Tewksbury, MA, USA).
Plant collection
Leaves of T. diffusa Willd ex Schult. var. aphrodisiaca Ward Urb. and T. diffusa Willd. ex Schult. var. diffusa were collected in the stage of flowering and fruiting in Imala, Culiacán, Sinaloa, Mexico (24° 51› 35» N; 107° 13’ 1” W; 92 masl) from 2016-2017. The varieties were identified at the Universidad Autónoma de Sinaloa (UAS) in Culiacán, Sinaloa and Centro de Investigaciones Biológicas del Noroeste (CIBNOR) La Paz, B.C.S. Mexico. Samples with access numbers HCIB30319 for Tdd and HCIB300069 for Tda were deposited in CIBNOR herbarium.
Extract preparation
The leaves, previously washed with tap water and dried in the shade for seven days, were pulverized and macerated with methanol or hexane, in proportion 1:10, under constant agitation of 175 rpm (Shaker DOS-10L, SKY LINE, ELMI, CA, USA) for 24 h. Then, the extracts were filtered and concentrated under vacuum in a rotary evaporator (BUCHI, CH) at 335 mbar, 45 °C and 35 rpm, and at 360 mbar, 40 °C and 35 rpm for the methanolic and hexanic extract, respectively. The concentrated extracts were stored at 4 °C until use.
High-performance thin-layer chromatography
The HPTLC analysis was performed based on the methodology proposed by Reich (2012). Damiana methanolic and hexanic extracts (5 μL of a 1:10 solution in methanol or hexane, respectively) and standard solutions naringin, chlorogenic acid, apigenin and diosgenin (4 μL of a 1:1 solution in methanol or hexane, respectively) were loaded as 10-mm band length and 15-mm distance between each band in a 10 x 10-cm glass plates HPTLC 60F254 (CAMAG, Merck, DE) using a semi-automatic applicator Linomat V (Camag, Muttenz, CH) which was controlled with VisionCATS 2.4 software. The plate was developed in a double channel chamber (CAMAG, Merck, DE) with the respective mobile phase up to 80 mm. The FEUM (2013) methodology was followed by separating the phenolic compounds from the methanolic extract, which consisted of developing the plate with the mobile phase: ethyl acetate, acetic acid, formic acid and water (100:11:11:27) with a previous saturation of 20 min. The developed plate was let to dry at room temperature and heated at 100 °C for three min. The bands were derivatized spraying with 2-aminoethyl diphenyl borate 1 % in methanol (NP) and with polyethylene glycol 4000 5 % in ethanol (PEG). For the hexanic extract, the mobile phase used was hexane, acetic acid (7.2: 2.9) and the plate was developed under the same conditions as the methanolic extract, but derivatization was performed with anisaldehyde in concentrated sulfuric acid (170 mL of cold methanol, 20 mL of glacial acetic acid, 10 mL sulfuric acid, and 1 mL of anisaldehyde) (Sophia et al., 2011). The bands obtained on the plate were observed on UV light at 254 and 366 nm (CAMAG, Merck, DE) and detected with a TLC scanner (CAMAG, Merck, DE) at 254 and 366 nm for the methanolic extract, and 500 nm for the hexanic extract (Sophia et al., 2011; Seasotiya et al., 2014).
Antimicrobial susceptibility assays
Extract preparation
The extracts were prepared following Akujobi et al. (2004) and Alaribe et al. (2011) methodologies. The extract concentrations used were 5, 10, 15, 20 and 30 mg/mL for both varieties. As for the methanolic extract, it was dissolved in 30 % dimethylsulfoxide (DMSO) while the hexanic extract was dissolved in 30 % DMSO with 5 % Tween 80.
Disc diffusion method
The initial inoculum of each microorganism tested was uniformly inoculated with a sterile swab on the surface of Müeller-Hinton agar plates (BD Bioxon, MX). Once the plates were inoculated, a 1-cm diameter filter paper disc containing 0.5, 1, 1.5, 2 and 3 mg of extract, was placed independently on the agar. Ciprofloxacin at 5 μg was used as positive control for the bacterial test and nystatin for the yeast test. DMSO 30 % and DMSO 30 % with Tween 80 at 5 % were used as negative controls for the methanolic and hexanic extract, respectively. The plates were incubated at 37 °C for 24 h. The antimicrobial activity was determined by measuring the inhibition zone diameter (Ahmad & Beg, 2001; CLSI, 2017). For each evaluation, five repetitions were made.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The MIC was determined by the microdilution assay, using 96-well microplates, inoculated with 10 μL of microbial suspension 1.5 X 108 CFU/mL, 140 µL of culture medium, and 0.05, 0.1, 0.25, 0.5, 1, 1.5, 2, 3 and 5 mg respectively. Absorbance was measured at 600 nm using a SINERGY HT microplate spectrophotometer (BioTek Winooski, VT, USA) at 37 °C at the beginning of the experiment and at 24 h post-incubation. The MIC was determined at the lowest concentration of the extract at which no growth was observed. Based on the MIC, MBC was determined, by streaking in selective media for each microorganism tested: Hektoen enteric agar (BD Difco) for Klebsiella pneumoniae, TSA for Enterococcus faeccalis, Baird-Parker agar (BD Difco) for Staphylococcus aureus, ECC (CHROMagar) for Escherichia coli and TSA for Candida albicans. All plates were finally incubated at 37 °C for 24 h, and the presence of growth was observed. MBC was determined as the minimum concentration of the extract capable of killing the analyzed microorganism. The MIC and MBC assays were performed in triplicate with two repetitions for each assay (Cha et al., 2016).
Results and Discussion
Chromatographic profile of methanolic extract
Crude extracts were used for the analyses. In Figure 1, profiles of the two varieties are shown, before (a) and after (b) derivatizing the metabolites with the NP-PEG developer. After derivatization, four yellow bands were observed in both varieties with greater intensity and definition in Tda variety; a yellow to brown band was also observed at daylight. At 254 nm, eight and seven yellow bands were observed in Tda and Tdd, respectively. At 366 nm, the methanolic extract chromatograms of T. diffusa leaves of both varieties revealed yellow to orange band colorations, these results confirmed the presence of flavonoids, which were in accordance to the FEUM (2013).
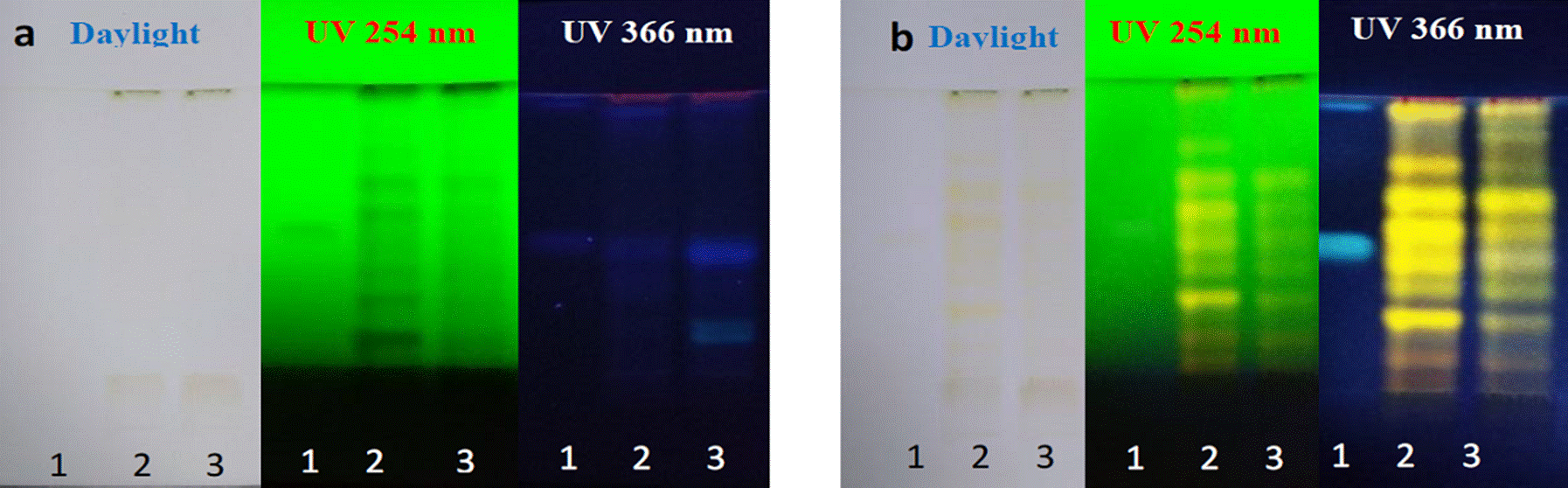
Figure 1 Chromatographic profile of Tda and Tdd methanol extract. Line 1: standards naringin, chloro genic acid and apigenin (1 mg/mL), line 2: Tda and line 3: Tdd. a) Plates before derivatizing, b) Plates after derivatizing.
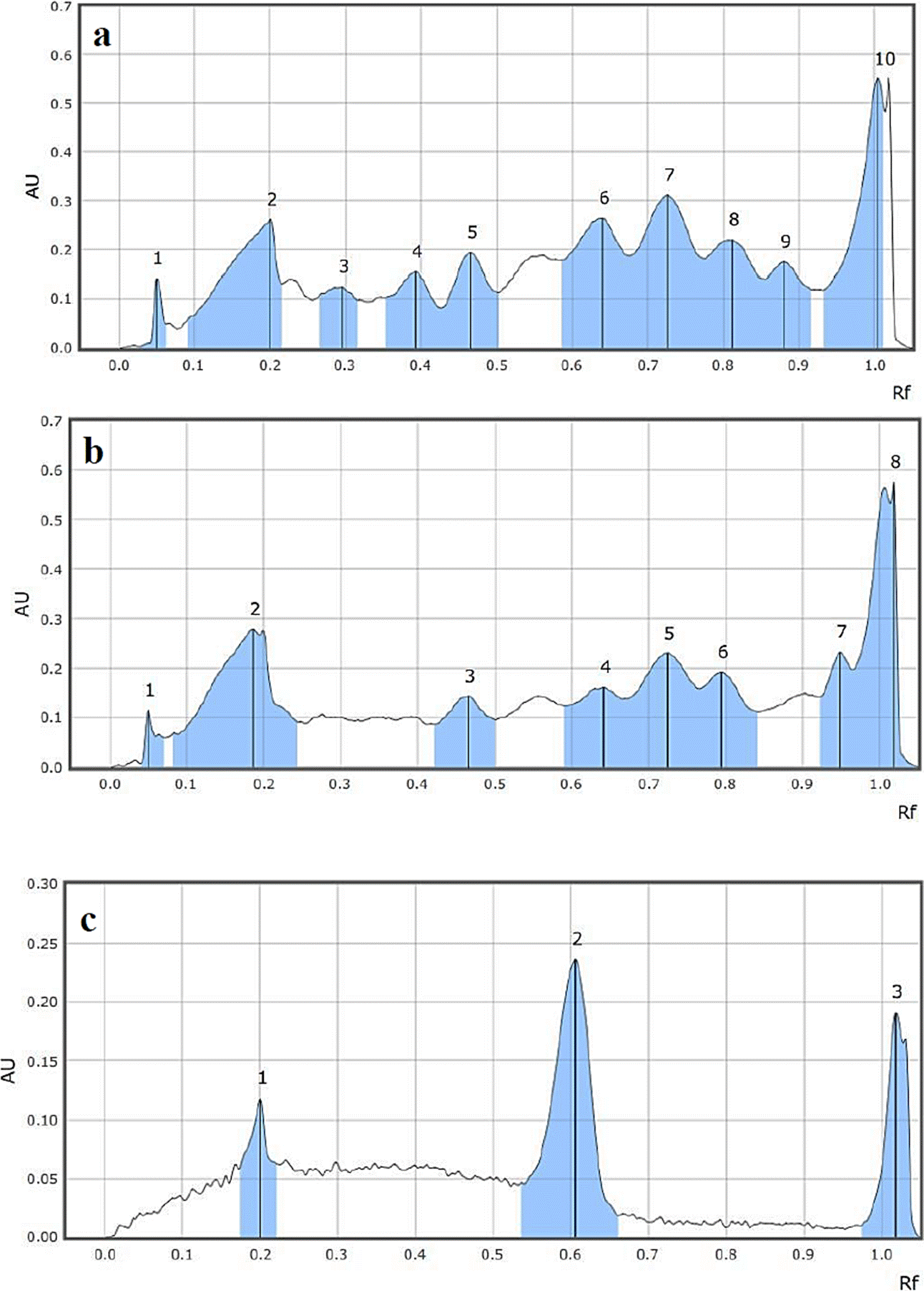
The HPTLC chromatograms generated for the standards and the methanolic extract of Tda and Tdd are shown in Figure 2. The 366 nm chromatogram in Tda showed ten peaks (2A) while that of Tdd showed eight (2B). These peaks may represent groups of metabolites, since crude extracts were used.
Chromatographic profile of the hexanic extract
In Figure 3, profiles of the hexanic extract of the two varieties are shown, before (a) and after (b) deriving the metabolites with anisaldehyde in sulfuric acid, revealed bands from blue to violet colorations (Figure 3b), which corresponded to terpenoid natural metabolites (Sophia et al., 2011). The chromatograms obtained by HPTLC of the hexanic extracts revealed nine peaks for the two varieties (Figure 4) and differences in retention by the stationary phase, shown in Table 2.
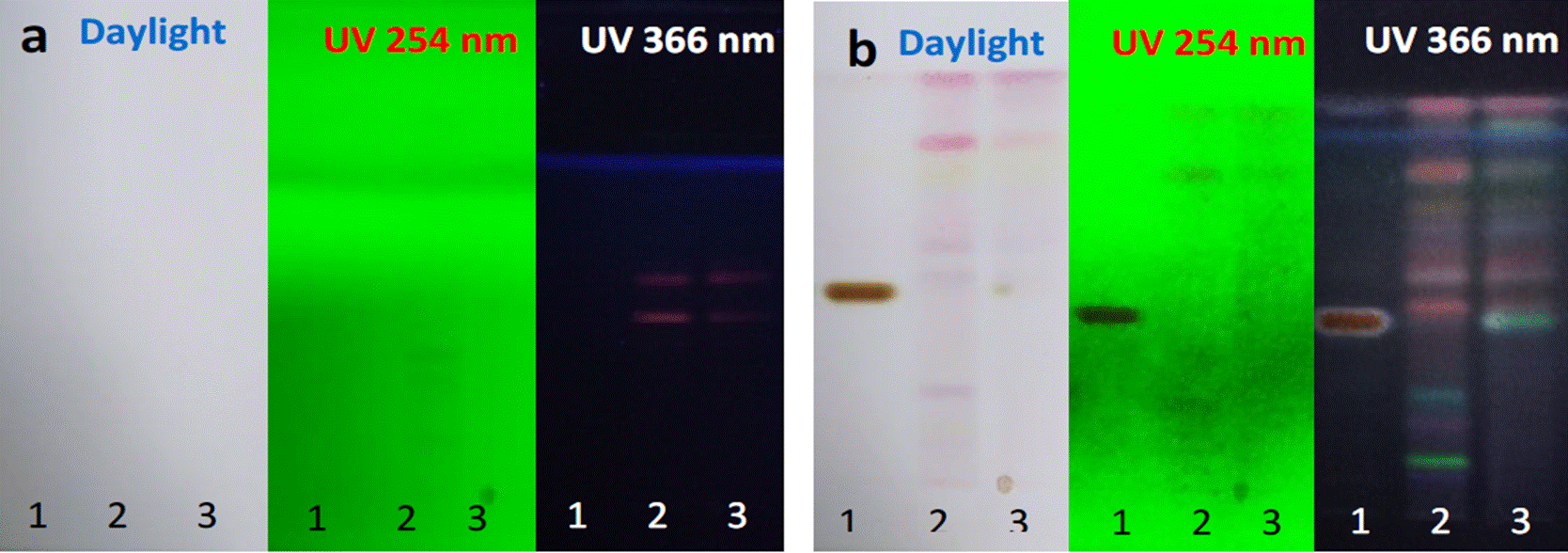
Figure 3 Chromatographic profile of Tda and Tdd hexanic extract. Line 1: standard diosgenin (1 mg/mL), line 2: Tda and line 3: Tdd. a) Plates before derivatizing, b) Plates after derivatizing.

Figure 4 a) Hexanic chromatogram of Tda by HPTLC, b) Hexanic chromatogram of Tdd, c) Chromatogram of standard diosgenin.
Table 1 Peaks height, areas and times retention (Rf), of the chromatograms of the methanolic extracts of Tda and Tdd and standards.
| Turnera diffusa var. aphrodisiaca | Turnera diffusa var. diffusa | Standards | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak | Rf | H | % area | Peak | Rf | H | % área | Peak | Rf | H | % area |
| 1 | 0.050 | 0.1428 | 5.94 | 1 | 0.050 | 0.1158 | 6.00 | 1 | 0.200 | 0.1176 | 21.62 |
| 2 | 0.200 | 0.2626 | 10.92 | 2 | 0.186 | 0.2789 | 14.44 | 2 | 0.606 | 0.2357 | 43.31 |
| 3 | 0.296 | 0.1252 | 5.21 | 3 | 0.466 | 0.1435 | 7.43 | 3 | 1.019 | 0.1908 | 35.07 |
| 4 | 0.393 | 0.1563 | 6.50 | 4 | 0.641 | 0.1620 | 8.39 | ||||
| 5 | 0.466 | 0.1943 | 8.08 | 5 | 0.724 | 0.2309 | 11.96 | ||||
| 6 | 0.640 | 0.2647 | 11.00 | 6 | 0.794 | 0.1922 | 9.95 | ||||
| 7 | 0.726 | 0.3120 | 12.97 | 7 | 0.949 | 0.2321 | 12.02 | ||||
| 8 | 0.811 | 0.2193 | 9.12 | 8 | 1.019 | 0.5759 | 29.82 | ||||
| 9 | 0.880 | 0.1757 | 7.30 | ||||||||
| 10 | 1.004 | 0.5521 | 22.96 | ||||||||
Table 2 Peaks height, areas and times retention (Rf), of the chromatograms of the hexanic extracts of Tda and Tdd and the diosgenin standard.
| Turnera diffusa var. diffusa | Turnera diffusa var. aphrodisiaca | Standard | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak | Rf | H | % de area | Peak | Rf | H | % de area | Peak | Rf | H | % area |
| 1 | 0.154 | 0.0328 | 4.51 | 1 | 0.153 | 0.0347 | 4.61 | 1 | 0.483 | 0.6093 | 100.00 |
| 2 | 0.222 | 0.0419 | 5.77 | 2 | 0.19 | 0.0314 | 4.17 | ||||
| 3 | 0.34 | 0.0338 | 4.65 | 3 | 0.247 | 0.0607 | 8.05 | ||||
| 4 | 0.453 | 0.12 | 16.52 | 4 | 0.478 | 0.0543 | 7.2 | ||||
| 5 | 0.544 | 0.1185 | 16.31 | 5 | 0.544 | 0.1071 | 14.2 | ||||
| 6 | 0.649 | 0.0585 | 8.05 | 6 | 0.603 | 0.0757 | 10.05 | ||||
| 7 | 0.699 | 0.049 | 6.74 | 7 | 0.69 | 0.0271 | 3.6 | ||||
| 8 | 0.786 | 0.2199 | 30.27 | 8 | 0.788 | 0.3326 | 44.11 | ||||
| 9 | 0.899 | 0.0521 | 7.17 | 9 | 0.901 | 0.0303 | 4.02 | ||||
It is well known that damiana has sesquiterpenes (caryophyllene), triterpenes (squalene and sisosterol), and polyterpenes (ficaprenol) (Zhao et al., 2007), whose compounds correspond to the chemical groups seen in this study.
Microbial susceptibility
Table 3 shows the results obtained from the antibacterial and antifungal activity evaluation by disc diffusion method of the crude methanolic and hexanic extracts of Tda and Tdd against S. aureus, E. faecalis, E. coli, K. pneumoniae and C. albicans. When the results were compared with control (ciprofloxacin for bacteria and nystatin for yeast), the methanolic and hexanic extracts showed similar or inferior capacity to the positive control. The 3 mg of the methanolic extract of Tdd showed an inhibition halo of 14.49 mm in the test against S. aureus, showing the best activity but no significant differences within the concentrations of 2.5, 2.0 and 1.5 mg of the same extract. The hexanic extract of Tdd was the only one that showed activity against E. coli, without significant differences between 1-3 mg and no activity against E. faecalis while the Tda and Tdd methanolic extract at 2.5 and 3 mg had the best results on this bacterium. As for K. pneumonia, all the extracts showed inhibitory activity, but they did not show statistically significant differences when compared with the control group. Only the hexanic extracts of the two varieties (Tda and Tdd) show antimicrobial activity at 3 mg against C. albicans.
Table 3 Antimicrobial effect of methanolic and hexanic extracts of Tda and Tdd.
| Variety | Extract | mg | Inhibition zone (mm) | ||||
|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | Escherichia coli | Enterococcus faecalis | Klebsiella pneumoniae | Candida albicans | |||
| Turnera diffusa var. diffusa | Methanolic | 1 1.5 | 12.56c,d,e 14.07b,c,d | NA NA | 10.53d,e 11.15c,d | 10.28c 10.50c | NA NA |
| 2 | 14.24b,c | NA | 11.34c | 10.86c | NA | ||
| 2.5 | 14.49b,c | NA | 11.82b,c | 10.96c | NA | ||
| 3 | 14.89b | NA | 12.15b | 11.01c | NA | ||
| Hexanic | 1 | 10.38f | 10.64b | NA | 10.18c | NA | |
| 1.5 | 10.71e,f | 10.74b | NA | 10.19c | NA | ||
| 2 | 10.82e,f | 10.66b | NA | 10.24c | NA | ||
| 2.5 | 11.28e,f | 10.64b | NA | 10.22c | NA | ||
| 3 | 11.74e,f | 10.76b | NA | 10.24c | 11.72b | ||
| Turnera diffusa var. aphrodisiaca | Methanolic | 1 1.5 | 10.34f 10.71e,f | NA NA | 10.08e 10.10e | 10.24c 10.29c | NA NA |
| 2 | 11.45e,f | NA | 10.11e | 10.40c | NA | ||
| 2.5 | 12.07d,e,f | NA | 10.31e | 10.42c | NA | ||
| 3 | 12.49c,d,e | NA | 10.43d,e | 10.45c | NA | ||
| Hexanic | 1 | NA | NA | NA | 10.11c | NA | |
| 1.5 | NA | NA | NA | 10.36c | NA | ||
| 2 | NA | NA | NA | 10.20 c | NA | ||
| 2.5 | NA | NA | NA | 10.20c | NA | ||
| 3 | NA | NA | NA | 10.30c | 12.09b | ||
| Control | Antibiotic | 5µg | 29.77ª | 31.42ª | 22.24ª | 40.17ª | 19.38ª |
*Different letters mean significant differences between treatments. Level of significance with the Tuckey test 95 %, n=5, NA: no activity.
All the statistical analyses had a square R of 98.00-99.80, with a 95 % level of significance.
Although some extracts showed no inhibitory activity in the antimicrobial activity test by the disc diffusion method, the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) assays were performed for all the extracts. For C. albicans, where only the highest concentrations of hexanic extracts showed activity, the MIC and MBC were found at 0.05 mg for the methanolic and hexanic extracts of the two varieties. As for E. coli and K. pneumoniae, they were recorded from 0.05 to 0.1 mg. The MIC was found from 2-3 mg for the methanolic extract with respect to S. aureus and E. faecalis. On the other hand, the hexanic extract of the two varieties for S. aureus and E. faecalis was found at 0.5 mg and 1.5 mg, respectively. Also, the MBC for Enterococcus faecalis was not observed for the hexanic extracts even though the highest concentration of 5 mg was used (Data not shown) (Table 4).
Table 4 Minimum inhibition concentration and minimum bactericidal concentration of methanolic and hexanic extracts of Tda and Tdd.
| Microorganism | MIC/MBC (mg/mL) | |||
|---|---|---|---|---|
| Turnera diffusa var. diffusa | Turnera diffusa var. aphrodisiaca | |||
| Methanolic | Hexanic | Methanolic | Hexanic | |
| Staphylococcus aureus | 3/3 | 0.5/3 | 2/2 | 0.5/3 |
| Escherichia coli | 0.1/0.1 | 0.05/0.1 | 0.05/0.1 | 0.05/0.1 |
| Enterococcus faecalis | 3/3 | 1.5/NA | 3/3 | 1.5/NA |
| Klebsiella pneumoniae | 0.05/0.1 | 0.05/0.05 | 0.05/0.1 | 0.05/0.05 |
| Candida albicans | 0.05/0.05 | 0.05/0.05 | 0.05/0.05 | 0.05/0.05 |
*NA: No activity.
The in-vitro antimicrobial evaluation of the Tda and Tdd methanolic extracts showed that both were effective against E. faecalis, K. pneumoniae and S. aureus, but the Tdd methanolic extract was statistically more effective (Tabla 3). Although, the Tda and Tdd methanolic extracts showed no visible activity against C. albicans using the disc diffusion method, the extracts of both varieties showed antimicrobial activity at 0.05 mg when the microdilution method was performed. It could have been related with the way the metabolites were diffused on the agar by using the diffusion method, which might not have been as homogenous as the microdilution method, since the metabolites are place in a homogenous aqueous medium.
On the other hand, the hexanic extracts of both varieties showed inhibition against K. pneumonia and C. albicans at a concentration of 3 mg, but no significant differences among them were observed (Table 4). Escherichia coli and S. aureus showed halos of inhibition from 10.6412.09 mm only in the Tdd hexanic extract using the diffusion method; however, both hexanic extracts of Tda and Tdd showed more effecacy with the microdilution method. Moreover, lower concentrations of hexanic extracts than methanolic extract were needed to have a higher efficacy on all the microorganisms tested, except for E. faecalis. The antimicrobial activity of methanolic extracts has been demonstrated against Gram-positive and Gram-negative bacteria where flavonoids were responsible for the activity (Taleb-contini et al., 2003). The metabolites present in the methanolic extract are mainly flavonoids (Figure 1), for which their antimicrobial activity has been demonstrated both in Gram-positive and Gram-negative bacteria; furthermore, a structureactivity relationship has been observed on flavonoids, of which the most active are those that have dihidroxy groups at C3’ and C4’ positions, the position C6’ unsubstituted and C7’ with a hidroxy (Taleb-contini et al., 2003). In addition, apigenin, another flavonoid present in damiana (Cushnie & Lamb, 2005; Nayaka et al., 2014; Cha et al., 2016) has also been proven to have antimicrobial activity; thus, our study has demonstrated that the flavonoids found in both Tda and Tdd varieties were responsible for the antimicrobial activity against the urinary tract pathogens analyzed.
The methanolic extracts of our study also showed antimicrobial activity against both Gram-positive and Gram-negative bacteria showing maximum activity against E. coli and K. pneumonia according to the MIC obtained. In relation to the statistical analysis, the methanolic extracts of Tdd had significant differences compared to those of Tda. These differences might have been related with the type of metabolites present in each extract, as it was observed in the chromatographic analysis; the extracts had differences in peaks 3 and 4 of Tda, which were not found in Tdd, and peak 7 of Tdd that was not shared with Tda (Figure 2, Table 1). In addition, at 366 nm without derivatization, the chromatogram showed two bands (blue to green color) that were unique for Tdd. Thus, beside the flavonoids present they may have other metabolites that are causing the maximum activity. However, more studies are needed to make this assumption.
Finally, the antimicrobial activity of the metabolite terpenes and terpenoids have also been evaluated, demonstrating that terpenes have better activity when they are in a crude extract than individually isolated (Nazzaro et al., 2013). Thus, the crude oil of the hexanic extracts in this study might have shown this activity on all the microorganisms evaluated, except for E. faecalis. The MIC and MBC obtained did not show significant differences against the microorganisms tested; however the chromatographic analysis of the Tda and Tdd hexanic extracts showed statistical differences. Therefore, the metabolites responsible for the inhibition activity of both varieties may not be those that are different between the varieties but those that are shared between them. Nevertheless, more studies are needed to identify the metabolites responsible for the antimicrobial activity in each variety and establish the relationship of each metabolite structure with the bioactive metabolites.
Conclusion
The proper identification of medicinal plant extracts is required for safety, quality, and efficacy of the human consumption products. This is the first invitro study that has shown the antimicrobial activity of methanolic and hexanic extracts of two varieties of T. diffusa (T. diffusa var. aphrodisiaca and T. diffusa var. diffusa) against bacteria and fungi causing urinary tract infections. With the HPTLC analysis the nature of the compounds that carry out the bioactivity was identified. This study served as a baseline to identify the metabolites present in each extract; as a follow-up, further analyses of the metabolites responsible for the antimicrobial activity should be performed, especially in Klebsiella pneumoniae because it is a pathogen related to disorders for which damiana has been used as an herbal remedy.











 texto en
texto en