Introduction
The Annonaceae family is composed of 108 genera and 2,400 species, distributed in tropical and subtropical climates (Hernández-Fuentes et al., 2016; Couvreur et al., 2012). In Mexico, in the states of Chiapas, Veracruz, Tabasco, Guerrero, Oaxaca, Nayarit, Michoacán, Yucatán, and Campeche, the genus Annona is the most important for the production of edible fruits (Andrés & Andrés, 2011; Pino, 2010), 15 species have been recorded and also the Atemoya, which is a variety derived from the cross of Annona cherimola x Annona squamosa (atemoya); the species cultivated for commercialization are soursop (Annona muricata L.), custard apple (Annona cherimola Mill.) and saramuyo (Annona squamosa L.) (Hernández-Fuentes et al., 2016). Efforts are being made to rescue the anona (Annona reticulata L.) and encourage its cultivation for the production of fruits since these are rich in carbohydrates, proteins, calcium, phosphorus, iron, thiamin, niacin, riboflavin, magnesium, ascorbic acid, and carotenoids, which constitutes an alternative for fruit production (Andrés & Andrés, 2011). Additionally, the Food and Agriculture Organization of the United Nations (FAO, 2012) indicates the use of crops that allow countries to take advantage of their potential, to promote economic development, reduce hunger and poverty, as well as the need to consider the anona in situ conservation activities (Andrés & Andrés, 2011). In this context, the objective of this review is to collect information on the generalities, pharmacology, and phytochemicals of A. reticulata to highlight its economic, social, and medicinal importance.
Methodology
This research focused on the collection of information with the words Annona reticulata; in the Elsevier, Scielo, Wiley online library, Redalyc, Springer and Scopus, and ScienceDirect databases, from 1991 to August 2022, taking as a source of information scientific articles published in indexed and refereed journals.
Results and Discussion
Botanical description. According to the Integrated Taxonomic Information System (ITIS), the taxonomic description of the anona (Annona reticulata L.) is as follows Table 1:
Table 1 Taxonomy of the Annona reticulata L.
| Kingdom | Plantae |
|---|---|
| Division | Tracheophyta |
| Subdivision | Spermatophytina |
| Class | Magnoliopsida |
| Order | Magnoliales |
| Family | Annonaceae |
| Genus | Annona L. |
| Species | Annona reticulata L. |
Source: Integrated Taxonomic Information System (ITIS, 2022)
Generalities
Custard apple (A. reticulata) is native to Guatemala and Belize (Pino, 2010) and is distributed throughout America, Africa, and Asia (Chavan et al., 2014). In Mexico, the soursop is found in the tropical regions of Veracruz, Tabasco, Yucatán, Oaxaca, Chiapas, Guerrero, Sinaloa, Puebla, Quintana Roo and Michoacán, at an altitude between 50 and 1150 masl. They develop in tropical and subtropical climates, between 0 and 1,500 m altitude in areas of Central America and South America, in Guatemala and Ecuador they are found below 1,220 masl and 1,500 masl, respectively, and in the Philippines and Siri Lanka for below 800 masl and 915 masl, respectively (Chavan et al., 2014). They adapt to conditions of low rainfall (dry season) and resist low temperatures (-2.78 °C to 2.22 °C), remaining dormant during the winter without suffering serious damage (Morton, 2013), still reporting fruit production. Although conditions may be favorable for its cultivation, soursop is found mainly in backyard orchards or the wild along rural roads (Andrés & Andrés, 2011). It is observed that this fruit tree is widely distributed and easily adaptable to adverse conditions, ecophysiologically speaking. The propagation of the anona is by seed (sexual), although it can also be asexual; grafts have been made between the soursops themselves or as soursop, saramuyo, and atemoya rootstocks (Chavan et al., 2014; Morton, 2013). This fruit tree is an important vehicle to propagate other species of the same family.
The anona tree is between 3 and 10 m tall, has an extended or rounded crown, its trunk measures 25 to 35 cm in diameter, and is considered the most vigorous of the Annona genus (Figure 1). It has many lateral branches, cylindrical stems with lenticels, and very short brown tomenta (Figure 1). The leaves are lanceolate to oblong-lanceolate, dark green, and measure 25 to 30 cm long and 7 cm wide (Chavan et al., 2014; Lim, 2012; Andrés & Andrés, 2011; Waghulde et al., 2021) (Figure 1). The inflorescences are found in drooping clusters and are grouped from 2 to 10, with external narrow fleshy petals that never open completely and pedicels that measure between 1.5 and 3 cm in length (Andrés & Andrés, 2011; Chavan et al., 2014) (Figure 1). The inflorescences present inefficient pollination, due to the slow opening of the petals, the position of the gynoecium to the androecium, the low number of pollinators, and the fact that they do not make uniform contact with the area of the gynoecium curve (Pinto, 1995) so it is necessary to practice artificial or manual pollination (Andrés & Andrés, 2011).

Source: Own elaboration
Figure 1 Tree (A), branches (B), leaves (C) and inflorescences (D) of A. reticulata L.
The fruit weighs between 0.1 and 1.0 kg, is heart-shaped, almost spherical or irregular, and measures 10 to 12 cm in length and 8 to 16 cm in diameter. The color of the epicarp, the shape of the areoles, and the type of reticulation in the fruits can vary (Pino, 2010). Vidal et al. (2015) describe red, yellow, purple, and pink epicarp colors.
According to Chavan et al. (2014), the epicarp is thin and yellow to brown when ripe, and reticulated. Andrés & Andrés (2011), mention that the epicarp is coriaceous and reddish yellow, with areoles above the carpels. Morton (2013) reports that the epicarp is thin but resistant and can be yellow-brown, pink, reddish, or reddish-brown when the fruits are ripe and with a slight, moderate, or marked reticulation. Pareek et al. (2011) mention that the maturity index of cherimoya, atemoya, saramuyo, and anona includes changes in the color of the epicarp, from dark green to light green or yellow-green. Finally, Lim (2012) mentions that the fruits have more or less smooth, rhomboid or hexagonal areoles separated by a marked reticulation, not tuberculate, with a reddish-brown or red epicarp when ripe (Figure 2).
The pulp is creamy-white, somewhat granular, and moderately juicy (Morton, 2013; Chavan et al., 2014). It has a pointed central fibrous core that is attached to the thick stem that extends to more than half of the fruit, this being a particularity of the fruit (Chavan et al., 2014). The seeds are small, about 1.25 cm long, oblong, laterally compressed, smooth, hard, shiny, dark brown or black, and there are commonly 40 to 76 seeds per fruit (Morton, 2013; Andrés & Andrés, 2011; Lim, 2012) (Figure 3). Chavan et al. (2014) mention that the seeds are viable for more than 12 months in dry air storage at 5°C.
Phenology. The phenological characteristics of cultivated plants provide useful tools to understand the relationships between plants and their environment. For anona, bud development is between June and April, leaf display from April to December, and senescence from December to April. As for the flowers, their growth occurs in November-December and flowering in December. The development of the fruits goes from December to February and fruiting from March to May (González-Esquinca et al., 2016), finding that it is an annual harvest fruit tree.
Pharmaceutical uses
The structural organs of the anona tree, such as the leaves, pulp, bark, root, and seeds, are used as medicinal sources since they have pharmacological properties such as anthelmintic, analgesic, anti-inflammatory, antihyperglycemic, antipyretic, antioxidant, antimicrobial, healing and cytotoxic; in addition to containing phytochemicals such as tannins, alkaloids, phenols, glycosides, flavonoids and steroids (Jamkhande & Wattamwar, 2015; Ngbolua et al. 2018). Chithra et al. (2016) evaluated the phytochemical components of soursop and anona, concluding that the fruits of both have terpenoids, tannins, alkaloids, and ascorbic acid, potential in the field of pharmacology as antioxidants and antibacterials for the development of possible drugs to cure some diseases such as diabetes, cancer, acquired immunodeficiency syndrome, and various degenerative diseases. It is an important fruit tree for its potential in the pharmaceutical industry since all its structural organs can be used for this purpose.
Leaves. Various bioactive compounds with important biological activities have been found in the leaves (Table 2). In this regard, Zaman & Pathak (2013) reported the presence of phytochemicals such as alkaloids, tannins, phenolic compounds, flavonoids, glycosides, and steroids in methanolic extracts of leaves of anona. On the other hand, Rahman et al. (2011) have reported that methanolic extracts from anona leaves have antihyperglycemic activity in mice, measuring glucose tolerance and blood glucose levels by the glucose oxidase method, obtaining a reduction in blood glucose level of 56.1 %, being an alternative for the treatment of diabetic patients; anti-inflammatory activity has also been demonstrated in rats, possibly due to the presence of acetogenins or the inhibitory activity of cyclooxygenase-2 (Bhardwaj et al. 2019). In this regard, Pathak et al. (2021) observed anti-inflammatory and antioxidant activity in methanolic extracts in anona leaves due to quercetin and gallic acid. In addition, in leaf extracts, the terpenoid has been chemically investigated: anonaretin A (Chavan et al., 2014) among others; bioactive phytocompounds such as quinones, coumarins, steroids, tannins, phenols, glycosides, alkaloids, terpenoids, flavonoids, and saponins have been found (Sangeetha et al., 2014), these are antioxidant, antibacterial and antimicrobial agents (Ahirwar & Tembhre, 2021). Mazumdar et al. (2022) found β-sitosterol and quercetin in ethanolic extracts of anona leaves, which increased the proliferation of lymphocytes (CD4, CD8, and B cells) and improved the intracellular expression of IL-2 and IL-6, so it can be a potential alternative for various diseases related to the immune system. Sankpal (2022) identified 33 phytocompounds, such as phenolics, flavonoids, alkaloids, tannins, glycosides, and terpenoids in methanolic extracts of the leaves, most of which have been reported to have important biological activities with antioxidant properties (copaene), antimicrobial ((-)-spatulenol), anticancer (p-dioxane-2,3-diol) and anti-inflammatory (caryophyllene). Likewise, a study carried out on three species of Annona (soursop, saramuyo, and anona) to determine the antioxidant capacity by the DPPH ABTS, nitric oxide and hydroxyl methods, observing that the extracts of anona leaves showed higher antioxidant capacity compared to Saramuyo (Baskar et al., 2007). The presence of acetogenins in anona leaves, such as 9-one anoreticuin, squamona, solamin, anomonisin, and rolliniastatin-2, has been reported (Roham et al., 2016; Anaya-Esparza et al., 2020). These compounds have antitumor activity in cancer cells of the breast, prostate, liver, colon, and lung, among others. Leaf extracts are an important source of phytochemicals that act on cancer cells from different organs or cell lines.
Pulp. Phytochemical compounds have been reported in the pulp of the anona fruit (Shetty et al., 2020) (Table 2), 17 polyphenols were quantified in methanolic extracts by HPLC-MS/MS, finding higher concentrations of salicylic acid, 3- hydroxybenzoic acid, 2,4-dihydroxybenzoic acid and gallic acid, in comparison to the reported in soursop and custard apple with antioxidant activity. Polysaccharides were extracted from the epicarp and pulp of anona, showing antioxidant activity by the DPPH method and antimicrobial activity through the release of tetrahydrocurcumin; therefore, they can be used for application on wounds (Senthilkumar et al., 2022; Shetty et al., 2020). The presence of catechins in the pulp has been reported, with in vitro antioxidant activity which was measured by the DPPH method, and in vitro anticancer activity by the 3-(4,5-dimethylthiazol-2-yl)-2 bromide methodology, 5-diphenyltetrazole (MTT), against breast cells (Rayar & Manivannan, 2016). In addition, in the pulp of anona, aromatic phytochemicals have been reported through CG-MS, Wong & Khoo (1993) identified 47 of these, mainly terpenoids (98.3 %) and monoterpenoids (91.2 %); of volatile compounds, the most abundant are terpene-4-ol (70.5 %) and α-terpineol (10 %). Jamkhande & Wattamwar (2015) report pinene, myrcene, limonene, terpinene-4-ol, and germacrene Pino et al. (2019) analyzed the flavor composition of four commercial Cuban soursop cultivars and identified 180 volatile compounds, of these 120 were reported for the first time in the pulp of this fruit; among the identified compounds, α-pinene, β-pinene, myrcene, limonene, terpinene-4-ol and germacrene stand out. Bhardwaj et al. (2019) reported the existence of essential oils in the leaves, roots and fruits of anona, such as β-pinene, spatulenol, elemol, bisabolene, (E,E)-farnesyl acetate, benzyl benzoate, and the arthurmerone. There is an important source of these for use in the pharmacological area through the extraction of compounds with antioxidant, antimicrobial, and anticancer activity from the anona pulp.
Cortex. The bark of the tree is used as a uterotonic, antispermatogenic, anti infertility, antifungal, antiplatelet, abortifacient, antiovulatory, diuretic, antiseptic, anticonvulsant, vermicidal, analgesic as a growth disturbing agent (Pathak & Zaman, 2013). Phytochemicals have been reported in this structural organ (Table 2). Saad et al. (1991) isolated reticulatacin and bulatacin acetogenins from bark extracts by NMR; in addition to two diterpenes and an alkaloid called limodenine. Hisham et al. (1994) elucidated the structure of acetogenin: reticulacinone, isolated from bark extracts of soursop stems by NMR. Bhalke & Chavan (2011) evaluated custard apple bark extracts in mice, reporting analgesic activity and mild to moderate central nervous system depressant activity, probably due to increased concentration of gamma aminobutyric acid in the brain. Obenu et al. (2022) extracted secondary metabolites in extracts with n-hexane in the bark of anona, such as steroids, flavonoids, and alkaloids; however, its antioxidant capacity, determined by DPPH, was very weak. Phytochemicals with different pharmacological activities have been identified in the bark of anona trees, although some with low antioxidant capacity.
Root. As for the soursop root, it is known to have alkaloids such as anonaine, limodenine, norushinsunine and reticulin (murcinine), with biological properties (Morton, 2013; Anaya-Esparza et al., 2020) (Table 2). Bharadwaj et al. (2019) have reported that a methanolic extract, within a carbopol gel, analyzed by GC-MS, has the potential to effectively treat skin cancer. Suresh et al. (2011) reported that anona root extracts significantly decrease tumor growth in mice, attributing this to the presence of acetogenins and alkaloids, so they can be used as a chemopreventive agent in cancer therapy. Phytochemical compounds with biological importance against some types of cancer have been extracted from the root.
Seeds. Phytochemical antitumor compounds have been identified in anona seeds by NMR. Maeda et al. (1993) characterized a series of tryptamine acyl alkaloids and Chang et al. (1998) isolated a cytotoxic acetogenin (7-lactone acetogenin), and anoretichuin, 9-one anoreticuin, cis/trans-bulatationone, bulatacin, cis/trans-murisolinone, and squamocin (Table 2), in ethyl acetate extract from seeds of anona, these acetogenins caused death in different cancer cell lines (Hep.G2, Hep.2, 3, 15, KB, and CCM2), including bladder cancer (T24). Bulatacin has been reported to cause cancer cell death in leukemia and breast cancer (Ravimanickam et al., 2018) and anonacin against bladder cancer (Yuan et al., 2003), both found in the seeds. Anaya-Esparza et al. (2020) reported the presence of steroids in anona seeds: canphane, α-copaene, β-elemene, β-caryophyllene, β-bisabolene, δ-cadinene, and germacrene D, by GC-MS. In hexanic extracts of anona seed, acetogenins with anti-angiogenic activity were obtained in mice by GC-MS, decreasing tumor growth and metastasis (Hassan et al. 2021). The seeds contain a wide variety of antitumor compounds, which act on different cell lines, with the potential to be used in treatments for humans.
Table 2 Phytochemicals reported in different organs of A. reticulata L.
| Phytochemical | Organ | References |
|---|---|---|
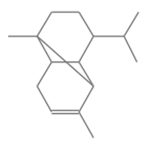 Copaene Copaene |
Leaves | Sankpal (2022) |
 Caryophyllene Caryophyllene |
Leaves | Sankpal (2022) |
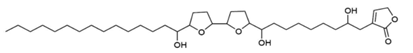 Rolliniastatin-2 Rolliniastatin-2 |
Leaves | Jamkhande & Wattamwar (2015) Ngbolua et al. (2018) Bhalke & Chavan (2011) |
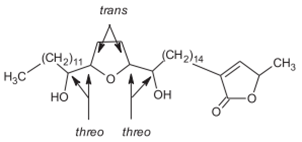 Reticulatacin Reticulatacin |
Cortex | Jamkhande & Wattamwar (2015) Ngbolua et al. (2018) |
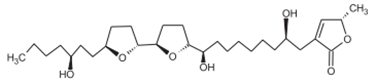 Bulatacin Bulatacin |
Cortex Seeds | Jamkhande & Wattamwar (2015) Ngbolua et al. (2018) Bhalke & Chavan (2011) |
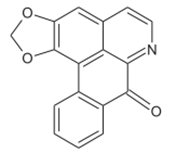 Liriodenine Liriodenine |
Cortex and root | Jamkhande & Wattamwar (2015) Ngbolua et al. (2018) |
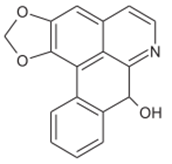 Norushinsunin Norushinsunin |
Root | Jamkhande & Wattamwar (2015) Ngbolua et al. (2018) |
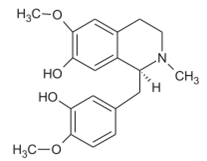 Reticulin Reticulin |
Root | Jamkhande & Wattamwar (2015) Ngbolua et al. (2018) |
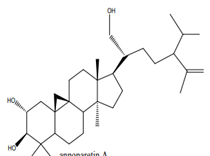 Anonaretin A Anonaretin A |
Leaves | Ngbolua et al. (2018) |
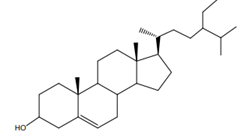 β-sitosterol β-sitosterol |
Leaves | Ngbolua et al. (2018) |
 α-pinene α-pinene |
Pulp | Bhardwaj et al. (2019) |
 β-pinene β-pinene |
Pulp | Bhardwaj et al.(2019) |
 Myrcene Myrcene |
Pulp | Bhardwaj et al.(2019) |
 Limonene Limonene |
Pulp | Bhardwaj et al. (2019) |
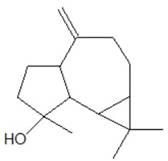 (-)-Spatunol (-)-Spatunol |
Leaves and pulp | Sankpal (2022) |
Source: Own elaboration based on Jamkhande & Wattamwar, (2015)
Food uses
Custard apple pulp is commonly consumed fresh or can be added to smoothies, custards, ice cream, and to make sauces for cakes and puddings (Morton, 2013); It is rich in carbohydrates, fats, proteins, vitamin A, vitamin C (Sasidharan & Jayadey, 2017; Jamkhande & Wattamwar, 2015; Morton, 2013) and minerals such as Ca, P, K, Mg, Na, Cl, S, Mn, Zn, Fe, Cu, Se, Co, Ni and Cr (Leterme et al., 2006) (Table 3). Moo-Huchin et al. (2014) indicate that anona fruits represent great potential for the development of new food products with bioactive properties. Senadeera et al. (2018) added anona, soursop, and saramuyo pulp to yogurts, increasing the antioxidant capacity, analyzed by the DPPH and FRAP methods. Leterme et al. (2006) found that soursop contains 0.373 mg/g of vitamin C compared to 0.206 mg/g in soursop and considered that some chemical components found in this fruit show anticancer properties against bladder cancer and other cancer cells. Custard apple pulp has a large number of compounds that give it nutritional importance for human consumption and gives it the potential to be used in the food industry.
Table 3 Nutritional content per 100g fresh pulp*
| Quantity | Units | |
|---|---|---|
| Energy | 80 - 101 | Kcal |
| Moisture | 68.3 - 80.1 | G |
| Protein | 1.17 - 2.47 | G |
| Total lipids | 0.5 - 0.6 | G |
| Ash | 0.5 - 1.11 | G |
| Carbohydrates | 20 - 25.2 | G |
| Crude fiber | 0.9 - 6.6 | G |
| Calcium | 17.6 - 27 | Mg |
| Iron | 0.42 - 1.14 | Mg |
| Phosphorus | 14.7 - 32.1 | Mg |
| Carotenes | 0.007 - 0.018 | Mg |
| Thiamin | 0.075 - 0.119 | Mg |
| Riboflavin | 0.086 - 0.175 | Mg |
| Niacin | 0.528 - 1.190 | Mg |
| Ascorbic acid | 15.0 - 44.4 | Mg |
| Nicotinic acid | 0.5 | Mg |
* Maximum and minimum levels of analysis carried out on pulp, in Central America and the Philippines. Source: Morton (2013).
Parek et al. (2011) published data on the nutritional content in the anona pulp, reporting lipid and carbohydrate values below the minimum values (Table 3). In 2010, the United States Department of Agriculture (USDA) reported the composition of nutrients in anona pulp: 0.231 g of saturated fatty acids, 18 mg of magnesium, 2.4 g of total dietary fiber, 19.2 g of vitamin C, 382 mg potassium, 0.135 mg pantothenic acid, 0.221 mg pyridoxine, 4 mg sodium, 33 U vitamin A, 0.007 g tryptophan, 0.037 g lysine, 0.004 g methionine, niacin below lower value and calcium above the upper value according to what is reported in Table 3. A large amount of nutrients contained in the anona pulp is observed, which gives it importance for its consumption by humans.
Toxicity
The bark of the anona tree contains 0.12 % of the alkaloid anonaine, which can cause paralysis in frogs; branch sap is irritating and can severely damage the eyes (Morton, 2013). However, Shivanna et al. (2019) indicate that the aqueous extracts of anona leaves supplied to mice did not have toxic effects on hematological and biochemical parameters. Furthermore, there were no adverse histopathological effects on the liver, kidney, and pancreas; therefore, these extracts could be used in food formulations with bioactive compounds.
Conclusions
The A. reticulata tree has potential for cultivation, since all its structural organs can be used from it because they have anticancer, anti-inflammatory, antihyperglycemic, analgesic, and antioxidant properties. The fruit is important for its nutrients, having the potential for the development of new food products with bioactive properties due to the high levels of antioxidants. The bioactive compounds present in the different organs of the anona plant have significant potential for possible industrialization and pharmaceutical use. The toxicity of the compounds present in the different organs of the soursop has not been sufficiently studied, so it represents an opportunity for future research.











 nueva página del texto (beta)
nueva página del texto (beta)





