Introduction
Metals are natural constituents of the environment. However, indiscriminate use in industrial activities has caused an alteration of their geochemical cycles and biochemical balance (Dixit et al., 2015). This is due to the excessive release of materials such as cadmium, lead, copper, chromium, zinc, arsenic, and mercury, among others. These metals cannot be broken down into non-toxic forms and they tend to accumulate in living organisms. Contamination of soil and water by these metals has become one of the main health problems in the world.
Some metals are toxic even at very low concentrations; some are carcinogenic and mutagenic (Nordberg, Fowler, & Nordberg, 2015). Within this group, arsenic, lead and cadmium are the most toxic substances for the environment and human health (Agency for Toxic Substances and Disease Registry [ATSDR], 2019). Moreover, it has been found that soil contamination by metals is a serious problem for agriculture because it negatively affects the yield and quality of crops (Danesh, Tajbakhsh, Goltapeh, & Varma, 2013). The main sources of metals are anthropogenic activities, such as the manufacture of pesticides and batteries, tanning and mining. Mining is the activity which contributes more to soil and water contamination by toxic metals ().
There are several physicochemical methodologies devised to remove metals from the environment. These include chemical precipitation, oxidation-reduction, ion exchange, reverse osmosis, membrane technology, evaporation, and electrochemical treatments. The main disadvantage of these methods is that they are very expensive and inefficient when metal concentrations are very low (for instance less than 100 mg∙L-1 in water) (Ahluwalia & Goyal 2007). Therefore, it is crucial to search for less expensive strategies that help to reduce the presence of toxic metals in soils and water. In this sense, filamentous fungi have been considered for biotechnological processes that allow reducing the presence of these pollutants in the environment (Bennet, Wunch, & Faison, 2002). It has been seen that sites contaminated with toxic metals are usually a source of filamentous fungi tolerant to these substances. Toxic metal tolerance is considered as the ability of an organism to survive in the presence of toxic metals using a response mechanism (Oladipo, Awotoye, Olayinka, Bezuidenhout, & Maboeta, 2018).
Aspergillus and Penicillium species have been shown to be the most tolerant to toxic metals (Ezzouhri, Castro, Moya, Espinola, & Lairini, 2009; Mohammadian, Ahari, Arzanlou, Oustan, & Khazaei, 2017; Oladipo et al., 2018). Also these are the species most common in arid or semiarid regions (Mouchacca, 2005; Oliveira, Cavalcanti, Fernandes, & Lima, 2013). Tolerance depends on factors such as the fungus species, metal and concentration of the contaminant, although environmental factors also influence it (Rose & Devi, 2018). In this work, two species of Aspergillus were isolated and identified from a mine soil located in a semiarid region, then their tolerance index to metals was evaluated.
Materials and methods
Samples and reagents
Tailings samples (15 cm depth) were collected from a gold mine located in Sonoran Desert, Mexico (110° 34' 32.84" W, 28° 48' 11.52" N) during the summer (June) of 2017. The climate is hot and dry, with temperatures often reaching 50 °C during the day, with a mean annual temperature of 24 °C and 450 mm of precipitation rates (Servicio Meteorológico Nacional [SMN], 2020). This site has been identified as contaminated soil with toxic metals (Secretaría de Medio Ambiente y Recursos Naturales [SEMARNAT], 2017). Samples (100 g) were collected according to standard methods for contaminated soils (United States Environmental Protection Agency [USEPA], 1991) at five points within a surface area of 1 ha. The samples were deposited in sterile containers and transported to the laboratory for analysis. Metal salts (AgNO3, CdCl2, K2Cr2O7, CuSO4, HgCl2, (CH3COO)2 Pb(3H2O and ZnSO4(7H2O) were used as source of toxic metals: Ag+, Cd2+, Cr6+, Cu2+, Hg2+, Pb2+ and Zn2+. Potato dextrose agar (PDA, DifcoTM) was used as culture medium.
Tailings composition analysis
The composition of metals (elemental analysis) in tailings samples was determined by energy dispersive spectroscopy (EDS) through transmission electron microscopy (TEM). The samples were dried overnight at 60 °C and examined using a nickel rack (samples holder). Elemental analysis was performed using a Field Emission Electron Microscopy JEM-2010F.
Isolation of fungal strains from contaminated soil
Samples of 1 g of tailings were deposited in 100 mL of distilled sterilized water; the mixture was stirred for 20 min at 25 °C and serial dilutions were made (10-1 to 10-4). After taking 100 μL aliquots of each dilution, they were placed in Petri dishes with PDA and incubated at 28 °C for seven days. The developed colonies were selected, and the sowing procedure was repeated in Petri dishes with PDA, until the isolation and purification of fungal strain was achieved.
Preparation of isolated spore suspension
Each isolated colony was inoculated separately in 250 mL Erlenmeyer flasks, which contained 50 mL of PDA, and were incubated for seven days at 28 °C. Subsequently, 100 mL of distilled water was added at room temperature and carefully stirred for 5 min with a magnetic stirrer. The spore count was performed with a Neubauer chamber. A suspension of approximately 108 conidia∙mL-1 were prepared to make inoculates in culture medium supplemented with the toxic metals to be evaluated.
Fungus inoculation
Each metal salt was prepared individually at a concentration of 1 mM. In the center of these Petri plates, containing solidified PDA,the spore suspension (108) was deposited in an orifice to obtain colonies of circular shape. Petri plates were incubated at 28 °C and at seven days after inoculation the radius of the colonies was measured; all tests were performed in triplicate.
Identification of isolated fungi
The strains of isolated fungi were identified based on main morphological characteristics of the colonies, considering macroscopic characteristics such as color, shape, and type of colony, as well as the most important microscopic structures such as spores and conidiophore. In addition, the identification of the strains was complemented by 18S rRNA gene amplification according to the method described by White, Burns, Lee, and Taylor (1990). A partial sequence of the 18S rRNA gene was amplified using primers ITS1 and ITS4. The result of the 18S rRNA gene sequence was compared with those available in the GenBank public databases (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Tolerance index
Fungal tolerance to the studied metals was evaluated by comparing the mycelium radii in culture medium with and without the respective metal (Valix, Tang, & Malik, 2001). The tolerance index is defined as mycelium radius with metal (cm)/mycelium radius without metal (cm).
Different metal salt concentrations were used (1, 5, 10, 15 and 20 mM) according to a multi-level categorical full factorial experimental design. The inocula of approximately 108 spores∙mL-1 were prepared in PDA culture medium. Inoculated plates were incubated at 28 °C for seven days. The tolerance index was determined on exponential phase of growth.
Minimum inhibitory concentrations and dose-response behavior
The minimum inhibitory concentration (MIC) is the lowest concentration at which a substance inhibits the growth of a microorganism (Rose & Devi, 2018). In this studio, the minimum inhibitory concentrations were obtained from the tolerance index experiments. Curves of dose (metal concentration)-response (tolerance index) behavior for each metal were obtained (Ezzouhri et al., 2009).
Statistical analysis
Data was analyzed by means of parametrical and non-parametrical methods of uni-variate statistical analysis. The normal distribution of data and homogeneity of variance were checked using the Kolmogorov-Smirnov and Levene tests, respectively. The influence of factors was evaluated by analysis of variance (One-way ANOVA) with post hoc Scheffé test. Significance was defined as P < 0.01. Results are presented as mean ± the standard deviation from the mean (n = 3). Results were also analyzed by non-parametric Kruskal-Wallis ANOVA method. OriginLab software 8.0 (OriginLab Corporation, 2008) was used for statistical analysis.
Results
Elemental composition of tailings
The elemental composition obtained by TEM-EDS indicate the presence of 13 elements (O, Si, Al, Mg, S, Ca, Fe, K, Ni, Zn, Mn, Pb and As) in tailings sample. The presence of Pb, As and Mn stands out due to their potential toxicity to a great diversity of living organisms, from microorganisms to human. According to the TEM-EDS analysis, they are found in a proportion of 0.14, 0.11 and 0.10 %, respectively (Table 1).
Fungi isolation and identification of tolerant strains
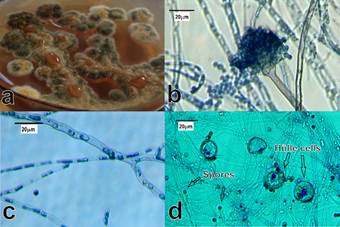
Fungal strains tolerant to toxic metals (1 mM Ag+, Cd2+, Cr6+, Cu2+, Hg2+, Pb2+ and Zn2+) were isolated from the mining tailing samples. The strains were identified morphologically (Figures 1 and 2) and genotypically.
Filamentous fungi were identified as Aspergillus flavus (Accession no. MN031598.1) and Aspergillus nidulans (Accession no. LM653124.1) based on molecular data according to the search in GenBank BLAST, with 96.74 % and 99.81 % similarity, respectively (BLAST, 2020). The length of the ITS4 sequence of the 18S RNA gene was 576 bp for A. flavus and 1 110 bp for A. nidulans.
The main macro and microscopic morphological characteristics of A. flavus are shown in Figure 1. The colonies grew rapidly at 28 °C, an initially white mycelium (first two days) turned green on the fifth day (Figure 1a). The conidiophores (Figure 1b) were 250 μm long; the spherical vesicles were completely covered by conidia and conidial biseriate head. Additionally, branched mycelium with septate, hyaline hyphae (Figure 1c) with a diameter greater than 1 μm and abundant globose conidia with size of 2.5 to 3 μm (Figure 1d) were observed. These morphological characteristics are consistent with those previously reported for A. flavus (Iheanacho et al., 2014).

Figure 1 Macro and microscopic morphological characteristics of Aspergillus flavus isolated from mining soil. a) Mycelium, b) Conidiophore, c) Hypha, d) Conidia.
The macroscopic characteristic of the A. nidulans colonies were slightly different from those of A. flavus (Figure 2). During the first 48 h at 28 °C, the mycelium displayed a yellowish appearance; it turned dark green after five to seven days (Figure 2a). The conidiophores were 150 μm long, the spherical vesicles were partially covered (three quarters of their area) by the conidia (Figure 2b). On the other hand, the mycelium was branched with septate and hyaline hyphae (Figure 2c). The globose conidia were 2.5 to 3 μm in diameter. In addition, A. nidulans presented abundant Hülle cells which are a type of cells related to the formation of cleistothecia (closed spore-bearing structures), that produce certain species of Aspergillus (Figure 2d). The observed, morphological characteristics were consistent with those previously reported for A. nidulans (Chen et al., 2016; Larone, 2011).
Minimum inhibitory concentration of tolerant strains
MIC is defined as the lowest concentration of some substance (metals in this study) that inhibits the growth of a microorganism (Ezzouhri et al., 2009). MIC’s for A. flavus and A. nidulans are summarized in Table 2. The most toxic metals for A. flavus were Ag+, Cd2+ and Hg2+; in these cases, the MIC was between 5 and 10 mM. The MIC with Cu2+ was between 15 and 20 mM. Interestingly, the growth of A. flavus was not inhibited at the highest concentration (20 mM) of Pb2+, Zn2+ and Cr6+. On the other hand, the metals that most easily inhibited A. nidulans were Ag+, Cu2+ and Hg2+ (1 < MIC < 5), while the MIC for Cd2+, Pb2+ and Zn2+ was between 15 and 20 mM and with Cr6+ fungus growth was not inhibited at the higher concentration tested (20 mM).
Table 2 Minimum inhibitory concentrations (MIC) of Aspergillus flavus and A. nidulans at concentrations 1, 5, 10, 15 and 20 mM of AgNO3, CdCl2, K2Cr2O7, CuSO4, HgCl2, (CH3COO)2Pb(3H2O and ZnSO4(7H2O as a source of the metals.
| Metal | Aspergillus flavus | Aspergillus nidulans |
|---|---|---|
| Ag+ | 5 < MIC < 10 | 1 < MIC < 5 |
| Cd2+ | 5 < MIC < 10 | 15 < MIC < 20 |
| Cr6+ | MIC > 20 | MIC > 20 |
| Cu2+ | 10 < MIC < 15 | 1 < MIC < 5 |
| Hg2+ | 5 < MIC < 10 | 1 < MIC < 5 |
| Pb2+ | MIC > 20 | 15 < MIC < 20 |
| Zn2+ | MIC > 20 | 15 < MIC < 20 |
Dose-response behavior
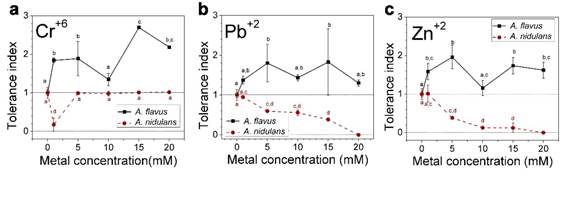
In ecotoxicological studies, dose-response relationships are fundamental for the understanding of the capacity of organisms to tolerate toxic exposures to chemical, biological, and physical agents (Agathokleous & Calabrese, 2019). In this study, the tolerance index (response) was used for assessing the behavior of the studied fungi at different doses of toxic metals. Results of statistical analysis can be observed in the dose-response graphs for each metal in Figures 3 to 5.
According to Oladipo et al. (2018), metal tolerance index for fungi can be classified as follows: ≥1 very high metal tolerance, 0.8-0.99 high metal tolerance, 0.6-0.79 moderate metal tolerance, 0.4-0.59 low metal tolerance and 0.0-0.39 very low metal tolerance. Based on this, A. flavus showed very high tolerance to the metals Cr6+, Pb2+ and Zn2+ at all the concentrations evaluated, since all tolerance index values were greater than 1 (Figure 3). In addition, significant differences were observed between the control and different concentrations of metals. At 1, 5, 10 and 15 mM of Cu2+, the tolerance index of A. flavus was very high (Figure 4). The values are statistically equal, although there are significant differences to the control sample. Only the highest concentration of copper (20 mM) inhibited the growth of A. flavus.
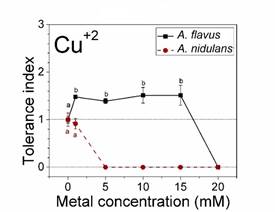
Figure 4 Dose-response behavior of Aspergillus flavus and A. nidulans. Tolerance indices with different letters denote significant differences (P < 0.01) between metal concentrations (one-way ANOVA with Scheffé post hoc testing and non-parametrical Kruskal-Wallis testing).
The tolerance index with Ag+, Cd2+ and Hg2+ was very high only at 1 and 5 mM (Figure 5). The fungus no longer grew at higher concentrations of these metals. The statistical analysis showed significant differences between the tolerance index of A. flavus at 1 and 5 mM of Ag+ respect to control (Figure 5a).

Figure 5 Dose-response behavior of Aspergillus flavus and A. nidulans. Tolerance indices with different letters denote significant differences (P < 0.01) between metal concentrations (One-way ANOVA with Scheffé post hoc testing and non-parametrical Kruskal-Wallis testing).
On the other hand, A. nidulans displayed a different behavior, with very high or high tolerance index to Cu2+, Ag+ and Hg2+ at 1 mM (Figure 5). At higher concentrations, the tolerance index decreased abruptly. In presence of Pb2+, Zn2+ and Cd2+ the tolerance at 1 mM was very high to moderate, decreasing gradually until the complete inhibition of growth at 20 mM (Figure 3).
Discussion
Tolerant fungus species
Our research shows that the two fungal strains, isolated from the same contaminated source, developed different levels of resistance to toxic metals. The high and moderate tolerance of the strains to the metals evaluated is relevant, since lead, mercury, cadmium, and chromium have been classified as priority substances due to their toxic characteristics (ATSDR, 2019).
Soil contains microbial communities of great diversity, particularly fungi found mainly in the upper 10 cm. According to reports of fungi isolated from soil, species of genera Fusarium, Penicillium, Trichoderma, Aspergillus and Geotrichum predominate. Theys have a cosmopolite distribution (Ezzouhri et al., 2009; Jiang, Wang, Xue, Cao, & Zhang, 2016; Mohammadian et al., 2017; Oladipo et al., 2018). However, the mycological diversity in environments with presence of toxic substances is usually poor than in healthy environments. In the present work only the Aspergillus genus was found.
The presence and success of an organism in an ecosystem depend both on its nutritional needs and on its environmental tolerance. The limits of tolerance to specific stressors are those that largely determine the inhabitants in extreme environments, including those contaminated with toxic substances. Some microorganisms are specially adapted to extreme habitats, where other microorganisms cannot survive (Dixit et al., 2015).
Studies on filamentous fungi in the soil of the Sonoran Desert are few, one such study is that of Ranzoni (1968), who reported several species of anamorphic fungi, among them several of the genus Penicillium and and Aspergillus, including A. flavus and A. nidulans. These results are similar those reported for other regions of semi-arid soils. Oliveira et al. (2013) found that the most abundant genera in a semi-arid region of Brazil are Aspergillus and Penicillium. Abdullah and Al-Bader (1990) reported a predominance of Aspergillus species on thermophilic and thermotolerant fungi in Iraq. The study of Mouchacca (2005) shows the fungi found in arid regions in the Middle East between 1940 and 2000 and noted the prevalence of anamorphic fungi (seven species of the genus Aspergillus).
In other countries, the presence of filamentous fungi has been confirmed in mines contaminated with metals: Nigeria (Oladipo et al., 2018), Iran (Mohammadian et al., 2017), China (Jiang et al., 2016) and Korea (Babu, Shim, Bang, Shea, & Oh, 2014). The species of indigenous filamentous fungi isolated from contaminated sites have in common a high to moderate tolerance index to different toxic metals. This extraordinary characteristic can be explained by the different strategies developed by these microorganisms to tolerate toxic metals and avoid cell damage (Gadd, 1993). Among such strategies one can list chelation and cell wall binding (extracellular sequestration) and intracellular physical sequestration of metal by binding to proteins and other ligands.
On the other hand, it has been reported that toxic metal stress may also be reduced by melanin synthesis. In a general sense, melanin refers to dark pigments synthesized by organisms of all biological kingdoms, including fungi (Nosanchuk & Casadevall, 2003). In this regard, filamentous fungi with dark-colored mycelium (black, brown or deep green) have been predominantly found in the Sonoran Desert (Ranzoni, 1968). Melanins have been suggested to play many roles in fungal biology (Cordero & Casadevall, 2017). Given their many unique properties, it seems likely that melanins help fungi survive a range of biotic and abiotic stresses. The latter include high UV radiation, reactive oxygen species and toxic metals. Melanins are polymerized from phenolic and/or indolic compounds. The result is negatively charged, hydrophobic pigments of high molecular weight (White, 1958). Melanins form granules that may accumulate at the cell surface or be released into the extracellular space (Malofe, Solhaug, Minibayera, & Beckett, 2019). In the Sonora Desert filamentous fungi with dark-colored mycelium (intense green, brown, or black) have been predominantly found (Ranzoni, 1968). Melanins exhibit high binding affinity for many different metal ions (Cordero & Casadevall, 2017). Carboxyl, amine, and hydroxyl functional groups of the pigment can potentially bind metals. Therefore, the importance of melanins in metal tolerance is a topic of interest for future research.
Each species can use several strategies. In addition, its large surface/volume ratio and its intense metabolic activity filamentous fungi are very efficient vectors to accumulate metals (Dixit et al., 2015).
Dose-response behavior
A remarkable high tolerance to toxic metals by Aspergillus species has been reported (Thippeswamy, Shivakumar, & Krishnappa, 2014). In this research Cu2+ was very toxic to A. nidulans, it only grew at a concentration of 1 mM. However, other studies have shown that some fungal species are resilient to this metal (Oladipo et al., 2018). It has been shown that the tolerance to copper is due to an active process that involves the synthesis of a metallothionein (Kermasha, Pellerin, Rovel, Goetghebeur, & Metche, 1993). The metallothionein is a low molecular weight protein rich in cysteine that allows the union of copper, cadmium, and zinc; once bound these metals become innocuous.
Hg2+ is toxic, but A. nidulans and A. flavus are still able to grow at 5 and 1 mM, respectively. It has been reported that other fungal species are capable to tolerate and accumulate this metal (Dhawale, Lane, & Dhawale, 1996; Kurniati, Arfarita, & Imai, 2014). Regarding Cd2+, it is one of the most toxic metals for the A. flavus isolated in this research. This result agrees with other experiments that have reported a strong inhibition of fungi exposed to minimal concentrations of cadmium salts (Joo & Hussein, 2012; Oladipo et al., 2018). In contrast, A. nidulans had resisted higher concentrations of this metal as reported in other works; for instance, Massaccesi, Romero, Cazau, and Bucsinszky (2002) showed that several species of filamentous fungi, isolated from sediments contaminated by industrial activities, removed up to 70 % of Cd2+ in 13 days. These results demonstrate the adaptability of fungal microorganisms, as well as their potential for use in bioremediation.
Pb2+, Cr6+ and Zn2+ exerted a different effect on the growth of A. flavus; the highest concentration of these metals evaluated in this work was not enough to inhibit the growth of the microorganism. The same behavior has been observed in yeasts isolated from coal mines (Castro-Silva et al., 2003). Despite this, it has also been observed that Zn2+ can have toxic effects in certain species of fungi such as Penicillium atramentosum 25SL, which decreased its growth when exposed to 40 mM solutions (Levinskaite, 2001). It has been suggested that the mechanism by which some fungal species immobilize or capture Zn2+ is the intracellular and extracellular exchange in the form of polyphosphate precipitation. Although Zn2+ is essential for all organisms, at very high concentrations it can have a toxic effect (Balsalobre et al., 2003). For certain species of fungi, exposure to toxic substances in their habitat can induce adaptive changes that allow them to remain viable under these stressful conditions (Valix et al., 2001). It is known that the species Aspergillus spp. has developed an enzymatic mechanism to detoxify Cr6+; this mechanism is mediated by an antioxidant enzyme system involving peroxidases, catalases and peroxide ascorbate (Chakraborty, Mukherjee, & Das, 2012; Srivastava & Thakur, 2006).
Life on Earth has existed since its beginning in extreme environments, where cells have been exposed to free radicals and various toxic substances (Calabrese et al., 2007). Interestingly, were observed that the tolerance behavior of A. flavus when exposed to toxic metals was stimulated (values above the control). Particularly the fungus showed stimulation at low doses of Cu2+ and Ag+ (Figures 4 and 5a). According to Calabrese and Blain (2009), this behavior corresponds to hormesis, a characteristic phenomenon that in toxicology describes biphasic dose response processes where cells or organisms (animals, fungi or plants) benefit from exposition to low doses of toxic or harmful environmental factors. This process is followed by inhibition and toxic effects at higher doses, as observed in Figures 4 and 5. Life on earth has existed since its inception in extreme environments, in which cells have been exposed to free radicals and various toxic substances. For this reason, organisms have developed complex mechanisms to deal with environmental hazards (Calabrese et al., 2007). The mechanism that explains hormesis (conditioning, adaptive response, and self-protection) establishes that at low levels of stress, metabolic pathways that already exist at the molecular and cellular level are autoregulated. In the case of fungi, the mechanisms that help them to resist the presence of toxic metals are hormetics routes that involve proteins and binding peptides, such as phytochelatins and metallothioneins (Sácký et al., 2014), which allow them to incorporate toxic metals into their cells. On the other hand, there are proteins with enzyme functions, for example, those of the cytochrome P450 superfamily and laccase mainly (Sharma, Dangi, & Shukla, 2018), used by fungi to biotransform metals to less toxic chemical forms. For A. nidulans it was not observed hormesis with the metals at the different concentrations.
Statistical analysis
The data obtained from the complete factorial experimental design were analyzed by different statistical methods used in toxicology research. The analysis of such methods helps us to choose the adequate statistical test to avoid inflating the type I error, when the null hypothesis is true, and it is rejected (null hypothesis: means are equal) in our interpretation of the results. Multi-way ANOVA, multivariate linear regression analysis and log normal regression analysis were not appropriate, high quantity of zeros when the doses of the metal were inhibitory and for the high variability when the growth of the fungus was stimulated above the control. Therefore, statistical analysis of one factor at the time was performed for determining the significance and the effect of concentration for the A. flavus and A. nidulans at specific metal.
Then, the Kolmogorov-Smirnov test performed. This revealed that the data were normally distributed, except for zero tolerance index, however, the Levene test revealed that some data were heteroscedastic. It is interesting that, for one-way ANOVA, homoscedasticity or normality assumptions are rarely tested in studies of toxicology (Na, Yang, Bae, & Lim, 2014).
In this case, one-way ANOVA was complemented with the Scheffé test, which is a more conservative post hoc test, and were used a conservative level of significance (P < 0.01) the most appropriate response to a significant ANOVA (Oliveira et al., 2014). The Scheffé procedure is the only multiple comparison procedure that is entirely coherent with ANOVA results. That is, with the Scheffe procedure, one is guarantee that if the ANOVA suggested a difference between groups (groups in this study: different concentrations of metals for each fungus) then at least one of the Scheffé comparisons will be significant at the same level (Ruxton & Beauchamp, 2008).
In addition, non-parametrical Kruskal-Wallis testing was performed for corroborate significant differences at each metal concentration. However, Kruskal-Wallis is based on differences of medians and ranges. Both, parametrical and non-parametrical test had the same results. The behavior of the two fungal species is statistically different, and it also depends on the metal and the concentration.
Conclusions
Aspergillus flavus and A. nidulans fungi are present in tailings in the semiarid region of the Sonoran Desert. The isolated filamentous fungi display different levels of tolerance to toxic metals (Ag2+, Cd2+, Cr6+, Cu2+, Hg2+, Pb2+ and Zn2+) which depend on the species, the type of metal and concentration. Aspergillus flavus is the fungus with the highest tolerance to all metals, with growth observed even at concentrations of 20 mM of Cr6+, Pb2+ and Zn2+. In future research, it will be interesting to search for the mechanisms that allow A. flavus its high level of tolerance to toxic metals. On the other hand, A. nidulans proved to be highly tolerant to Cr6+ up to a concentration of 20 mM, and to Ag+, Zn2+, Pb2+ and Cu2+ up to 1 mM. The tolerance level observed by these fungi, especially to Cr6+, Pb2+ and Zn2+, suggests their potential use to remove these metals.











 texto en
texto en