Introduction
The COVID-19 pandemic caused an increase in environmental disturbance due to the excessive use of plastic materials, both in food and for personal safety (Benson et al., 2021). The increasing demand for packaged food has increased the consumption of single-use packaging (Janairo, 2021). Therefore, several studies have focused on the development of biodegradable, biocompostable and environmentally friendly packaging (Zhang & Sablani, 2021).
Biopolymer films represent an environmentally friendly packaging alternative. These films are formed by biopolymer matrices with different functionalities, such as moisture barrier properties, active substance carriers and biocompatibility to contain different food. Among the advantages of films made with biopolymers is their rapid biodegradability in the environment (Trajkovska-Petkoska et al., 2021).
Gelatin is one of the most versatile biopolymers in film formation. This polypeptide could be combined easily with lipids and carbohydrates to form a polymeric network due to its gelling properties (Tyuftin & Kerry, 2021). It has also been observed that gelatin films allow the incorporation of different types of additives, with the purpose of extending the shelf life of food products by reducing microbial growth (Roy & Rhim, 2020).
Essential oils, antioxidants and extracts from organic residues, have been added in biopolymeric matrices to delay spoilage caused by microorganisms, with additional benefits in the hygienic-sanitary process of food (Mir et al., 2018). Natural extracts from residues of horticultural products are sources of bioactive compounds, and have been studied as additives in film formation, such is the case of extracts of mango peel (Chaiwarit et al., 2020), orange (Terzioğlu et al., 2021), lime (Rodsamran & Sothornvit, 2019), pineapple (Kumar et al., 2021b), among others.
Coconut shell is a natural resource commonly used as an input for the elaboration of heavy metal removing filters (Sengupta & Basu, 2016); it also represents a high source of fiber and active compounds. However, its effect as an additive in biopolymeric matrices has not been studied. In this regard, the objective of the present study was to evaluate the effect of a coconut shell extract (CSE) added to gelatin-carboxymethylcellulose (G-CMC) biopolymeric films on its physicochemical and antifungal properties, to determine its potential use as a packaging material for fruit and vegetable products.
Materials and methods
Materials and reagents
The reagents used to prepare the films were gelatin type B, carboxymethylcellulose (CMC) and glycerol (Merck, Germany), in addition to potato dextrose agar (PDA) and potassium nitrate (Meyer, Mexico). All materials used were analytical reagent grade.
Collection and preparation of coconut shell extract
Coconut (Cocos nucifera L.) fruits were purchased at consumption maturity from a local market in Texcoco, Estado de México. Harvest was performed 24 h before purchase. The fruits were transported in plastic containers to the Laboratorio de Biomateriales del Centro de Investigación en Ciencia Aplicada y Tecnología Avanzada - Unidad Legaria, del Instituto Politécnico Nacional, where those with mechanical damage or infection by phytopathogens were removed.
To obtain the coconut shell extract, approximately 15 fruits were selected, defibrated and the endocarp was removed. Defibrated samples were ground using a disc mill (148-2, The Bauer Co., USA) and dried in a digital oven (Fe-294AD, Feligneo, Mexico) at 60 ± 2 °C for 24 h. The extraction was performed considering the method described by Nagarajan et al. (2015), with some modifications. A total of 15 g of the dried material was mixed in 500 mL of 30 % (m/v) ethanol. The mixture was sonicated in an ultrasonic bath (TI-H-5, Elma, Germany), centrifuged (model K, International Equipment Co., USA) at 1 750 rpm for 30 min and filtered with Whatman No. 1 paper (Whatman® International, Ltd., England). Finally, the filtered mixture was concentrated at 40 ± 2 °C using a rotary evaporator (RE-500, Yamato, Japan) and freeze-dried (FreeZone 4.5, Labconco™, USA), thus obtaining the aqueous-ethanolic coconut shell extract (CSE). The CSE was stored in hermetically sealed amber jars at 53 % relative humidity (RH).
Preparation of active films
Gelatin (G), CMC, and glycerol were dissolved in distilled water using a ratio of 1:1:0.6, respectively. The solution was heated at 90 °C in a stirring hot plate (SP1311325Q, Thermolyne™, USA) with constant stirring (600 rpm) for 1 h. The solution was cooled at room temperature (25 °C), and then different concentrations of CSE were added: G-CMC (control, without addition of extract), 200/CSE (200 mg·L-1 extract), 300/CSE (300 mg·L-1 extract) and 400/CSE (400 mg·L-1 extract). Finally, 25 mL of the solution were poured into Petri dishes (1.5 x 12 cm) and placed in a digital oven at 60 °C for 24 h. The dried films were removed from the dishes and stored in hermetically sealed bags without exposure to light until analysis. Prior to each characterization, the films were conditioned in a desiccator at 25 °C and 53 % RH.
Characterization of the films
Thickness and mechanical properties
The thickness of the films was measured with a digital micrometer (H-2781, Mitutoyo, Japan), with an accuracy of 0.01 mm. Measurements were taken at ten different positions on each film, and the average was recorded in µm.
The mechanical properties measured were puncture force (N), and tensile strength (MPa) (American Society for Testing and Materials [ASTM], 1995a), both with a texture analyzer (TA-XT2i, Stable Micro Systems, England) and 25 N fixed load cell. To determine the puncture force, 2.5 cm diameter circular samples were cut from each film. The samples were fixed in circular cells adapted for the test. For tensile strength, rectangular specimens of 2 x 4 cm2 were cut and placed in the equipment with grip cells. The measurement was performed at a speed of 1 mm·s-1. Finally, this parameter was calculated using Equation 1.
Where F max is the maximum force supported by the film (N) and A is the cross-sectional area of the film (m2).
Water vapor permeability (WVP)
Water vapor permeability (WVP; g·m-1·s-1·Pa-1) was determined according to the modified ASTM (1995b) standard method. Containers of 3 cm diameter with a capacity of 120 mL were adapted. Films covered the top of each container and were hermetically sealed with paraffin wax; subsequently, 25 mL of a saturated potassium nitrate solution (40 %) were injected into each container and stored in a controlled chamber at 28 °C and 97 % RH. The containers were weighed every 8 h on a digital balance (Ve-300, Velab™, Mexico) with 0.01 mg resolution. The transpiration rate was determined by linear regression analysis (slope of weight loss by time), and permeability was calculated using Equation 2.
Where r v is the transpiration rate (g·m-2·s-1), P v* is the saturation vapor pressure (Pa), RH 1 is the relative humidity of the chamber (dimensionless), RH2 is the relative humidity of the container (dimensionless) and T is the film thickness (m).
Scanning electron microscopy (SEM)
The morphology of the films was observed in a scanning electron microscope (SEM) (JSM 6390 LV, JEOL, Japan) with an accelerating voltage of 10 kV. For this purpose, 4 mm2 samples were cut from each film and fixed in cylindrical sample holders with double-sided adhesive carbon tape. The samples were coated with gold by sputtering (Desk IV, Denton Vacuum, USA) for 120 s. The images collected were of the cross-section of the films at magnifications of 1000x and 1500x.
Color
The color of the films was analyzed as described by Nallan-Chakravartula et al. (2020). A CIE Lab high-definition colorimeter (NH300, 3NH, China) was used, and the whiteness index (WI) and yellowness index (YI) were calculated from L* (luminosity), a* (greenness/redness) and b* (blueness/yellowish) values with Equations 3 and 4, respectively:
A white standard (L* = 94.512, a* = 0.417 and b* = 0.350) was used to calibrate the equipment.
UV light transmittance and opacity
To measure UV light transmittance, 1 x 3 cm2 pieces were cut from each film and conditioned in a measuring cell with a spectrophotometer (Lambda 35 UV-Vis, Perkin Elmer®, USA) (Kchaou et al., 2020). The measurement range was set from 200 to 800 nm. Film opacity (abs·mm-1) was determined by Equation 5.
Where Abs 600 is the absorbance at 600 nm and x is the thickness of the films (mm).
Fourier transform infrared spectroscopy (FTIR)
The spectra of the films were recorded on an FTIR spectrophotometer (Cary 630, Agilent Technologies, USA), with a resolution of 2 cm-1 and a scan rate of 32 scans·min-1. Measurements were carried out from 4 000 to 650 cm-1.
Determination of the in vitro antifungal activity of coconut shell extract
The antifungal activity of CSE was evaluated using concentrations of 0, 200, 300, and 400 mg·L-1. Each concentration was applied on a sterile disc of filter paper (Whatman No. 1) of 25 mm diameter, fixed in the central part of a Petri dish with PDA. The dishes were pre-inoculated with a suspension of 106 spores·mL-1 of Aspergillus niger and Rhizopus stolonifer in 20 mL of culture medium, and stored in a digital incubator (IC 403CW, Yamato, Japan) at 25 °C and 97 % RH for 48 h. After the incubation period, the inhibition halo around the disc (mm) was determined using a vernier (500-196-30, Mitutoyo, Japan). The fungal cultures used were collected at the Laboratorio de Microbiología de la Universidad Autónoma Chapingo using the method of Luksiene and Buchovec (2019).
Statistical analysis
One-way ANOVA analyses were carried out to evaluate the physicochemical and antifungal properties of the films and Tukey's mean comparison tests (P ≤ 0.05). MINITAB® statistical software was used for the analyses. All analyses were performed in triplicate, and data are reported as the mean ± standard deviation.
Results and discussion
Thickness and mechanical properties
Film thickness changed significantly (P ≤ 0.05) among treatments (Table 1). The films with 200/CSE, 300/CSE, and 400/CSE increased 3.4, 11.9 and 14.3 µm compared to the control, respectively. These results suggest compatibility between the gelatin peptides and the extract components, which resulted in the development and formation of a thicker compact matrix. A similar effect was reported by Ahmad et al. (2012) on gelatin films with bergamot and lemongrass extracts. Initially, these authors observed a disruption in the polypeptide chains of gelatin by the presence of the extract; then, these polypeptides were able to interact and form bonds with the phenolic compounds due to the hydrophilic nature of the extract, resulting in a structure with greater size. It should be noted that the thickness of the films tends to increase by incorporating external agents related to the biopolymeric solution since otherwise, the agent could saturate the solution and, consequently, develop thin, fragile, brittle structures with non-soluble residues (Xue et al., 2021). Furthermore, it is essential to obtain a product with greater thickness as packaging material and subsequent handling.
Table 1 Thickness, puncture force, tensile strength, water vapor permeability (WVP) and opacity of gelatin-carboxymethylcellulose (G-CMC) films and those added with different concentrations of coconut shell extract (CSE).
| Treatment | Thickness (µm) | Puncture force (N) | Tensile strength (MPa) | WVP (× 10-11, g·m-1·s-1·Pa-1) | Opacity (abs·mm-1) |
|---|---|---|---|---|---|
| Control (G-CMC) | 23.4 ± 0.3 a | 24.5 ± 1.3 a | 14.8 ± 1.4 a | 3.3 ± 0.2 a | 2.76 ± 0.1 a |
| 200 mg·L-1 (200/CSE) | 26.8 ± 1.1 b | 34.5 ± 0.9 b | 16.7 ± 1.2 ab | 3.7 ± 0.1 a | 4.91 ± 0.1 b |
| 300 mg·L-1 (300/CSE) | 35.3 ± 0.6 c | 30.1 ± 0.7 c | 20.1 ± 1.9 b | 5.3 ± 0.1 b | 5.16 ± 0.1 c |
| 400 mg·L-1 (400/CSE) | 37.7 ± 0.8 d | 29.4 ± 0.7 c | 16.3 ± 1.0 a | 6.1 ± 0.1 c | 5.30 ± 0.2 c |
Means with the same letter in each column are not statistically different (Tukey, P ≤ 0.05).
The puncture force of the G-CMC film was 24.5 N, with significant differences (P < 0.05) compared to the other treatments (Table 1). The highest force obtained was 34.5 N with 200/CSE. The stability of the matrix may be due to the electrostatic interactions and to the hydrogen bonds established between the hydroxyl groups of the polyphenols of the extract and the hydrophilic acceptor molecules of the gelatin in the biopolymeric matrix (Hoque et al., 2011). Therefore, the increase in the strength of the films with CSE could be related to the formation of a stable biopolymeric network according to the extract concentration. On the other hand, puncture force decreased significantly (P < 0.05) in the 300/CSE and 400/CSE treatments compared to 200/CSE. As mentioned above, at high concentrations of external agents, the solution saturates the available bonds and generates a dense structure. Similar results were obtained by Kan et al. (2019) on chitosan/gelatin films with Crataegus pinnatifida extract.
Similar to the previous analysis, the tensile strength decreased significantly when incorporating 400 mg·L-1 of CSE, compared to the treatment with 300 mg·L-1. This decrease could be due to the high concentration of phenolic compounds, which generated a heterogeneous structure with discontinuous areas in the film. These results agree with those reported by Kchaou et al. (2020). Furthermore, they indicate that mechanical properties decrease with the incorporation of natural extracts in quantities greater than the saturation of the solution or when generating incompatibility with the biopolymers of the matrix because it creates a decrease in the density of intermolecular interactions and an increase in the free volume between the gelatin chains, which causes fragility in the films.
The highest puncture force and tensile strength were achieved when the extract was applied at low and medium concentrations (200 and 300 mg·L-1). This phenomenon occurs because of the stability of the biopolymeric network generated through electrostatic interactions and hydrogen bonds between the extract and the biopolymeric matrix. On the other hand, the maximum extract concentration (400 mg·L-1) decreases the mechanical properties, since the compounds in the extract reduce the density of intermolecular interactions, which generates a discontinuous polymeric network. Therefore, the incorporation of CSE into the biopolymeric matrix modified both the mechanical properties and the thickness of the active films without compromising their formation, handling, and demolding. Higher values of resistance and puncture strength are desirable in developing biopolymeric films aimed at food packaging because they reflect the net load they can support during handling.
WVP
The lowest WVP value was reported in the control films (3.3 × 10-11 g·m-1·s-1·Pa-1), while films with CSE increased their value by 0.4, 2, and 2.8 × 10-11 g·m-1·s-1·Pa-1, with 200/CSE, 300/CSE, and 400/CSE, respectively (Table 1). The incorporation of CSE significantly (P < 0.05) affected the WVP values. The increasing values were proportional to the concentration of extract added to the film-forming solution. The high permeability can be explained by the sorption, diffusion, and desorption processes, where polymers of a hydrophilic nature transit and may increase that parameter (Xu et al., 2020). First, the phenomenon consists of sorption and solubilization of biopolymers, followed by diffusion of water molecules in the matrix and desorption or evaporation (drying phase) of water molecules on the film surface.
In the present study, the concentration of biopolymers, plasticizer, and water were the same; therefore, the increase in permeability was caused by the concentration of the added extract. Dou et al. (2018) found that WVP in gelatin films was affected by the incorporation of plant extracts, as a dense network structure was formed between the polymers and the extract, thereby decreasing the permeability. In another study, Malherbi et al. (2019) found that WVP in gelatin films with guabiroba pulp extract had variable results regarding the concentration of the added extract.
The results of the present study confirm that WVP in gelatin films is related to the proportion of the extract added to the solution. Thus, as the amount of extract increases, the heterogeneous areas generated by G-CMC-CSE interactions increase, which triggers the discontinuity in the polymeric network and increases WVP. The measurement of this parameter is decisive in films for food preservation, due to the need for protection and a barrier against the concentration of water vapor from the environment and the food.
SEM
In the micrographs of the control film, a smooth, uniform structure with no visible cracks is observed (Figure 1a), reflecting good compatibility and homogeneity between polymers in the matrix. This homogeneity is attributed to the affinity among gelatin, CMC, and glycerol in aqueous medium. Water favors the formation of molecular interactions such as hydrogen bonds among these biopolymers (Nazmi et al., 2017).

Figure 1 Scanning electron microscopy images of the cross-section of gelatin-carboxymethylcellulose (G-CMC) films and those with different concentrations of coconut shell extract (CSE) at 1000x and 1500x magnification.
The homogeneous structure of the film was affected when 200 mg·L-1 of CSE were added (Figure 1b). Roughness is evidence of dispersion of the extract in the matrix, where hydrophobic interactions of CSE and the formation of hydrophilic interactions with the phenolic compounds in the extract are involved (Nor Adilah et al., 2020).
An intensification of roughness and craters can be seen in the cross-section images of the 300/CSE and 400/CSE films (Figures 1c and 1d), with irregular and discontinuous sections. This heterogeneous surface is consistent with increasing extract concentration. Similar results were reported by Rasid et al. (2018) on gelatin films with extract ofCentella asiaticaL. Thus, the roughness and cracks observed in films intensified as the extract increased. Furthermore, these changes are correlated with the formation of intermolecular bonds between polymers (FTIR section). Finally, although the addition of extract increased the complexity in the matrix, the discontinuities in the biopolymeric network caused by CSE addition did not compromise the formation of films.
Color
The highest values of luminosity (L*) and parameter a* (greenness/redness) were obtained with the control treatment (Figure 2). In contrast, films with CSE showed a significant (P < 0.05) decrease in L* values and, consequently, an increase in a* and b* (blueness/yellowish) values. This indicates that films lost luminosity and acquired a yellowish tone, which agrees with the YI results, because as the amount of CSE in the G-CMC matrix increased, the level of brownness in the film increased.

Figure 2 Luminosity (L*), color parameters a* and b*, whiteness index (WI) and yellowness index (YI) of gelatin-carboxymethylcellulose (G-CMC) films and those added with different concentrations of coconut shell extract (CSE).
The results were consistent with the WI, where a significant effect was also observed according to the CSE concentration. The lowest value of that parameter was observed in the treatment with the highest CSE concentration (Figure 2). This indicated that the color of the control films, being colorless and translucent, can change its hue with a minimum of additive concentration. In this case, the observed changes in color parameters were mainly attributed to the presence of phenolic compounds of CSE, which presented a brown coloration upon incorporation (Li et al., 2021). Therefore, the addition of CSE into the G-CMC matrix significantly modified the color parameters of the films. The development of new materials with color contribution can reduce light exposure, and thus avoid oxidative damage to food products, suggesting an improved packaging technology.
Light transmittance and opacity
The incorporation of CSE in the gelatin and carboxymethylcellulose matrix caused significant changes (P < 0.05) in the transmittance values according to the added extract concentration (Figure 3). The transmittance in films with CSE increased from 350 nm, while in the control film it was from 200 nm; i.e., a translucent film was obtained in the entire light spectrum studied. Although the G-CMC film was translucent and glossy, the incorporation of the extract changed the transparency of the film to a brownish-yellow color.

Figure 3 UV-Vis light transmittance of gelatin-carboxymethylcellulose (G-CMC) films and those with different concentrations of coconut shell extract (CSE).
The color contributed by the CSE could be due to the high content of lignin, which is characterized by absorbing UV light, as demonstrated in several studies (Shikinaka et al., 2020). In food packaging, materials that create a barrier to UV-Vis light are desirable to reduce lipid peroxidation, nutrient loss and thereby prevent the generation of unpleasant odors and flavors (Ge et al., 2018). Therefore, the films with CSE obtained in the present study have an advantage as UV light blockers.
The results obtained by UV-Vis indicate higher opacity as the concentration of the CSE increases (Table 1). Both transparency and opacity of films are important optical properties in materials intended for food packaging. High absorbance indicates higher transparency and thus lower opacity (Khedri et al., 2021). Since this is a light color contributed by CSE, the films maintained relatively low opacity (Figure 4). A similar result was reported by Nor Adilah et al. (2020) on gelatin films with mango peel extract, where the yellowish tone observed was attributed to the carotenoids present in the fruit due to its ripening state.

Figure 4 Opacity of gelatin-carboxymethylcellulose (G-CMC) films and those with different concentrations of coconut shell (CSE).
Having a film with higher opacity for the packaging material industry might seem a disadvantage, since a translucent material is desirable; however, in foods with high fatty acid content, such as avocado, cheese, meat, among others, it is essential to handle the food with a material that avoids oxidation against UV light (Michelin et al., 2020). In this sense, CSE could be a natural additive alternative to decrease UV light transmittance and increase opacity in G-CMC films and, with this, create a packaging material with barrier properties at wavelengths from 200 to 350 nm.
FTIR
In the FTIR spectra of the G-CMC film and its interactions with CSE at different concentrations, characteristic bands of gelatin such as amide A at 3 270 cm-1, amide B at 2 930 cm-1, amide I at 1 634 cm-1, amide II at 1 534 cm-1and amide III at 1 240 cm-1 were detected (Figure 5), which represent the vibrational modes of the peptide bond of gelatin. These findings are consistent with that reported by Rasid et al. (2018). Additionally, all films showed similar bands in the range of 3 200 to 3 500 cm-1, attributed to amide A interactions (N−H stretching vibrations) and intermolecular and intramolecular interactions of the ‒OH groups of the polymeric components (gelatin, carboxymethylcellulose and glycerol). In the films with CSE, an increase in the stretching of the ‒OH band is shown, which could be due to the number of compounds of phenolic nature in the extract (Tanwar et al., 2021).
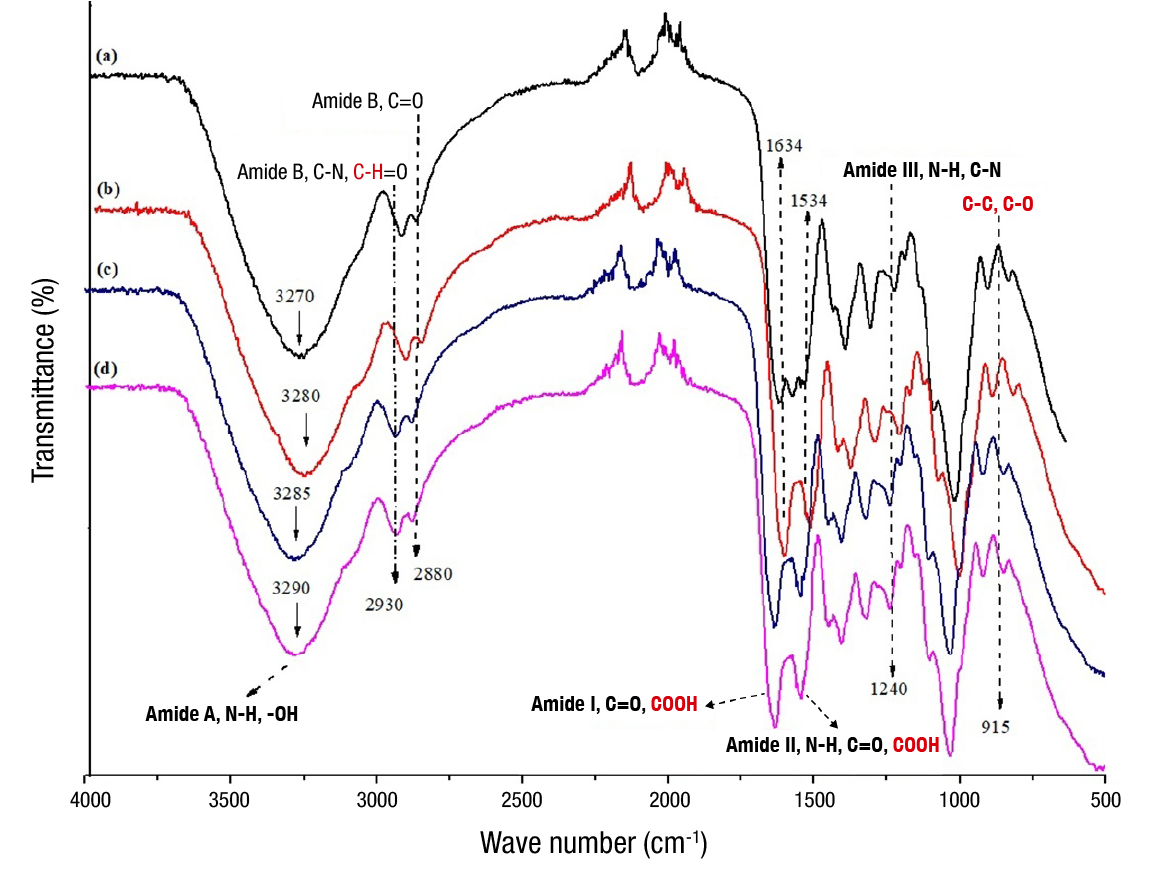
Figure 5 Fourier transform infrared spectroscopy spectra of gelatin-carboxymethylcellulose (G-CMC) films and those with different concentrations of coconut shell extract (CSE): a) G-CMC (control, without adding CSE), b) 200 mg·L-1 of CSE (200/CSE), c) 300 mg·L-1 of CSE (300/CSE) and d) 400 mg·L-1 of CSE (400/CSE).
The peaks at 2 930 cm-1 and 2 880 cm-1 are characteristic of amide B (C−N stretching and C=O asymmetric stretching vibrations). The C−H interaction in the 2 930 cm-1 band represents the methane ring of the CMC hydrogen atoms, which is consistent with that reported by Kowalczyk et al. (2020). The band 1 634 cm-1 is attributed to amide I, which represents the C=O bonds of gelatin, and the COOH-coupled hydrogen bond of CMC. The band present at 1 534 cm-1 refers to amide II of gelatin (N−H and C=O interactions). Similarly, amide II could be interacting with the −COOH group of CMC (Esteghlal et al., 2018). At 1 240 cm-1 amide III is identified with N−H and C−N bonds, and in the band present at 915 cm-1, C−C and C−O interactions of CMC are observed (Kowalczyk et al., 2020).
The study of the FTIR spectra was carried out to evaluate and determine the interactions between the compounds involved in the polymeric solution. The incorporation of CSE in the G-CMC films favored intermolecular and intramolecular interactions by non-covalent bonds between the −OH groups of the compounds.
In vitro antifungal activity
The G-CMC film showed no inhibition of the growth of R. stolonifer and A. niger strains (Table 2), indicating that this film has no antifungal properties against the strains studied. These results are consistent with that reported by Sahraee et al. (2017) on gelatin films. The above shows that biopolymers lack of inhibitory activity.
Table 2 Antifungal activity of gelatin-carboxymethylcellulose (G-CMC) film and those with different concentrations of coconut shell extract (CSE).
| Treatments | Inhibition halo (mm)** | |
|---|---|---|
| Rhizopus stolonifer | Aspergillus niger | |
| Control (G-CMC) | 0.0 ± 0.0 a* | 0.0 ± 0.0 a* |
| 200 mg·L-1 (200/CSE) | 64.17 (± 0.2) b | 64.10 (± 0.3) b |
| 300 mg·L-1 (300/CSE) | 64.10 (± 0.1) b | 64.87 (± 0.1) c |
| 400 mg·L-1 (400/CSE) | 65.0 (± 0.0) c | 65.0 (± 0.0) d |
* No zone of inhibition, even the fungal pathogen grew on top of the disc. ** Inhibition halo diameter, except for the 25 mm disc diameter. Means with the same letter in each column are not statistically different (Tukey, P ≤ 0.05).
The treatments 200/CSE and 300/CSE showed antifungal activity by presenting an inhibition halo of 64.17 and 64.10 mm for R. stolonifer, and 64.10 and 64.87 mm for A. niger, respectively. Furthermore, increasing the concentration of CSE to 400 mg·L-1 resulted in higher inhibitory activity against both fungal strains (Table 2). The antifungal activity of coconut shell is related to the presence of a high content of catechins. In addition, many studies have reported a high content of lignin and condensed tannins in coconut shell, to which antibacterial (Candida albicans, Escherichia coli and Staphylococcus aureus), antiviral and anticancer properties have been attributed (Prakash et al., 2018).
The plausible mechanism of the biocidal activity of CSE, reported as a lignin-rich source, is centered on the cell membrane, as it causes damage and rupture of bacterial cells and, consequently, death of the microorganism (Yang et al., 2016). Moreover, the antimicrobial effect depends on the type of extraction, climatic condition, harvesting period, among other factors (Kumar et al., 2021a). Other studies have confirmed the antimicrobial effect of lignin extracted from various biological sources (de Sousa-Nascimento et al., 2021). Therefore, the incorporation of CSE into G-CMC films represents a packaging alternative with fungicidal action to preserve the shelf life of foods by controlling deterioration caused by the proliferation of phytopathogens.
Conclusions
This study revealed that adding CSE significantly increased the thickness of the films; in addition, it modified the mechanical and vapor permeability properties of the G-CMC films. CSE gave a brown color to the translucent films, increasing the opacity and having a protective effect against UV light. The microstructure was modified by adding CSE; however, the extract had no effect on the formation and release of the films. Furthermore, CSE showed high inhibitory capacity against R. stolonifer and A. niger strains. Therefore, the films represent a promising material as a substitute for conventional packaging, since, in addition to their characteristics, they can reduce food deterioration caused by fungi.











 texto en
texto en 



