Introduction
Bladder stones, composed of struvite and carbonate-apatite, were recovered from an Egyptian mummy dated back to 4,800 years BC.1 Observations related to lithiasis of probable infectious origin can be found in the writings of Hippocrates (460-370 BC) and Avicenna (980-1037 AD), as well as in more recent publications from the 17th to the 20th century.1
The first paper in which a clear definition of encrusted cystitis is given, describing the signs and symptoms, cystoscopic findings, histopathology, pathogenesis, prognosis, diagnosis and treatment of this entity was published by Françoise in 1914.2
Encrusted cystitis is a chronic disease of the urinary bladder with inflammation and calcareous deposits encrusted in the mucosa, generally accompanied by alkaline urine, sometimes with a strong ammonia-like odor, leukocyturia and, occasionally, hematuria of varying intensity. One of the most noticeable characteristics of encrusted cystitis is the intense pain that patients suffer and the difficulty of its management without proper diagnosis and treatment. In the words of Françoise: "Encrusted cystitis may not be a death sentence, but it is a torture for life".2
Encrusted pyelitis can be defined as the presence of calcareous incrustations in the wall of the renal pelvis with severe inflammation of the adjacent tissues and may be associated with struvite staghorn calculi and urological manipulations.3
Encrusted cystitis and encrusted pyelitis share very common aspects in terms of etiology, pathogenesis, diagnosis and treatment, differing only in the location of the lesions, although sometimes both processes may occur together.
Urease as an initiating factor of lithogenesis
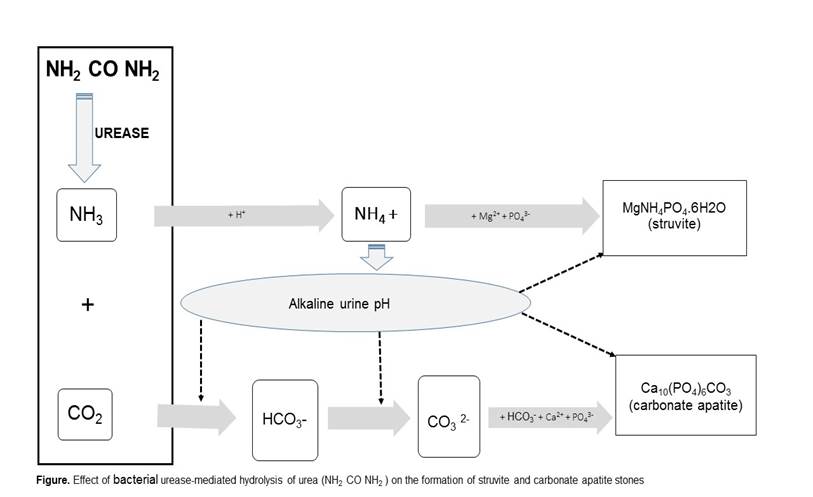
Both cystitis and pyelitis are associated with deposits of calcareous salts (triple phosphate), causing severe lesions that are difficult to repair. These deposits are caused by urease, an enzyme of microbial origin. By hydrolyzing urea into CO2 and ammonia (NH3), urease alkalinizes the urine by sequestering H+ ions, leading to the formation of ammonium (NH4+). In addition to the toxic effect of this compound on the urothelium, the high pH favors the conversion of CO2 to bicarbonate, which facilitates the formation of magnesium ammonium phosphate or struvite (MgNH4PO4·6H2O) and carbonate-apatite [(Ca10(PO4)6(CO3)] as depicted in Figure 1.
The role of urease in the genesis of struvite stones has been clearly demonstrated In experimental models,1,4-6 even by intrarenal injection of this enzyme alone.7 Furthermore, the formation of these stones can be prevented or reduced by administering urease inhibitors such as acetohydroxamic acid, both in experimental models and in human clinical trials.1,3-6
First suspicions of bacteria being causative agents of the diseases
At the beginning of the 20th century, a possible correlation between infection and the formation of certain calculi in the urinary tract was established.8,9 This correlation was much stronger if the microorganism involved had the capacity to hydrolyze urea. “Bacillus proteus urinae" was already described as having this property and in 1901 five cases of pyelonephritis with struvite incrustations were published;8 "B. Proteus" was isolated in three cases, and staphylococci in the remaining two.9 In 1925, Hager and Magath stressed the importance of bacteria with the capacity to hydrolyze urea in the etiology of encrusted cystitis, and indicated "Salmonella ammoniae" as the trigger of the process.10
Regarding bacteria with the capacity to hydrolyze urea, it is interesting to mention the work of Shaw and Hill in 1925 who reported the isolation, from the exudate of a wound due to surgical drainage,11 of a bacterium with great ureolytic capacity which they called "Corynebacterium thompsoni". Later, in 1937, cases of encrusted cystitis were published with a description of ureolytic bacteria including "streptococci", "hemolytic streptococci", "streptococci of the viridans group" and "coli bacilli".12 In 1938, Burns reported the microorganisms isolated in 31 cases of infectious calculi, finding a predominance of "Bacillus proteus",13 although he also isolated "staphylococci" and diphtheroids in two cases each. In 1939, Thompson and Schulte reported the isolation,14 in cases of alkaline cystitis, of diphtheroids and urease-positive micrococci. Similar findings were confirmed by Cifuentes in 1947,15 who found diphtheroids in pure culture in two cases among 15 patients with encrusted cystitis, and diphtheroids associated with other microorganisms in six other patients. In contrast, he found Proteus species in two cases and only one of these in pure culture.15
In the 1950’s and 1960’s, coinciding with the widespread use of antibiotics, preventive measures and improvement of urological procedures, there was a decrease in the number of published cases of encrusted cystitis and a certain lack of interest in this pathology. However, with the generalized use of bladder radiotherapy and the application of cyclophosphamide in the 1960’s and 1970’s for the treatment of some bladder tumors,16,17 an increase in incidence was observed, since the tumors themselves and the therapy used to combat them favored the development of encrusted cystitis. Of the 22 cases published by Jameson,16 9 suffered from bladder tumors. Among some of these cases, the microorganisms most often involved were "B. Proteus" alone or associated with Escherichia coli and Pseudomonas aeruginosa which were treated, with greater or lesser success, with nalidixic acid.16 In 1978 Rodríguez-Tolrá and Serrate published 5 cases of encrusted cystitis, one of them associated with a bladder tumor. Proteus mirabilis was isolated from all patients, although the authors mentioned that in one case Corynebacterium spp. grew in the culture.18 In 1982, Aubert et al described two cases of encrusted cystitis with isolation of "Staphylococcus" associated with "diphtheroids" and Corynebacterium sp.19
What's new since 1985?
Most publications prior to 1985 mentioning encrusted cystitis were associated with strongly ureolytic bacteria such as species of Proteus, Klebsiella pneumoniae, Pseudomonas aeruginosa, staphylococci (Staphylococcus aureus and other species), some species of streptococci and diphtheroids. There are published cases of encrusted cystitis with negative urine cultures or with isolation of bacteria that are not especially ureolytic, such as Escherichia coli, enterococci, and other organisms.15 Regardless of the doubts that may arise about the correct identification of the isolates, especially in very distant times, it is impossible to determine the clinical significance of such microorganisms that could suggest superinfections or that may not be representative of what really occurs in the tissues. There are also ureolytic organisms that may not be detected in routine urine cultures due to their slow growth (diphtheroids) or the need to use special culture media, such as Ureaplasma urealyticum,20 bacterial L-forms and anaerobic microorganisms.21,22
As mentioned in previous paragraphs, the isolation of diphtheroids from urology surgery wounds or urinary tract infections, especially associated with struvite lithiasis, has been the subject of publications since 1925 and has been cited in later articles.11,13-15,18,19 In some cases, the ureolytic capacity of many of these microorganisms was mentioned, but the isolated bacteria were not well characterized or identified.
In 1985, four cases of encrusted cystitis were described for the first time with the isolation of a new species of Corynebacterium, initially described as CDC group D2 and later characterized as Corynebacterium urealyticum.5,23,24 Since then, most published cases have been linked to this new species of Corynebacterium. It is difficult to know whether this is due to the loss of interest in publishing cases associated with "more classical" microorganisms (Proteus, Klebsiella, Pseudomonas aeruginosa, etc.) or that these have been, in some way, "wiped out" by the widespread use of antibiotics resulting in the selection of more antibiotic-resistant microorganisms such as C. urealyticum.25
Since the first description of C. urealyticum as a frequent agent of urinary tract infections and especially responsible for encrusted cystitis,25 there have been a series of publications involving this microorganism in infections of the upper urinary tract,26,27 and it has acquired prominence due to its participation in the so-called encrusted pyelitis.3 Encrusted pyelitis presents with calcareous incrustations in the wall of the renal pelvis with severe inflammation of the adjacent tissues, which may be secondary to the migration of the bacteria through the ascending ureteral route or a nephrostomy drainage.3,26-29 Encrustations in the renal collecting system require, as in encrusted cystitis, a pre-existing mucosal lesion, usually secondary to urological intervention. Urological complications in the course of renal transplantation and immunosuppression are predisposing factors for encrusted pyelitis.3,27-31 Transplant recipients suffering from encrusted pyelitis may present ureteral obstruction, obstructive uropathy, abscess formation and even organ loss.3 In the past 30 years, more cases of encrusted pyelitis alone or in association with encrusted cystitis have been published, in most cases involving C. urealyticum.3,27-31 In one of these cases gram-positive bacteria were observed in the urine but the culture, improperly performed, was negative and a good therapeutic result was obtained with vancomycin administration.31 In another well-documented case of encrusted pyelitis in a renal transplant patient, the urinary pH was strongly alkaline but the culture was negative until a second attempt was made in which C. urealyticum was isolated; a favorable outcome was obtained after administration of vancomycin and doxycycline and acidifying the urine.30
Since several decades, the following has been well established:
its ability to produce in vitro and in vivo struvite and apatite stones, as well as the efficacy of various experimental treatments;5,6
the sensitivity of the microorganism to different antimicrobials and the emergence of antibiotic resistance;6,27
the frequency with which this bacterium colonizes the healthy skin of different individuals;6,34
the classification, taxonomic status, genome sequence and proteomics of C. urealyticum.6,24
Epidemiology
C. urealyticum has been isolated from the skin of 25-37 % of healthy hospitalized elderly individuals, predominantly females.34 The incidence of C. urealyticum in urine cultures is highly variable and depends greatly on the culture media used for its detection and the observation time of the culture. A study carried out over a period of six years in a medium-sized university hospital yielded a total of 90 patients with bacteriuria caused by this microorganism (0.23 % of all urine cultures processed); the finding was considered clinically significant in 51 patients (56.6 %) and associated with struvite calculi in 14 (27 %).27 It is estimated that between 4-16 % of patients with C. urealyticum bacteriuria develop calculous complications.35 Recently, a spectacular increase in cases of bacteriuria due to this microorganism over a six-year period (2009-2014) was described in a Spanish hospital with an increase of up to 300 %, with 18 cases of encrusted uropathy, mainly pyelitis.36
Since 1987, numerous cases of urinary tract infections associated with C. urealyticum and struvite crystals or stones have been published in Australia, Belgium, China, France, Germany, Greece, India, Iran, Italy, Japan, Malaysia, Morocco, the Netherlands, New Zealand, Poland, Romania, Russia, Saudi Arabia, South Africa, Spain, Switzerland, Taiwan, Tunisia, Turkey, the United Kingdom and the United States. In addition to several interesting case reports, a number of review articles appeared since 1998 that can be consulted.27,29,33,35,37-40
Histopathology
In 1914, Françoise gave a brilliant histopathological description of encrusted cystitis in which he already reported the presence of inflammatory lesions with isolated microbial colonies,2 together with lymphocytes and polymorphonuclear leukocytes and some thrombosed vessels. Subsequent studies have confirmed the already known histopathological descriptions, although without reference to the microbial factor.15,18,19,27,33,35
Encrusted pyelitis, both in the native and in the transplanted kidney, shows similar alterations presenting microcalcifications and granulomas with multiple cells and sometimes parenchymal abscesses.3,33,35,37
Diagnosis
The diagnosis of both cystitis and encrusted pyelitis is based on knowledge of the symptoms presented by the patient as well as a rigorous clinical history that includes risk factors and urological antecedents. All this information must be complemented by routine analysis as well as by correct processing of urological samples for cytobacteriological study.2,3,23,26,27,35,37 Urine cultures should be carried out using appropriate media (blood agar) and extending the incubation period up to 72 h to allow the detection of slow-growing bacteria; this means that it is necessary to perform a real urine culture and not replace this study with other indirect and faster microbial detection procedures lacking the required sensitivity. In cases of strong suspicion of C. urealyticum infection, a rapid, sensitive and specific molecular test can be performed.41,42 Samples useful for bacterial detection include urine, biopsies, encrustations and stones.
Imaging techniques are very useful to diagnose these processes and range from simple abdominal radiography to ultrasound and CT scan. Plain radiography is not sufficiently sensitive and some encrustations may remain unnoticed. Ultrasound is a useful procedure for the diagnosis of both encrusted cystitis and pyelitis, and sometimes can detect ureterohydronephrosis. At present, a CT scan is considered the gold standard technique for the diagnosis of encrusted cystitis and pyelitis due to its high sensitivity and specificity. Cystoscopy and other endourological procedures can demonstrate calcified encrustations and may allow taking samples for histological and microbiological studies.3,23,26,27,29,33,35,38,39
Treatment
The optimal duration of treatment is not established because patients and their conditions are very different. Therefore, the treatment must be tailored to each case. In general, most patients require extended treatment, from weeks to months.
Treatment of encrusted cystitis and pyelitis requires three complementary approaches: elimination of calcareous incrustations and/or repair of the urinary tract; 2) treatment with antimicrobials directed against the microorganisms involved; and 3) acidification of the urine.
-
Elimination of calcareous incrustations and/or repair of the urinary tract.
Surgical removal of calculi in encrusted cystitis, by cystoscopy or cystotomy, was proposed by Françoise in his renowned paper.2 Currently, there is consensus that, if stone extraction is indicated, it should be done by cystoscopy.
In encrusted pyelitis, endourological extraction with fragmentation and removal of the calculi is difficult and dangerous,35 and therefore surgical removal, nephrostomy or ureteral stent placement is generally resorted to.3,29,37 One staghorn was successfully treated by percutaneous debulking and chemolysis.43 In some exceptional cases, conservative treatment of encrusted pyelitis has been achieved.44
-
Antimicrobials directed against the microorganisms involved.
The use of urinary antiseptics was recommended even before demonstrating the bacterial etiology of the process. Thus, Françoise proposed the administration of urotropin (hexamethylenetetramine) although it would not be expected to achieve good results since this drug requires an acidic environment to exhibit activity. With the same empirical objective, irrigations with oxycyanide, silver nitrate or iodine vapors were recommended.2
With the development of antibiotics and chemotherapeutics, there has been an important advance in the treatment of these pathologies, especially when the drugs are selected based on antibiogram data.
In the currently more common case that the microorganism involved is C. urealyticum, most clinical isolates of this microorganism show a high level of resistance to numerous antimicrobials, although they are consistently sensitive to glycopeptides (vancomycin and teicoplanin) and linezolid.6 The sensitivity of this bacterium to other antibiotics such as tetracyclines, macrolides, beta-lactams, aminoglycosides and fluoroquinolones is highly variable and, therefore, not advisable as empirical treatments if antibiogram results are not available.
In the treatment of encrusted cystitis and pyelitis caused by C. urealyticum, favorable outcomes have been obtained with vancomycin and teicoplanin.3,28-31,33,34,40,45,46 In some cases, other antimicrobials such as fluoroquinolones, tetracyclines, rifampicin, linezolid and macrolides have been used with varying results.23,26,27,33,35
Since bacterial urease plays a crucial role in the genesis of struvite calculi, inhibitors of this enzyme, such as acetohydroxamic acid, have been used as adjuvant treatment with promising results.4,27-30,35 Propionohydroxamic acid, another inhibitor of bacterial urease, has also been proposed.35
-
Acidification of the urine.
It is known that acidification of urine to a pH below 5.5 favors the dissolution of struvite stones, so this was an objective that was sought after for more than a century. Instillation of "lactic bacilli" by Newman,47 Caulk,48 and Redewill.49 not only acidified the urine but might also produce an antibiosis phenomenon that could antagonize more pathogenic invasive bacteria.
Other alternatives have been instillation or irrigation with lactic acid,2,18 acetic acid, lithium oxide,2 solution G,15,16,31,36 Thomas solution and dimethyl sulfoxide.30,31,36,40,46
Orally, phosphoric acid,12,15,19 citric acid, sodium citrate, arginine hydrochloride,15 mandelic acid,16 ammonium chloride,16,18,40, hydroxyurea, L-methionine,40,50 and cranberry juice have been used.40
Conclusions
Infectious encrusted uropathy is considered nowadays a rare but sometimes serious condition that undoubtedly is underdiagnosed. We have collected more than 100 publications from 29 countries dealing with this condition. Diagnosis is not always easy because many patients have an unspecific clinical presentation, many physicians are unaware of the disease, and many clinical laboratories are not prepared to detect C. urealyticum in clinical samples. Nevertheless, encrusted uropathy should be suspected in patients with risk factors even when the laboratory fails to isolate the organism from clinical samples. The urologist should insist that the laboratory search for C. urealyticum if the urine is alkaline and struvite crystals appear in the sediment. Pyuria and sometimes microhematuria are usually present, but clinical samples should be studied without delay as an alkaline environment may kill many cells.
In this review, we have tried to give a historical overview of infectious encrusted uropathy with emphasis on the importance of a correct, prompt diagnosis and treatment.











 nueva página del texto (beta)
nueva página del texto (beta)