Tropical dry forests (TDFs) account for over 42 % of the world’s tropical forests (Murphy & Lugo 1986), and the floristic region of the “Central Chiapas Depression” in Mexico is among the most diverse (Olson et al. 2001, Sousa 2010). One characteristic of this floristic region is the alternation of the rainy and dry seasons, which conditions plants to follow characteristic phenological patterns, including the length or the interactions of leaf development and the flowering and fruiting periods. In such environments, most species are deciduous, with leaf drop occurring at the beginning of the dry season and foliation after the first rains. The most common patterns include flowering in the rainy season (Bullock & Solís-Magallanes 1990); in other cases, the beginning of the reproductive stage occurs during the dry period (Singh & Kushwaha 2006). However, certain species show less common patterns, such as those with evergreen habits (Choat et al. 2005, Rojas-Jiménez 2007), including some Cordia species in Mexico and Central America. For example, Cordia alliodora has been classified as a wet-season deciduous tree (Choat et al. 2007).
Various leaf phenological patterns are observed in TDFs, and according to Borchert et al. (2002), these patterns are related to the distribution of precipitation throughout the year. Rivera & Borchert (2001) and Singh & Kushwaha (2005) have described multiple foliar strategies, which are classified as deciduous, brevideciduous, evergreen and leaf exchange.
Brevideciduous species only lose their leaves during a short period in the dry season, and most of them have similar strategies to respond to drought (Guazuma ulmifolia and Tecoma stans). Almost all of them possess a water-use mechanism, such as increasing stem water potential, to obtain more water, and they increase their root mass to perform these processes. In contrast, deciduous trees begin senescence and the consequent caducity early in the dry season; they respond to drought with water-saving strategies, such as closing stomata, decreasing photosynthetic activity, minimizing stem water potential to save water in the roots and stem, and loss of foliage, which occurs in some species of Tabebuia (Chaves et al. 2003, Singh & Kushwaha 2005, Elliot et al. 2006). In evergreen species (Couepia polyandra, Randia armata and Thouinidium decandrum), foliar bud break begins weeks before all the old leaves have abscised. In TDF cohabite species, in which senescence and the production of new leaves is almost simultaneous, they retain a portion of their foliage throughout the year (evergreen) (Huante et al., 2002, Rivera et al. 2002). These environments also contain species with foliar replacement strategies, including Pithecellobium saman, Simarouba glauca and Swietenia macrophylla (Borchert et al. 2002, Rivera et al. 2002)
Trees in TDFs display several phenological patterns caused by seasonal variations in rainfall, changes in temperature and solar radiation (Nanda et al. 2014). There are species that flower and fructify when foliar senescence and caducity occur during the dry season, but other species flower and fructify during the rainy season. Winter reproduction is frequently found in certain species, and it can be related to environmental factors, such as photoperiod (Borchert et al. 2004) or the presence of pollinators, as well as the dispersal syndrome of each species.
The TDF is an ecosystem with a high diversity of plant species that are useful to humans, including plants that serve as food or medicine such as Byrsonima crassifolia, Gliricidia sepium, and Guazuma ulmifolia (Miranda 2015). The TDF of the Central Chiapas Depression contains wild trees of the Annonaceae family with food potential, such as Annona lutescens Saff. (anona amarilla), A. macroprophyllata Donn. Sm. (papausa), A. reticulata L. (anona roja), and A. purpurea Moc. & Sessé ex Dunal (chincuya). Some of these species are also found in home gardens and within live fences (González-Esquinca 2001).
These Annona species grow in the same area, but they have different reproductive habits; in particular, they disperse their seeds at different times of the year. Direct observations over several years show that A. lutescens and A. reticulata bear fruit at the end of the dry season, whereas A. macroprophyllata and A. purpurea do so at the end of the rainy season and the beginning of the dry season. The contribution of these four TDF trees to forest functioning is poorly understood (Castro-Moreno et al. 2013). In this paper, we describe and classify the phenological and morphological changes associated with their seasonal cycle for the first time. This information will contribute to our understanding of the life cycles of these tree species and their ability to respond to seasonal changes.
Materials and methods
Study area. The study area is located between the municipalities of Chiapa de Corzo and Parral in Chiapas State, Mexico. The studied tree species were found growing in three disturbed areas of TDF (16°31’36’’ N, 92°58’25’’ W; 16°28’82’’ N, 92°57’48’’ W, and 16°21’47’’ N, 92°58’32’’ W). The climate in the region is warm and subhumid, with summer rains (AW1) and two seasons that are characterized as a dry season (October-May) and a rainy season (June-October). The average annual temperature is 25.8 °C, although maximum temperatures can reach 38 °C in exceptionally warm years, and the average annual rainfall is 921.9 mm (García 1988).
The climate of the study area was characterized using the precipitation and temperature data from Rivera de Zaragoza (municipality of Chiapa de Corzo) during 2013. The drought season corresponded to the months of November to April, when rainfall was below 46 mm, and maximum temperatures reached 26 °C. The rainy season occurred from June to September, with rainfalls of up to 265 mm and temperatures under 25 °C.
Phenological characterization. Considering that the studied tree species grow in disturbed areas, they were found in low densities. To perform this study, we selected 20 adult trees per species growing within the same area (approximately 5,000 m2) to avoid possible variations due to environmental conditions. The plants were identified and numbered, and they were observed during the third week of each month. During the flowering stages, trees were recorded each day from December 2012 until February 2014 and documented photographically. The vegetative and reproductive stages were characterized using the modified, extended “Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie” (BBCH) scale described by Hess et al. (1997). Although this scale is used for cultivated plants, we elected to use it because the studied Annona species are potentially cultivable. The scale proposed by Hess et al. (1997) considers a 2-digit code, where the cycle of plant development is divided into ten clearly recognizable and distinguishable phases referred to as principal growth stages and numbered from 0 to 9 (first number). The secondary growth stages (second number) are used to more accurately identify the progression in growth during each principal growth stage. The following principal growth stages were used in our study: bud development (BBCH 0), leaf development (BBCH 1), flower growth (BBCH 5), full flowering (BBCH 6), fruit growth (BBCH 7), fruiting (BBCH 8), and senescence (BBCH 9). The second number includes clearly recognizable external features (Tables 1-2) corresponding to the secondary stages of plant development. The herbarium samples were checked against the specimens deposited in the Herbarium Eizi Matuda (HEM) of the Universidad de Ciencias y Artes de Chiapas (UNICACH) using the following record numbers: A. lutescens (ARGE 352), A. macroprophyllata (ARGE 345), A. purpurea (ARGE 348), and A. reticulata (47155). To classify flowering and fruiting stages, the Newstrom et al. (1994) classification was used.
To determine the association between climatic factors (rainfall, monthly total and maximum, minimum and average temperature) and the phenology of the species, Spearman’s correlations were performed. For this purpose, data were grouped into reproductive stages (flowering and fruiting) and growth phases (leaf development and senescence).
Results
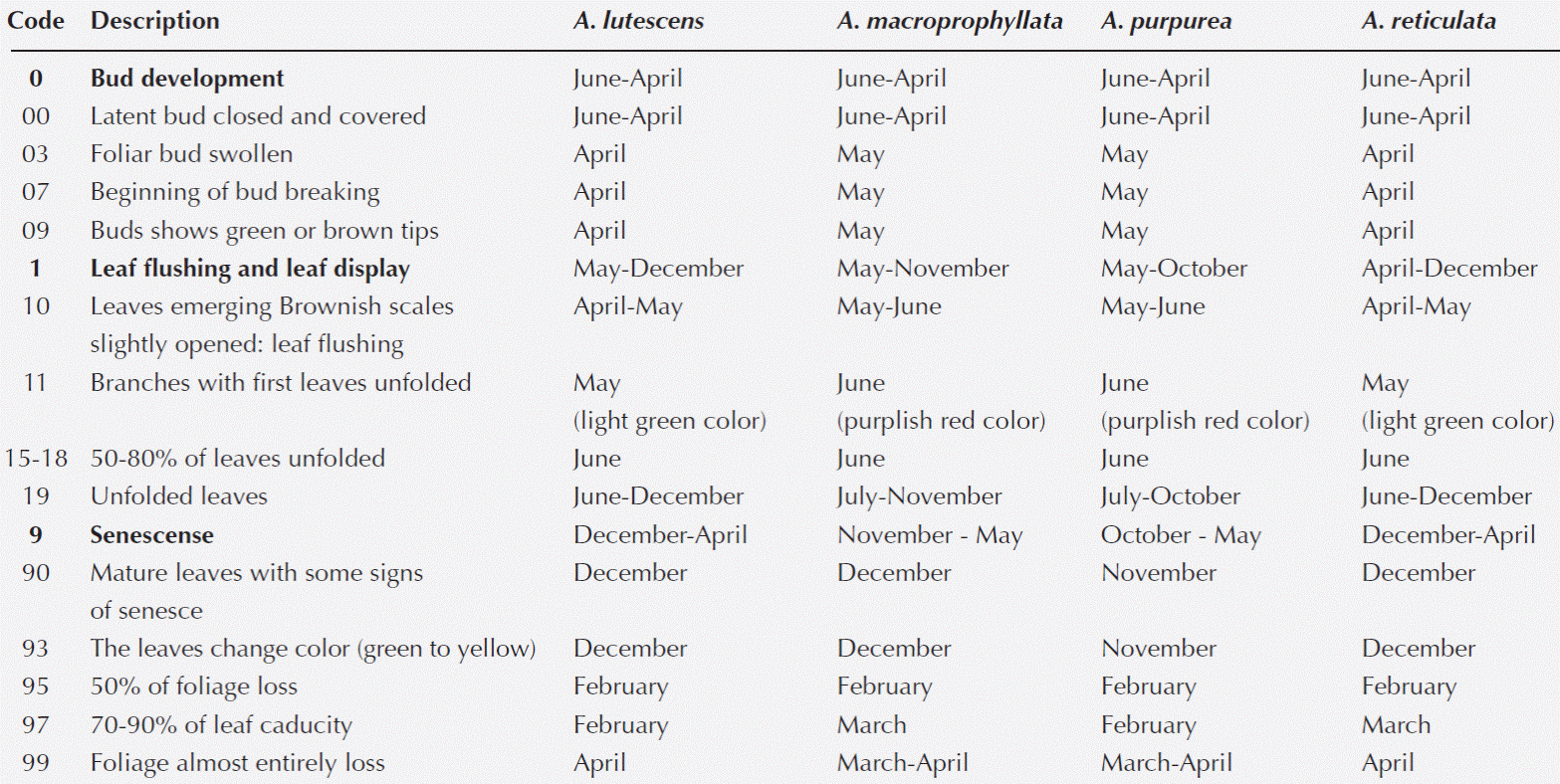
Foliar phenology. In all species, visibility of dormant buds was higher when the trees lost their leaves (BBCH 00); leaf bud development in Annona lutescens and A. reticulata began at the end of the dry season, while that of A. macroprophyllata and A. purpurea began during the transition from the dry season to the rainy season (BBCH 03) (Table 1). The branches produced mixed buds (vegetative and floral), and in all of the species, they were both terminal and axillary.
The leaf buds of Annona lutescens, A. macroprophyllata, and A. reticulata were usually coated with whitish laminar cataphylls (BBCH 03) that broke when the leaves emerged (Figure 1H). In contrast, A. purpurea buds were covered by two or more pubescent cataphylls (BBCH 03) that resembled a cap when the buds opened (BBCH 07, Figure 1L) and eventually showed the first green or brown buds (BBCH 09). In all of the species, the first leaves emerging from the buds were initially covered in abundant golden-brown hairs.

Figure 1 Some development state of Annona species. A-D, Annona lutescens. A, Flowers in distinct development state. B, Male flower. C, Fruit. D, Flower bud. E-H, Annona macroprophyllata. E, Female and male flowers. F, male flower. G, Developing fruit. H, Leaf bud. I-L, Annona purpurea. I, Flower female. J, the flower is used as chamber pollination by Coleoptera. K, Developing fruit. L, Leaves buds. M-P, Annona reticulata. M, Male flower. N, Female flower. O, Fruit. P, Branch with leaves and flower fertilized
Leaf development. Leaf flushing (branches with first leaves unfolded) in these trees began in May after the dry season and sometimes occurred before the rains, and the leaves emerged from the leaf buds after three to four days (BBCH 11). The leaf buds of Annona macroprophyllata were the only ones that produced an initial pair of nonpetiolate leaves, which were orbicular with brown hairs on the cataphylls and the margins. The presence of this type of leaf indicated the development of branches simultaneously bearing leaf buds and flowers, but the remaining branches, formed from apical buds, lacked floral buds. Twenty days after the rains began, the branches of the four species exhibited leaves in various stages of development, with the youngest leaves located apically (BBCH 12-19). When the rains ended, most of the trees displayed abundant foliage and fully developed branches (BBCH 19, BBCH 39).
Leaf senescence. At the onset of the drought season, the young plants started to show leaf senescence, and leaf senescence was detected later on the mature trees. Leaf senescence was characterized by the loss of leaf turgor, a gradual chlorosis up to the time of leaf fall, and the beginning of latency in the leaf buds. The end of vegetative activity occurred during this period, and no new leaves were formed (BBCH 91). At the beginning of the dry season, there were trees with symptoms of leaf senescence, and in all of the species, the leaves turned from green to yellow and then to brown (BBCH 93). The trees lost more than 50 % of their leaves (BBCH 95) during February, and by early March, they only retained 10 % of their senescent leaves (BBCH 97). Finally, during the last weeks of March and April, the trees became leafless (BBCH 99). The behaviour of the four species was similar, although Annona lutescens and A. reticulata usually retained some proportion of their foliage; these species appeared more drought resistant than the other two Annona species.
Reproductive phenology. Annona lutescens and A. reticulata flowered sporadically throughout the year, but most of the flowers were aborted. Full flowering occurred only during November and December (Table 2). The flowers that emerged at the beginning of the dry season were the only ones that produced fruit. The flowering of A. macroprophyllata and A. purpurea only occurred in May and June. Annona lutescens and A. reticulata fruited at the end of the drought season, but the other two species produced fruit during the middle and at the end of the rainy season.
Emergence of flowers and flowering. In Annona lutescens and A. reticulata, the development of flowers usually lasted from one to two weeks. The flower buds were green, visible and located on branches with leaves (BBCH 50, Figure 1D). Each flower bud contained from one to six flowers at different stages of development: barely visible, one-fourth of their final size (BBCH 52, Figure 1A, M), one-half of their final size (BBCH 55), and closed flowers at their final size (BBCH 59). The inflorescences were opposite to the leaves or implanted at the mid-part of an internode and were composed of several individual pedicellate flowers with very small triangular sepals and three greenish-yellow outer petals. In A. macroprophyllata, the leaves and flowers developed simultaneously, and the flowers were single and possessed a pedicel (Figure 1E-F). However, the flowers of A. purpurea were single or arranged in pairs (Figure 1I) and were subsessile and extra-axillary.
The flowers of all species exhibited dichogamy. They had a prefemale state (BBCH 60) when the petals were closed or slightly opened, and this stage lasted for 24 to 48 hrs. In the female state (BBCH 61, Figure 1E, N), the petals were partially opened, the stigma was visible and bright, and the release of scent was perceptible; the pollination period lasted up to three days. The male state (BBCH 65, Figure 1B, F, M) was observed when the petals were completely open and the visible stamens were separated, releasing the pollen. Annona macroprophyllata possessed red-brown pubescent petals, which were usually retained for several days (BBCH 68, Figure 1G), and a purple androecium that was distinguishable from the cream-coloured gynoecium. In A. lutescens and A. reticulata, the petals were yellowish-green, while A. reticulata was distinguished by red coloration at the base of the inner and the outer walls of the petals (Figure 1N). In both cases, the petals turned brown and fell off at the end of flowering. The petals of A. purpurea appeared velvety with purple on the inside and creamy yellow on the outside and dropped slightly earlier than the petals of the other species. This process may have been due to the numerous beetles that used the floral cavity for copulation (Figure 1J).
Growth of fruit and fruiting. With fruit development, the petals fell in all of the species with the exception of Annona macroprophyllata, whose petals tended to be more persistent (BBCH 70, Figure 1G). After this stage, the fruit that had not set fell off the plant, while those that had set grew (BBCH 71); fruit at various stages of development was visible on the same tree (Figure 1G, K). The fruit reached one-half of its final size and full size at approximately 70 days (BBCH 75) and 120 days (BBCH 79) after pollination, respectively. All of the species were found to have climacteric fruit (Table 2).
On all of the trees, it was possible to find fruit in the following stages of development: (1) fruit with incipient maturity that changed from green to yellow (Annona lutescens) or from green-purple to red (A. reticulata) (Figure 1C, O), with the fruit of A. macroprophyllata and A. purpurea remaining green or slightly brown (BBCH 81); (2) fruit with firm flesh mature enough for harvest (BBCH 87); and (3) fruit mature enough for consumption, with an intense aroma and colour and a smooth texture (BBCH 89). The fruit ripened quickly, so they could not be stored for more than four to seven days; in particular, the fruit of A. macroprophyllata broke open while still on the tree, showing a white or pink-coloured pulp.
The correlation analysis between climatic factors and phenology of Annona species shows that the reproductive phases of species are associated with changes in precipitation and minimum temperatures; these environmental factors also bind A. macroprophyllata and A. purpurea, as species requiring the presence of rain with decreasing temperature for fructification, whereas A. lutescens and A. reticulata do not require these conditions to bear fruit (Table 4). In contrast, the strategy of foliation is similar among the four species and is triggered by the start of precipitation and the consequent decrease in temperature (Table 5).
Table 4 Spearman correlation (and it p value) between reproductive phenology and climatic variables for the species of Annona

Discussion
In TDFs, many plant species may respond to the same climatic conditions in contrasting ways through different physiological, phenological, and morphological adaptations. In particular, our study shows that Annona species in the Central Chiapas Depression show different vegetative and reproductive phenological patterns (Tables 1 and 2). Our results also show that A. lutescens and A. reticulata could be brevideciduous species (Singh & Kushwaha 2005) because their foliar caducity period (three months) is shorter than that of A. macroprophyllata and A. purpurea, which are deciduous species (six months) (Figure 2).
The flowering of these species is annual and intermediate (Newstrom et al. 1994), with a duration of about two months, but trees of Annona lutescens and A. reticulata with flowers out of season can be observed.
Annona lutescens and A. reticulata start flowering at the beginning of the dry season and begin fruiting at the end of the dry season (Figure 2), and their seeds can germinate immediately after fruiting. However, A. macroprophyllata and A. purpurea had flowers at the end of the dry season and towards the beginning of the rainy season and began fruiting at the end of the rains. Their seeds likely remain dormant for at least six months or until the next rainy season starts (González-Esquinca et al. 2014).
These features suggest that the two groups of species have different climatic requirements (Tables 4 and 5) and that seed dormancy in Annona macroprophyllata and A. purpurea provides an escape from the extreme conditions found in the TDF during the dry season, increasing the probability of seedling survival during the following rainy season (Figure 3).
The reproductive responses of Annona lutescens and A. reticulata showed a negative Spearman correlation with rainy period, whereas A. macroprophyllata and A. purpurea showed positive Spearman correlations with the rainy period; these features allow us to suggest their categorization in early dry reproductive strategy (EDRS) and late dry reproductive strategy (LDRS) groups, respectively (Table 3). Note that phenological data for A. lutescens (Castro-Moreno et al. 2013) from another TDF area corroborate our classification of the species.
This study indicates that foliage display occurs during the rainy season in the four Annona species, which are typically deciduous species (Figure 3), but that leaf duration varies. Annona lutescens and A. reticulata, the EDRS species, were observed to tolerate drought longer than the other two species before reaching leaf senescence and expiration, i.e., they are the more desiccation-tolerant species. Shorter foliage displays were found in A. macroprophyllata and A. purpurea, the LDRS species. The results indicated that the leaves of A. lutescens and A. reticulata begin to senesce after three months of drought, and the trees only become almost leafless in the final dry months (brevideciduous). Additionally, leaf flushing begins days before the start of the rains. Perhaps the large density of lenticels on the stems of the EDRS species allows them to survive in drought (Figure 1D, H); in fact, desiccation tolerance has been reported in A. lutescens (Castro-Moreno et al. 2013), the most likely of the four Annona trees to have the highest drought resistance. Rivera et al. (2002) discussed the importance of increased photoperiod in certain species for initiating foliation before the rains, and these plants may also perceive increases in relative humidity or temperature prior to the establishment of the rainy season.
The above characteristics distinguish Annona lutescens and A. reticulata as species whose reproduction and survival strategies are synchronized with the climatic conditions of the TDF. By contrast, the characteristics of A. purpurea and A. macroprophyllata indicate lesser resistance to drought, which may explain their presence in ecotones of tropical sub-dry forest (Tables 4 and 5).
The phenology of Annona species has been previously described only for two cultivated species, cherimoya (A. cherimola, Cautin & Augusti, 2005) and the floral stages of soursop (A. muricata, Yamarte et al., 2004), and for a single wild species (A. lutescens, Castro-Moreno et al. 2013). An understanding of the vegetative and reproductive patterns of wild species and their relationships with the animals that depend on them (e.g., herbivores, pollinators, and fruit bats; Figure 1J) allows us to comprehend the response of plants to the climatic conditions of a particular area and facilitates the use of sustainable production and harvest strategies for wild edible species with the potential for cultivation, like those described in this study. Phenological characterizations also allow the establishment of study models to determine and predict the physiological and morphological responses that plants have developed to the changing environment over evolutionary history.
Further studies of the ecophysiology and morphology of these trees can potentially determine whether any of these adaptations may confer resistance to climate change, allowing the trees to serve as an alternative crop for reforestation.











 nueva página del texto (beta)
nueva página del texto (beta)