Cocoa (Theobroma cacao L.) is an allogamous, woody species of the family Malvaceae (de Almeida and Valle 2007), native to the rainforests of the Amazon basin and other tropical areas of Central and South America. Despite its center of origin, of the 8.6 million hectares planted worldwide only 17 % correspond to America and the Caribbean (Carr and Loockwood 2011), with a production of 4,23 million ton of cocoa in 2014-2015 (ICCO, 2015), i.e. an average production of 560 kg/ha. Its economic importance lies in being a small-scale cultivation from which depend 5-6 million farmers (de Almeida and Valle 2007; Carr and Loockwood 2011). Cocoa is a shade crop, intolerant to drought (Belsky and Siebert 2003) and its productivity is strongly affected by the distribution of rainfall and duration of drought periods (Balasimha et al. 1991; Bae et al. 2008).
There are three types or morphogenetically different groups of cocoa known as “Criollo” (native of Venezuela), “Forastero” (original from the Amazon basin) and “Trinitario” produced naturally in the island of Trinidad (a cross between Criollo and Forastero), which differ in quality of the almonds, vigor and yield (Criollo, high quality, and Forastero with different qualities and tastes; Cheesman 1944).
Forastero and Trinitario cocoa are considered of lesser quality but are widely grown worldwide because of some advantages in performance and resistance to diseases (Girón et al. 2007). Recently, Motamayor et al. (2008) suggested a new classification of cocoa germplasm grouped into 10 genetic groups, which reflects the diversity of cocoa and a better approach than the previous classification ( Motamayor et al. 2002). The Criollo cocoa identified within this group has a low genetic diversity (Motamayor et al. 2002) and its quality is considered one of the best in the world (Elwers et al. 2009) although this variety is susceptible to disease. Cocoa exhibits considerable genetic variability regarding morphological and physiological traits (Daymond et al. 2002a; b). However, studies of genotypic variation of photosynthetic traits in cocoa are limited (Daymond et al. 2011).
Most ecophysiological studies have been conducted in cocoa seedlings or saplings in laboratory, greenhouses and nurseries. Low photosynthetic rates (A) and stomatal conductance (gs) have been reported in cocoa trees with reductions due to water deficit, high vapor pressure deficit (VPD) and high photosynthetic photon flux density (PFD) among other abiotic factors that could cause stress (Deng et al. 1989; de Almeida and Valle 2007; Acheampong et al. 2013; Ávila-Lovera et al. 2016). There are limited studies on ecophysiological responses to water and light availability of different genotypes of adult cocoa trees, both in agricultural ecosystems and natural habitats (Moser et al. 2010; Araque et al. 2012; Jaimez et al. 2013; Ávila-Lovera et al. 2016).
The cultivation of cocoa in Venezuela is mainly located in three regions: Southwest, Northeast, representing the major production area (43 % of total production) and the North Central Coast (González-Jiménez 1999). Most of the plantations have some combinations of the three cocoa types creating mosaics of plants with differences in the quality and morphology of fruits. However, some plantations maintain highly homogeneous cocoa.
Although not recognized as a cocoa producing area in Venezuela, some old, abandoned plantations are found in “Cacao” and “Palma Real” mountain of Isla Margarita, Nueva Esparta state (Northeast). These agroforestry systems have combination of trees of mamey (Pouteria sapota), avocado (Persea americana), copey (Clusia rosea), breadfruit (Artocarpus communis) in the upper layer of the forest and cocoa in the lower. The first biometric and morphometric description of cocoa plants in this area, conducted by Girón et al. (2007), showed the existence of populations of high quality wild Criollo and Forastero plants (Figure 1A, B), considered representatives of the first plantations in the country. These plants must have particular physiological traits that have allowed them to grow for a long time in this habitat.
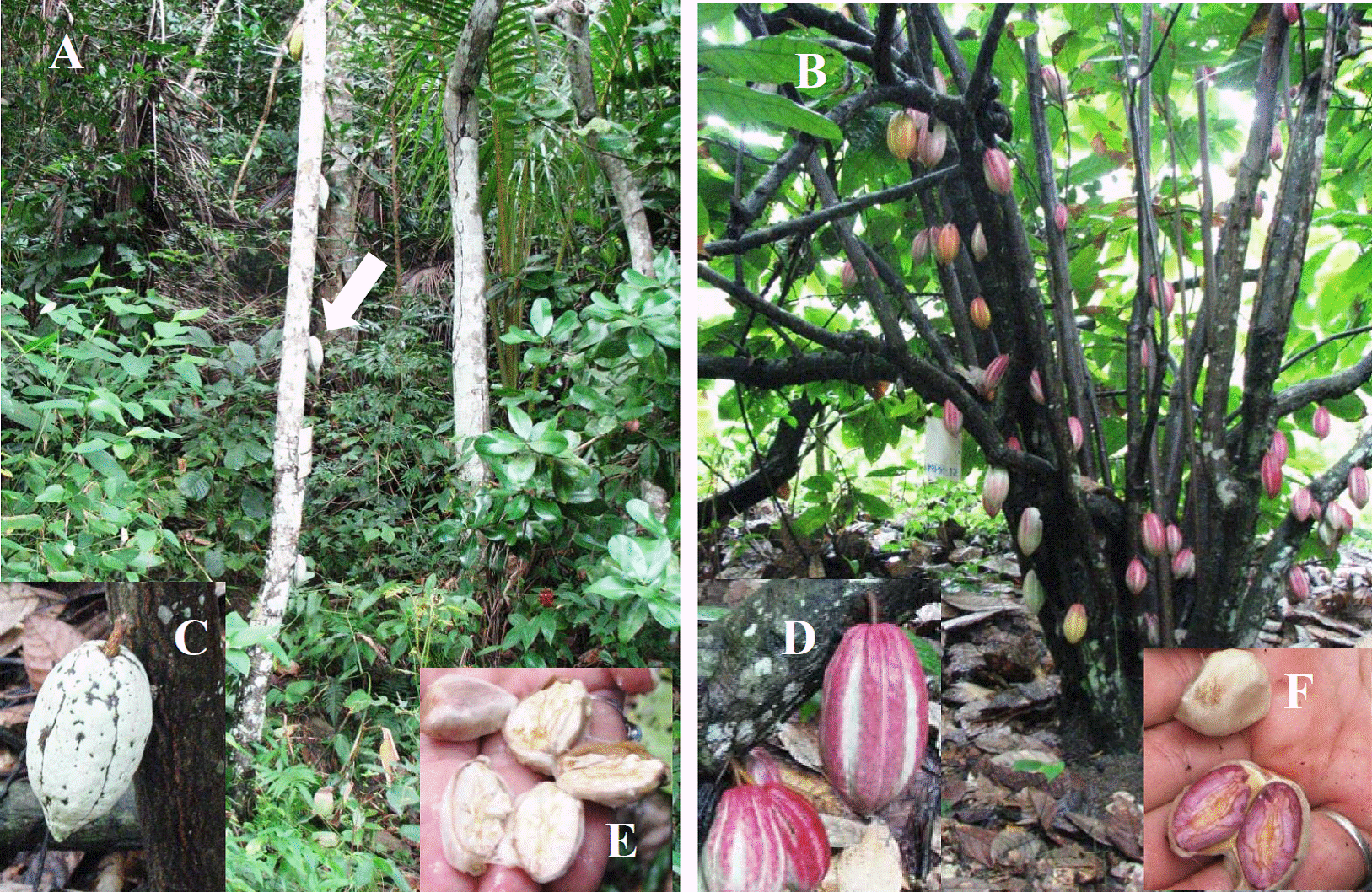
Figure 1. A), wild individuals (adults> 50 years old) of Criollo cocoa with main stem of approximately. 8-m-high; the arrow points to a Criollo cocoa pod B) individual of Forastero cocoa with profuse side-branching stems and large pod production; C) and D) detail of pods, E) and F) form and color of almonds of Criollo and Forastero cocoa, respectively.
The existence of this plantation for so many years in this location seems to confirm that in regions with little access to inorganic fertilizers, litter fall can maintain soil fertility (Isaac et al. 2007). Moreover, the reduction in wind speed and evapo-transpiration by trees that provide the shade reduce the water deficit and high temperature of air and soils during the dry season (Beer et al. 1998). Although in the island of Margarita, the climate is semiarid, mountain regions with 900 m or higher, such as the “Cacao” and “Palma Real” mountains, have microclimatic conditions that enable the development of this agricultural system. These cocoa plantations allow the study of the physiological characteristics of old Criollo cocoa and its differences with the cultivars recently introduced and currently used in Venezuela.
While many plantations in Venezuela are mainly of Trinitario and Forastero cultivars, the strategy established in the last decade is the gradual introduction of hybrids and Criollo cultivars with high almond quality in extended regions of the country. Physiological responses of Criollo cocoa to water deficit have been reported and osmotic adjustment importance in some cultivars is highlighted (Rada et al. 2005; Araque et al. 2012). Different cultivars from major cocoa areas of the country, including the island of Margarita, grown in a germplasm bank in the central region, showed significant seasonal and between-cultivar physiological differences (Pereyra 2007; Tezara et al. 2009).
The aim of this study was to assess physiological traits such as water potential, gas exchange photochemical activity of photosystem II and biochemical parameters of old cocoa Criollo and Forastero trees that have survived without agronomic management, to ascertain if there are physiological differences between them.
Materials and methods
Study area and plant material. The measurements were performed in adult individuals in a premontane rainforest (Ewel et al. 1976) located at 11° 01’ N, 63° 53’ W and 470 m in the mountain Palma Real, in Margarita Island, Edo. Nueva Esparta, Venezuela, in January 2008 and November 2009. The rainy season occurs between November and January and the dry season between June and August. Annual rainfall is 700-800 mm. This rainfall pattern and the occurrence of fog at high altitudes have allowed the establishment of tropical deciduous and transition forests with trees that can reach 40 m in height (Hoyos 1985).
Cocoa trees are shaded by several fruit trees randomly planted. The Criollo individuals (Figure 1A) were identified by fruit characteristics (Figure 1C), whitish cotyledons (Figure 1E), light green young leaves and pubescent leaf petioles. The Forastero cocoa (Figure 1B, 1D) had light to dark violet cotyledons (Figure 1F), dark new leaves (from red to violet) and glabrous leaf petioles. Microsatellite analysis showed that individuals corresponded to ancestral Criollo or Forastero cocoa (Marcano-de Segovia 2007).
Physiological measurements. All physiological measurements described below were made on fully expanded leaves of adult trees.
Water relations. Leaf water potential (Ψ) was measured at 06:00 and 12:00 h in leaves of five individuals of each type of cocoa (n = 5) with a pressure bomb (PMS, Corvallis, Oregon, USA).
Gas exchange. Measurements of gas exchange: photosynthetic rate, A; transpiration rate, E; stomatal conductance, gs; intercellular CO2concentration, Ci, and water use efficiency, WUE were performed with a portable infrared gas analyzer (CIRAS 2, PP Systems, Hitchin, UK) used in conjunction with an assimilation chamber (PLC, PP Systems, Hitchin, UK), at ambient CO2concentration (Ca) of 380 μmol mol-1, 21 % O2, PFD of 400 μmol m-2 s-1and a leaf temperature (TL) of 28 ± 0.5 °C. Gas exchange was measured between 9:00 and 11:00 h since previous measurements showed that A at maximum within these hours.
Curves of photosynthetic rate vs. intercellular CO
2
concentration (A/C
i
). Response curves of A to intercellular CO2 concentration (A/Ci curves) were done in four individuals of each type of cocoa (n = 4) by decreasing Ci from approximately 298 μmol mol-1 (at which A at Ca = 380 μmol mol-1 was initially measured) to zero and then progressively increasing Ci to 1,200 μmol mol-1 CO2. Measurements were made between 09:00 and11:00 h at 400 ± 10 μmol m-2 s-1 of PFD, 21 % O2 and TL of 28 ± 0.5 ºC. The A/Ci curves were fitted to the empirical equation
Curves of photosynthetic rate vs. photosynthetic photon flux density (A/ PFD). Response curves of A to PFD (A/PFD) were done in four individuals of each type of cocoa (n = 4) by decreasing PFD from 400 μmol m-2 s-1 (at which A was initially measured) to 0 and then progressively increasing it from to 1.500 μmol m-2 s-1 in eight steps, using the leaf microclimate control system of the CIRAS 2. Measurements were done between 09:00-11:00 h at 380 ± 10 μmol mol-1 of Ca, 21 % O2 and TL of 28 ± 0.5 ºC.
Photochemical activity of PSII. Chlorophyll a fluorescence was measured on attached dark-acclimated leaves (n = 6) with a PAM 2100 fluorometer (Walz, Effeltrich, Germany) using the protocol described by Genty et al. (1989). Maximum quantum yield of PSII (Fv/Fm) was measured in situ at predawn in dark-adapted leaves. Photochemistry activity response curves to PFD were done in four individuals (n = 4) in each cultivar. Relative quantum yield of PSII (ΦPSII) at steady state A was calculated as, ΦPSII = F’ m - F s / F’ m , where Fs and F’m are steady state and maximum fluorescence in light, respectively. Photochemical (qP) and non-photochemical (qN) quenching coefficients were calculated from measurements of fluorescence. Electron transport rate of PSII (J) was estimated as J = ΦPSII×PFD×a×0.5, where a is the fraction of incident PFD absorbed by the leaf (0.84).
Chlorophyll content and isotope ratios of carbon and nitrogen (δ 13 C, δ 15 N). Chlorophyll extraction was performed on an aliquot of fresh leaf samples (n = 5) of known area, using 80 % acetone cold (Bruinsma 1963). For each type of cocoa, leaf samples of four individuals (n = 4) were ground and then analyzed for carbon isotope ratio (δ13C), nitrogen isotope ratio (δ15N) and leaf N content at the University of Illinois-Chicago, using an elemental analyzer (Costech, Valencia, California) coupled to a Delta+XL isotope ratio mass spectrometer (Finnigan, Bremen, Germany) operated in continuous flow and run against NIST and lab standards to a precision of 0.05 ‰ for C and 0.15 ‰ for N.
Specific leaf area (SLA). Specific leaf area was measured in discs (n = 10) of the same leaves used for measurements of gas exchange. The discs were dried to constant weight at 70 °C and SLA determined as area of leaf discs/ dry mass.
Statistical Analysis. Results are presented as the mean of each variables measurements made in 2008 and 2009 ± standard error. The Statistica 5.5 statistical package was used for analysis of variance (ANOVA, with a significance level of p < 0.05) and Tukey test was used as post hoc test. The graphics were done using Sigmaplot 11.0.
Results
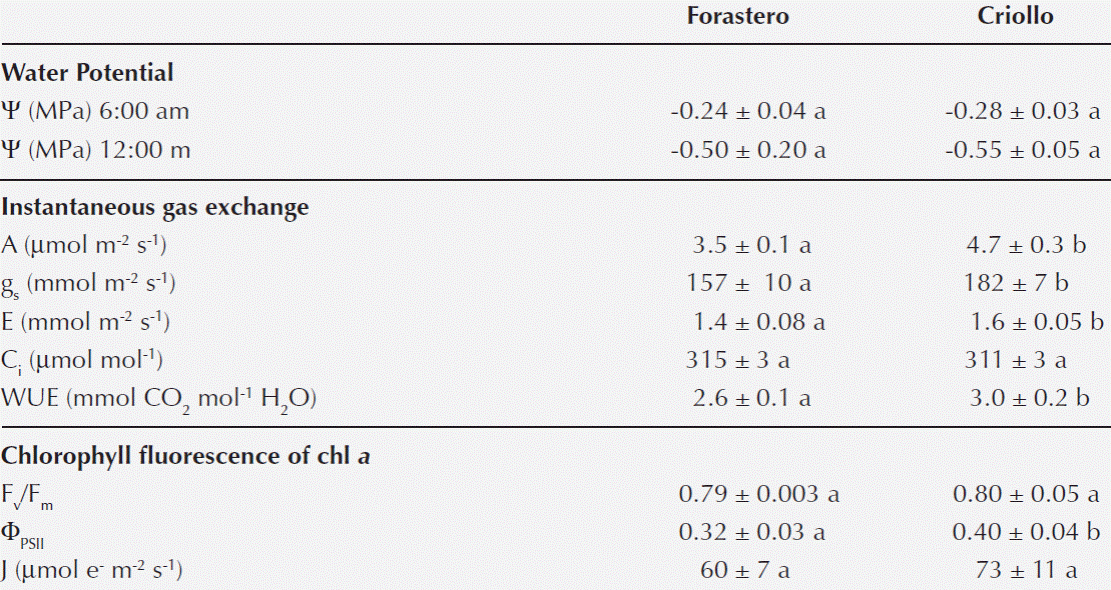
Water status, instantaneous gas exchange and photochemical activity. No significant differences were found in Ψ at dawn and noon between the two types of cocoa (Table 1). Criollo cocoa showed higher instantaneous values of A (34 %), gs (16 %), E (14 %) and WUE (15 %) than Forastero, while in both types of cocoa Ci was similar (Table 1). The maximum quantum yield (Fv/Fm) of dark-adapted leaves and the electron transport rate (J) measured at 400 μmol m-2 s-1 PFD was similar for the two types of cocoa, while the quantum yield of PSII (ΦPSII) was slightly higher in the Criollo cocoa (Table 1).
Table 1 Pre-dawn and midday leaf water potential (Ψ). Average photosynthetic rate (A), stomatal conductance (gs), transpiration (E), intercellular CO2 concentration (Ci), instantaneous water use efficiency (WUE), maximum quantum efficiency (Fv/Fm), relative quantum yield of photosystem II (ΦPSII) and electron transport rate (J) of Forastero and Criollo cocoa in the “Palma Real” Mountain, Margarita Island. The results are presented as means ± (SE). Different letters between columns indicate significant differences for each variable (p < 0.05).
Curves of Photosynthesis vs. intercellular CO
2
concentration (A/C
i
). The A/Ci curves indicated no significant difference in A at saturating [CO2] (
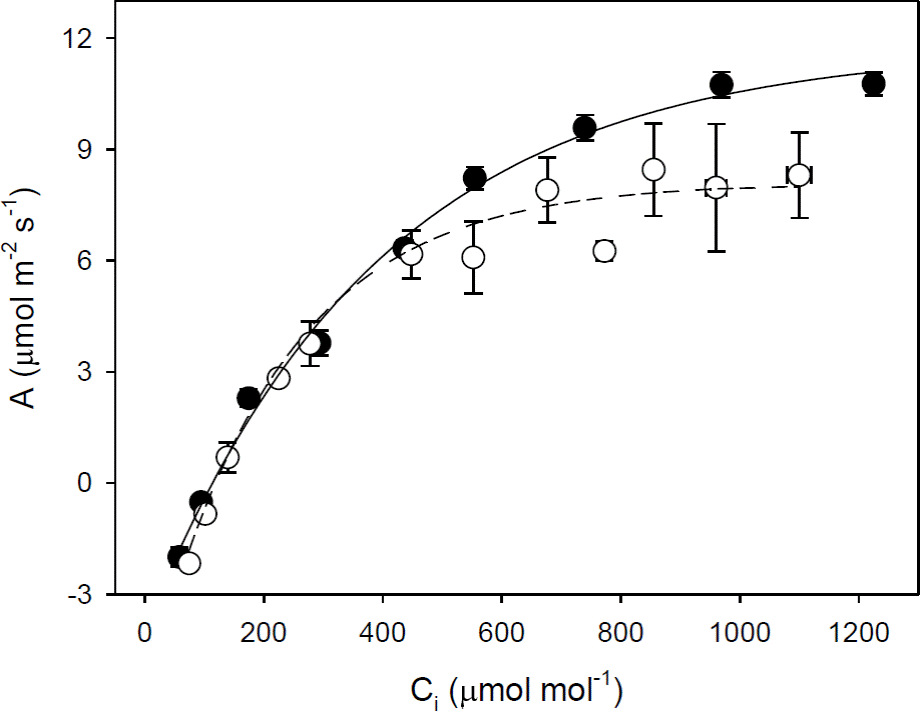
Figure 2 Curves of photosynthesis (A) vs intercellular CO2 concentration (Ci) of intact leaves of cocoa trees: Forastero (●) and Criollo (❍). Each value represents the mean ± SE, n = 4.
Table 2 Parameters of the photosynthetic response of to intercellular CO2
concentration (A/Ci), CO2-saturated photosynthetic
rate (
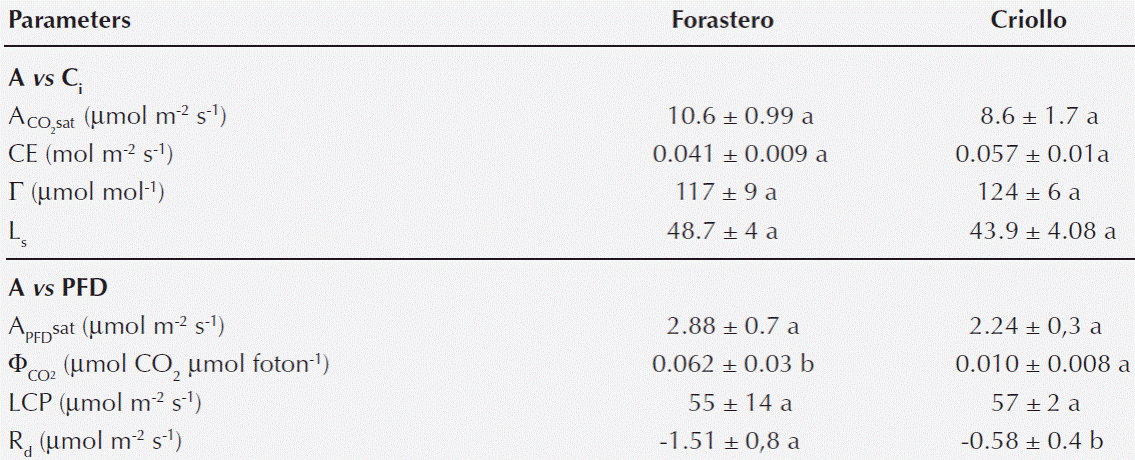
Curves of Photosynthesis vs. photosynthetic photon flux density (A/ PFD). Light curves indicated characteristics of shade plants for both types of cocoa, i.e. low rates of photosynthesis at saturating light (APFDsat), low light compensation point (LCP) and dark respiration (Rd) (Table 2). The A was saturated at PFD of 400 μmol m-2 s-1 (Figure 3). The photosynthetic rate at saturating light was similar in both types of cocoa (APFDsat). Both the apparent quantum yield of CO2 fixation (ΦCO2) and Rd were significant lower in the Criollo cocoa (Figure 3, Table 2).
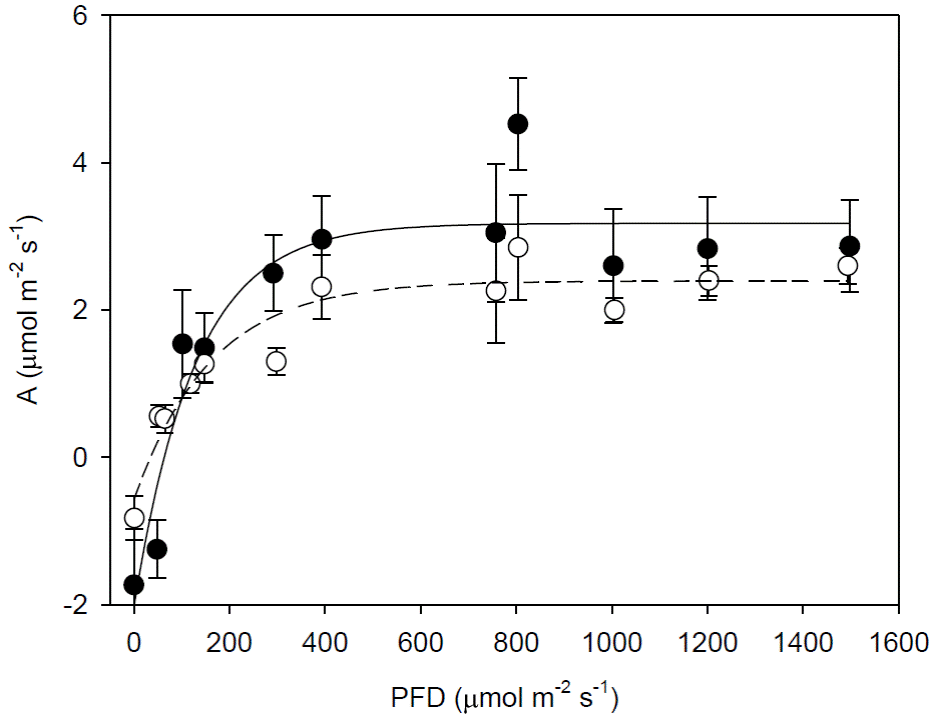
Figure 3 Curves of photosynthesis (A) vs photosynthetic photon flux density (PFD) of intact leaves of cocoa trees: Forastero cocoa tree (●) and Criollo (❍).Each value represents the mean ± SE, n = 4.
Response curves of photochemical variables to photosynthetic photon flux density. The fluorescence parameters of the two types of cocoa showed a similar response to PFD (Figure 4A, B, C, D). Maximum J was low (about 50 μmol e- m-2 s-1), decreases in ΦPSII and qP with the increase of the PFD (Figure 4B, C) were observed, while the amount of energy dissipated as heat (qN) increased, with values greater than 0.8 when PFD was 400 μmol m-2 s-1 (Figure 4D).
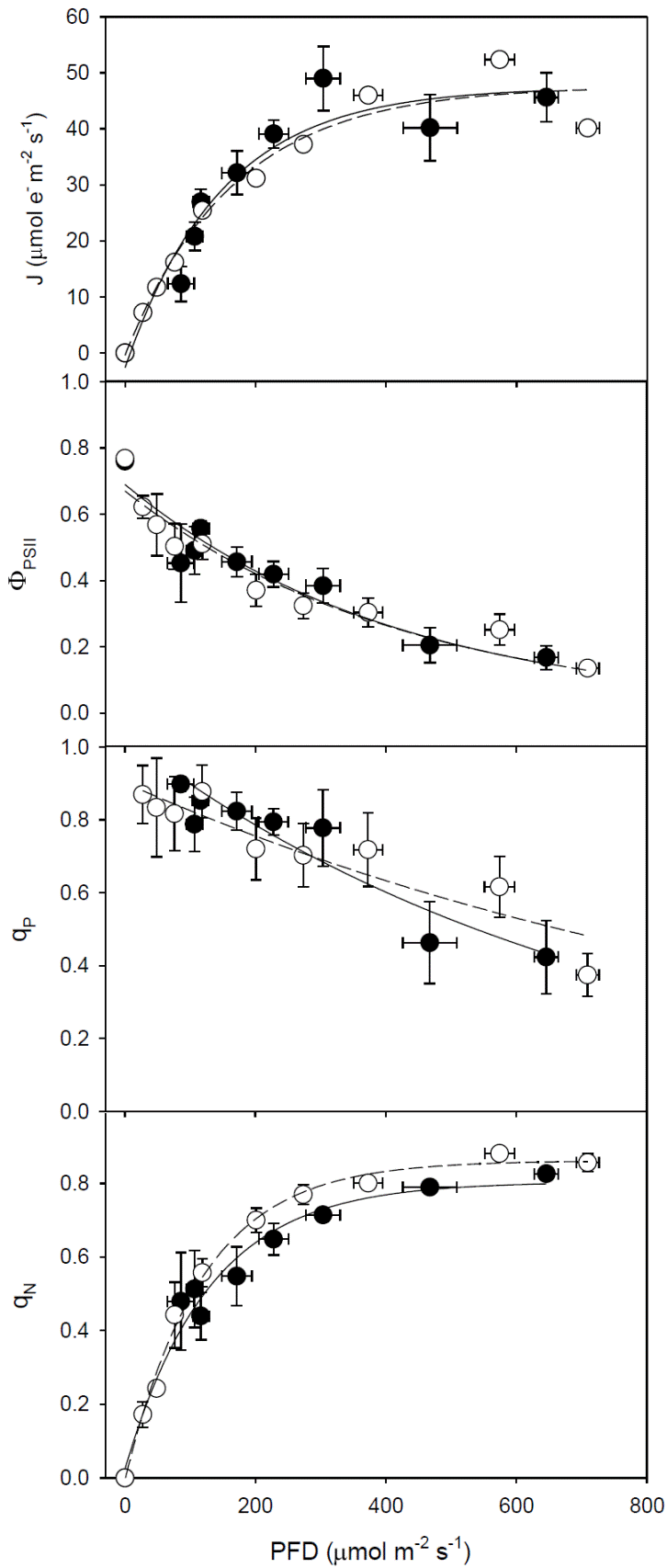
Figure 4 Fluorescence parameters vs the photon flux density: A) electron transport rate (J). B) quantum yield of PSII (ΦPSII). C) photochemical quenching coefficient (qP) and D) non-photochemical quenching coefficient (qN) in cocoa leaves Forastero (●) and Criollo (❍).
Chlorophyll content, isotope ratios of carbon and nitrogen and specific leaf area. No differences were found in the total Chl content or δ13C between the two types of cocoa, while a higher content of foliar N and δ15N were observed in the Criollo cocoa. Forastero showed a higher SLA compared with Criollo (Table 3).
Table 3 Total chlorophyll content (Chl(a+b)), leaf nitrogen content (N), nitrogen (δ15N) and carbon (δ13C) isotopic composition and specific leaf area (SLA) of Forastero and Criollo cocoa trees. The results are presented as means ± (SE). Different letters indicate significant differences for each parameter (p <0.05) between columns.
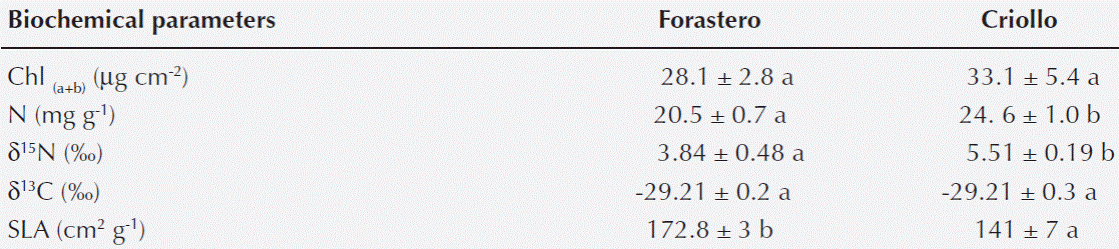
Discussion
This work reports a physiological comparison between Forastero and Criollo old cocoa growing wild without agricultural management, pruning or fertilization. Under these conditions it is possible to evaluate the performance of cocoa plants introduced over more than 50 years ago. The physiological information presented here can be used to contribute to the knowledge needed to recommend cocoa farmers in the management and the re-establishment of good quality materials. Morphological and production differences between Criollo and Forastero cocoa are well known (Girónet al. 2007). However, physiological features that help differentiate between both types of cocoa had not until now been well evaluated.
Water status. High values of Ψ early in the morning were in agreement to those reported for young plants in well-watered conditions in an agroforestry system (Jaimezet al. 2008; Tezara et al. 2009; Araqueet al. 2012) and in young trees in a germplasm bank (Pereyra 2007; Ávila-Lovera et al. 2016). By the time of the study (November and January) when monthly rainfall average was high, Ψ at noon of these trees indicated a low transpiration rate which may well be the result of an environment of low evaporative demand. In agroforestry systems, a reduction in wind speed and evapotranspiration contributes to lower water vapor gradients between the leaf and the atmosphere (Beer et al. 1998).
Instantaneous gas exchange. The very low average A observed for both cultivars might be associated with low gs. Similarly, A values have been found to range from 0.7 to 6.5 μmol m-2 s-1 with low gs (20 -150 mmol m-2 s-1) in different studies (Joly and Hahn 1989; Daymond et al. 2011; Araqueet al. 2012; de Almeida et al. 2014; Ávila-Lovera et al. 2016). Higher instantaneous A and lower E determine a significant higher WUE in Criollo compared to Forastero, suggesting that Criollo cocoa could grow well in regions with limited water availability. Values of A in Criollo and Forastero adult trees was similar to those in young cocoa trees (Pereyra 2007; Tezara et al. 2009; Ávila-Lovera et al. 2016) even with high values of gs.
The gs in both types of cocoa was low (50-200 mmol m-2s-1), which suggests that A may be limited by low stomatal opening. This fact was evidenced by the high values of Ls (the relative limitation of A due a gs, approximately 46 %), indicating that photosynthesis was reduced by approximately half with respect to the photosynthetic rate at infinite gs. These old cocoa trees showed values of Ls twice the reported in young cocoa by Ávila-Lovera et al. (2016), suggesting a greater stomatal control of the photosynthetic process.
The WUE shown by the two types of cocoa were similar to those reported for younger individuals of six cultivars from Margarita Island and eastern Venezuela grown in a germplasm bank (Pereyra 2007; Tezara et al. 2009) but higher than other four cultivars of Criollo cocoa in a agroforestry system (Araqueet al. 2012). The long-term WUE estimated by δ13C in adult trees (-29.2 ‰) was similar to those found in Criollo cultivars (Ávila-Lovera et al. 2016) and significantly higher to that reported for younger cocoa trees (-30 ‰) (Pereyra 2007; Tezara et al. 2009), indicating that adult trees could even present a better physiological performance during the rainy season.
A/C
i
curves. The A/Ci curves showed no differences in
Light curves and PSII activity. The parameters of A/PFD curves of the Criollo and Forastero cultivars indicated adaptation to shade, i.e. low APFDsat, low Rd and LCP, without differences in these parameters between types of cocoa. Values of APFDsat, ΦCO2 and Rd were similar to those reported for several cocoa genotypes under different conditions (de Almeida and Valle 2007; Baligar et al. 2008; Daymondet al. 2011; Bertoldeet al. 2012; de Almeida et al. 2014; Ávila-Lovera et al. 2016). The saturating PFD in both types of cocoa is relatively low, around 400 μmol m-2s-1, similar to previously reported data (300-600 μmol m-2 s-1, Joly and Hahn 1989; Balasimha et al. 1991; Almeida et al. 2014).
Apparent quantum yield (ΦCO2) of Criollo cocoa was lower (0.010 μmol (CO2) μmol (photon)-1) than Forastero and from other species of Theobroma (de Almeida et al. 2014) indicating a lower light use efficiency. This can probably be a characteristic of Criollo cocoa suggesting greater sensitivity to light and the importance of cultivating this type of cacao under shade conditions. Although cocoa can tolerate high PFD, productivity and sustainability of farming and control of other biotic stresses is more efficient at lower PFD (Tscharntkeet al. 2011).
There were no differences in Fv/Fm (values were around 0.80) between cocoa types, without showing signs of photoinhibition indicating that the potential capacity of photosystem II is similar in Criollo and Forastero cocoa, Criollo cocoa had higher ΦPSII than Forastero. The electron transport (J), equivalent to a value of A of 5-6 μmol m-2s-1, could partially explain the low rate of photosynthesis in cocoa, as was recently reported by Ávila-Lovera et al. (2016). A low rate of electron transport may cause a low rate of carboxylation due to a low synthesis of RuBP because of underproduction of photochemical compounds such as ATP and NADPH.
The response of fluorescence variables (J, ΦPSII, qP and qN) to PFD was similar in both types of cocoa indicating a similar photochemical capacity of PSII, which is consistent with the similarity in total chlorophyll content. Values of J, ΦPSII, qP and qN were similar to those reported in younger Criollo cocoa trees (Araque et al. 2012; Ávila-Lovera et al. 2016). Chlorophyll content was lower than that reported for eight genotypes of cocoa (Daymondet al. 2011).
Leaf N concentration and Specific leaf area. Foliar N values were higher than those reported for 8-year-old trees from agroforestry systems in Ghana (Isaac et al. 2007) without pre-fertilization during rainy periods. These contents were similar to those obtained by Araque et al. (2012) in fertilized three-year-old Criollo cocoa plants and five-year-old Criollo plants grown in a germplasm bank (Ávila-Lovera et al. 2016). It would be important to measure leaf N content during drought because decreases in leaf N concentration have been reported for Criollo cocoa (Araque et al. 2012; Ávila-Lovera et al. 2016). Foliar N expressed by weight was higher in the Criollo cocoa, which may confer a better nitrogen use as revealed by the higher A in this type of cocoa. The higher value of δ15N Criollo cocoa also suggests a higher content of soluble nitrogen available for photosynthesis (Evans 2001).
Forastero had the highest values of SLA, which is the result of a lower content of mechanical tissue, such as cellulose and lignin (Niinemets and Kull 1998). These values are similar to those reported in Margarita Island cocoa from a germplasm bank in the Central region of Venezuela (Pereyra 2007) and lower than reported for saplings grown in greenhouse conditions (Daymond et al. 2011).
Ecophysiological similarities and differences between the two types of cocoa. The increased water use efficiency of Criollo cacao, due to higher A and E compared to Forastero could make this variety suitable for cultivation in regions with periods of restricted water supply. Additionally, the δ13C was higher than their counterpart young trees in a germplasm bank from Central Venezuela, indicating a greater integrated WUE. Photosynthetic response to Ci and PFD and photosystem II activity of both types of cocoa was similar; in contrast, the differences in ΦCO2, leaf N content, SLA and instantaneous gas exchange indicate that Theobroma cacao exhibits physiological plasticity. This agrees with results of A, gs, and N by Daymond et al. (2011) in eight cocoa genotypes.
Conclusions
Trees of Criollo and Forastero cultivars over 50 years of age had a photosynthetic rate similar to their younger counterparts. The prevalence of these trees for many years in this environment, with annual intervals with low rainfall, indicates that these cultivars can grow successfully in geographic areas with seasonal drought. Furthermore, the results show that the best quality Criollo cocoa is not physiologically at a disadvantage with the Forastero, at least from the perspective of their photosynthetic performance.











 nueva página del texto (beta)
nueva página del texto (beta)


